Abstract
Ty1 elements are long terminal repeat (LTR) retrotransposons that reside within the genome of Saccharomyces cerevisiae. It has been known for many years that the 2′-5′ phosphodiesterase Dbr1p, which debranches intron lariats, is required for efficient Ty1 transposition. A recent report suggested the intriguing possibility that Ty1 RNA forms a lariat as a transposition intermediate. We set out to further investigate the nature of the proposed Ty1 lariat branchpoint. However, using a wide range of techniques we were unable to find any evidence for the proposed lariat structure. Furthermore, we demonstrate that some of the techniques used in the initial study describing the lariat are capable of incorrectly reporting a lariat structure. Thus, the role of the Dbr1 protein in Ty1 retrotransposition remains elusive.
Keywords: Ty1, retrotransposition, RNase protection, lariat
INTRODUCTION
Lariat RNAs are well-known intermediates in and products of pre-mRNA splicing. There are several well-described methods for establishing the presence and structure of such molecules, which consist of a circular portion, and a 3′’ “tail”. During pre-mRNA splicing, the circular portion of the lariat is generally produced via a transesterification reaction between the 2′-hydroxyl group of an internal residue (typically an adenosine) and the 5′ end of a precursor linear RNA (Padgett et al. 1984; Ruskin et al. 1984). Lariat RNA structures are also naturally formed by group II self-splicing introns (Michel and Ferat 1995), and have been synthetically created using both ribozymes (Tuschl et al. 2001) and deoxyribozymes (Wang and Silverman 2003). Due to their unusual shape, lariats migrate more slowly than do corresponding linear RNAs during gel electrophoresis (Ruskin et al. 1984; Ruskin and Green 1985).
A recent publication reported evidence suggesting that a very large (> 5000 nucleotides) lariat RNA was an intermediate in Ty1 retrotransposition (Cheng and Menees 2004). The proposed structure of this molecule suggested that it could not possibly have been produced by pre-mRNA splicing because (1) Ty1 contains no known introns and (2) the circular portion of the lariat appeared to have been formed from a linear capped precursor mRNA through some novel mechanism. Since detection methods for such large lariats are not well established, and because there was some doubt about whether the precise site of the 2′-5′ linkage in the putative lariat had been correctly mapped (Perlman and Boeke 2004), we evaluated several techniques for identifying and mapping such molecules.
We evaluated a number of lariat detection methods, including the detection of small, branched RNase H digestion products with predictable electrophoretic properties, as well as demonstrating that large circular molecules very similar in structure to that of the proposed intron lariat could be easily detected on formaldehyde agarose gels because of their altered electrophoretic mobilities. We were unable to detect molecules with such mobilities from in vivo Ty1 RNA preparations. Finally, we evaluated an RNase T1 protection method used by Cheng and Menees (2004) and found this method capable of erroneously reporting the presence of lariat structures when none were present. In short, we were unable to find evidence, using any of these methods, for the proposed lariat structure.
RESULTS AND DISCUSSION
Electrophoretic mobility of circular Ty1 RNA molecules
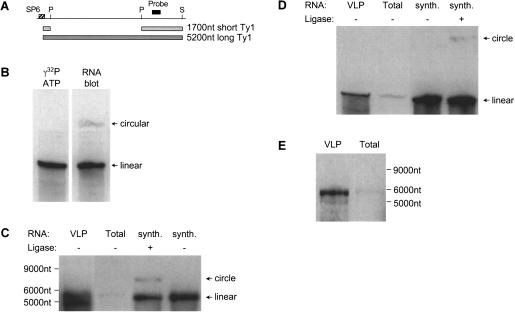
The lariat or circular structure of RNA has been frequently reported to impede its migration during denaturing polyacrylamide gel electrophoresis (Ruskin et al. 1984; Ruskin and Green 1985; Chapman and Boeke 1991; Chen and Sarnow 1995). To test the hypothesis that a lariat Ty1 RNA, similar to that described by Cheng and Menees (2004), would have a lower electrophoretic mobility than that predicted by its molecular weight, we prepared in vitro a large circular Ty1 RNA molecule (corresponding to Ty1 nucleotides 241–5463; all Ty1 coordinates are from Boeke et al. 1988) and examined its migration during gel electrophoresis. Such a molecule is very similar in size and overall topology to the proposed lariat and therefore should provide a straightforward and direct assay for the predicted Ty1 lariats. To produce circular Ty1 RNAs, linear RNA was in vitro transcribed in the presence of guanosine monophosphate (to ensure that the RNA would be a substrate for ligation), and the two ends were joined by DNA oligonucleotide-assisted ligation using T4 DNA ligase (Chen and Sarnow 1995, 1998). To confirm that we could in fact generate a circular molecule using this technique, we first analyzed the migration of a shortened 1.75-kb circularized Ty1 RNA molecule (with an internal deletion between Ty1 nucleotides 478 and 3946). This RNA circle is in the size range for which aberrant migration of yeast intron lariats has been previously documented (Michel and Ferat 1995). Following denaturing polyacrylamide gel electrophoresis, two major RNA species were observed in the sample containing DNA ligase. The first is the linear precursor, also present in the control reaction containing no ligase, and the second, a slower-migrating RNA species presumably representing the ligated form of the RNA (Fig. 1B ▶). To determine whether this novel RNA species was circular, we attempted to label the ligated RNA using T4 polynucleotide kinase, reasoning that only a linear RNA with a free 5′ phosphate group could incorporate radioactivity, whereas a circular molecule should resist such incorporation. Consistent with our prediction, no incorporation of labeled phosphate into the slower-migrating RNA species was observed, indicating that the product of the ligation reaction was in fact a circularized RNA. Furthermore, the electrophoretic mobility of this product was inconsistent with it being a linear dimer of the starting RNA.
FIGURE 1.
Electrophoretic mobility of in vitro circularized Ty1 RNA. (A) Schematic representation of the short (Ty1 nucleotides 241–478 adjacent to 3946–5463; Boeke et al. 1988) and long (Ty1 nucleotides 241–5463; Boeke et al. 1988) SP6 promoter driven Ty1 RNA in vitro transcription constructs. The positions of PvuII (P) and SnaBI (S) restriction sites, used respectively to create the deletion for the short Ty1 construct and to linearize the template prior to transcription are shown. The position of the Ty1-specific riboprobe (Ty1 nucleotides 4232–4356; Boeke et al. 1988) used in hybridizations is indicated by a solid box. (B) Northern blot to confirm circularization of short Ty1 construct. Transcribed short RNAs were in vitro ligated using T4 DNA ligase, dephosphorylated with calf alkaline phosphatase, and then labeled with either γ32P-ATP or ATP using T4 polynucleotide kinase. Following electrophoresis through denaturing polyacrylamide (2.5%/8 M urea), gel sections were exposed directly to X-ray film (γ-32P labeled) or electroblotted to nylon and probed with the Ty1-specific riboprobe. (C) Northern blot analysis of circularized long Ty1 RNA construct. Transcribed long Ty1 RNAs were in vitro ligated using T4 DNA ligase. Ligated RNAs were separated through 1.3% agarose formaldehyde-containing gels alongside 1 μg VLP and 10 μg total RNA from dbr1 mutant yeast. Northern blots were probed with the Ty1-specific riboprobe. The positions of Ambion Millennium Marker RNA molecular weight standards are indicated. (D) Experiment identical to C except that samples were separated through denaturing polyacrylamide (2.5%/8 M urea). (E) Formaldehyde agarose gel electrophoresis of 100 ng VLP and 5 μg total RNA from independent preparations of dbr1 mutant yeast cell RNA, separated and probed as in C. The positions of Ambion Millennium Marker RNA standards are indicated.
Analysis of the full-length construct also identifies a novel Ty1 RNA species that consistently migrates more slowly than its linear precursor on both formaldehyde agarose (1.3%–2%) and low-percentage denaturing polyacrylamide (2.5%/8 M urea) gels. In all cases the linear unligated Ty1 RNA has a mobility consistent with its molecular weight, whereas the novel species, inferred to be circular Ty1 RNA, migrates at a position between the 6-kb and 9-kb markers on formaldehyde agarose gels, and much slower than reliable size standards available to us during polyacrylamide gel electrophoresis (Fig. 1C,D ▶). The mobility on agarose clearly indicates that this ligation product is indeed circular, and not a tandem ligation of two linear RNAs. Furthermore, no “laddering” indicative of trans-joining of linear RNAs was observed. Based on these observations, we would expect that the predicted lariat form of endogenous Ty1 RNA should also demonstrate impeded mobility during gel electrophoresis. However, Ty1 RNA prepared from dbr1 mutant virus-like particles (VLPs) and total cellular fractions migrates strictly as predicted by its molecular weight (Fig. 1C–E ▶), indicating that it consists solely of a linear form. Identical electrophoretic behavior was observed for RNA prepared from wild-type samples (data not shown). While we cannot exclude the possibility that single nicks were introduced into lariat Ty1 molecules during RNA preparation, resulting in their migration as a linear species, the mobility of in vivo Ty1 RNA that we observed is consistent with that of previous reports, including the report describing the proposed lariat (Elder et al. 1983; Winston et al. 1984; Garfinkel et al. 1985; Cheng and Menees 2004). Furthermore we are unaware of any previous studies reporting aberrant Ty1 RNA electrophoretic mobility under denaturing conditions. All blots were overexposed to exclude the possibility that only a very small quantity of nonlinear Ty1 RNA was present in Ty1 VLPs. In summary, these data suggest that endogenous Ty1 RNA does not migrate as a large lariat or circular molecule.
RNase H digestion
To further investigate the nature of the putative Ty1 RNA branch point, we probed the structure of the Ty1 RNA by targeted RNase H cleavage and Northern analysis. Using two DNA oligonucleotides designed to target RNase H to unique sequences at the 5′ and 3′ ends of the Ty1 RNA, we predicted detection of a 355-nucleotide (nt) digestion fragment from linear Ty1 RNA and a novel 803-nt digestion fragment from a branched Ty1 RNA (such a molecule would be predicted to migrate at 803 nt or slower due to its branched nature), when hybridized with a unique 5′ end probe (Fig. 2A ▶). One major advantage of using a two-oligonucleotide digestion strategy to detect a lariat RNA is that successful digestion generates a novel RNA species that can be detected on a gel, in contrast to the product of single-oligonucleotide digestion, which Cheng and Menees (2004) report to be indistinguishable from an undigested Ty1 RNA. Furthermore, such a novel branched RNA species would also be observed in RNA samples that had undergone slight degradation or nicking during preparation. A similar strategy has been successfully employed by Chu et al. (1998) to study branch structure in group II introns.
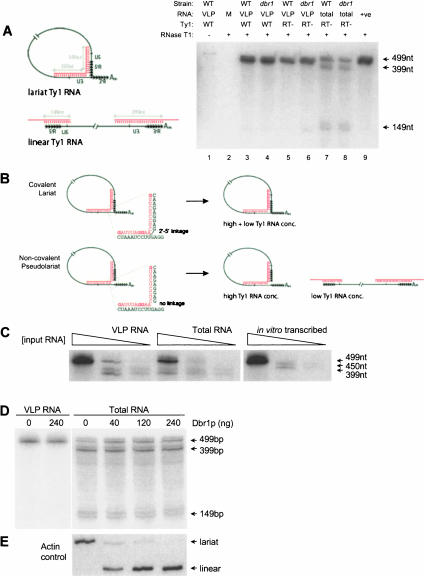
FIGURE 2.
Northern analysis of RNase H-digested Ty1 RNA. (A) Targeted RNase H digestion of Ty1 RNA with two DNA oligonucleotides. Schematics indicate the annealing position of targeting (C,D) and probe (1) oligonucleotides, and the predicted size of RNase H digestion fragments, excluding the size of the polyA tail expected to be present on the lariat Ty1 digestion fragment. RNAs were digested with increasing quantities of oligonucleotide: 0 ng, 1 ng, 2.5 ng, 5 ng, and 10 ng as indicated. Arrows indicate the positions of major RNase H digestion fragments. (B) Targeted RNase H digestion of Ty1 RNA with a single oligonucleotide. Schematics indicate the positions of single targeting (A–C) and probe (1 and 2) oligonucleotides used and the predicted sizes of RNase H digestion fragments, excluding the size of the polyA tail. RNAs were digested with 50 ng of the oligonucleotide indicated. The positions of major digestion fragments are indicated by arrows. It is important to note that the fragments labeled 839 nt and 941 nt do not include a size adjustment for the polyA tail. RNase H digestions shown in both A and B were separated on the same gel, and the Northern blot sequentially hybridized with probes 1 and 2.
RNase H cleavage of wild-type and dbr1 mutant VLP RNA generates only the 355-nt fragment and no discrete slower-migrating products (Fig. 2A ▶). This indicates that this segment of the Ty1 RNA lacks the predicted branch, and suggests that VLP RNA consists of a pure population of linear Ty1 molecules. Furthermore, a linear cleavage pattern was observed following digestion of dbr1 mutant total cellular RNA (data not shown). Also consistent with these results, RNase H-targeted digestion of the same RNAs with a single oligonucleotide, which is expected only to linearize the proposed lariat RNA species, generates only cleavage products of a size indicative of purely linear RNA being present (Fig. 2B ▶). These data suggest that, in contrast to the recent report by Cheng and Menees (2004), the Ty1 RNA present in various VLP preparations is linear and lacks the reported branch. As yet, we are unable to provide an explanation for the discrepancies between this RNase H study and that performed by Cheng and Menees (2004).
Primer extension
The 2′-5′ phosphodiester linkage of RNA lariat branch points effectively blocks reverse transcription from the 3′ side of the branch; this property has been used to map the branched nucleotide position of intron lariats by primer extension (Rodriguez et al. 1984; Padgett et al. 1985). To analyze the position of the putative Ty1 RNA branch point, we performed primer extension using oligonucleotides (6715 and 6716) designed specifically to anneal to polyadenylated Ty1 RNA by virtue of complementarity to both the extreme 3′ R sequences in the LTR and the polyA tail (Elder et al. 1983). Extension reactions performed using both wild-type and dbr1 mutant VLP RNAs demonstrated no apparent strong block to transcription by an RNA branch point, and clearly lacked the predicted 62-nt (6715) or 70-nt (6716) products corresponding to a block to transcription at the reported Ty1 RNA branch point (Fig. 3A ▶). Furthermore a smear of larger than expected extension products was seen, indicating that the polymerase proceeds until it became either randomly disassociated or inhibited by secondary structures distal to the purported branch site. As a control, we performed primer extension using an oligonucleotide (6673) specific to the 5′ end of the RNA that produces a defined extension product of 149 nt from linear Ty1 RNA, the reportedly predominant RNA species present in wild-type VLPs (Cheng and Menees 2004). Using the same RNA samples, this reaction produced a single abundant primer extension product of the expected size. These data do not support a branch point near the 3′ end of the Ty1 RNA molecule.
FIGURE 3.
(A) Primer extension analysis of Ty1 RNA. Schematic indicates the annealing position and orientation of primers (6673, 6715, and 6716) on both lariat and linear RNA templates. Samples numbered 1 and 3 represent two independent preparations of wild-type VLP RNA; samples numbered 2 and 4 represent two independent preparations of dbr1 mutant VLP RNA. RNA samples 1 and 2 were prepared from cells induced for 24 h, and samples 3 and 4 were from cells induced for 6 h. Samples numbered 5 and 6 are no RNA and no AMV RT negative controls, respectively. The positions of molecular weight markers are indicated. The arrowhead indicates the position of the positive control 149-nt primer extension product. (B) RLM-RACE analysis of RNA prepared from both wild-type and dbr1 mutant yeast strains expressing either a wild-type or RT mutant GAL-Ty1 construct. Reactions were performed both with and without pretreatment of the RNA with phosphatase and pyrophosphatase (Phos +/−). RLM-RACE RT-PCR was performed using a sense primer specific to the 5′ annealed RNA oligo and a gene-specific antisense primer. RACE products were cloned and sequenced for verification. As an internal control for reverse transcription and Ty1 RNA concentration, RT-PCR was performed using the same reactions with internal primers 6668 and 6669. (C) Semiquantitative RLM-RACE RT-PCR was performed exactly as described in B. The number of cycles for which RT-PCR was performed is indicated. (D) RT-PCR across the lariat branch-point. Ty1 RNA prepared from both wild-type and dbr1 mutant VLPs was subjected to RT-PCR using primers 6673 and 6675 spanning the putative branchpoint. The number of PCR cycles performed is indicated. RT-PCR products were cloned and sequenced for product verification. (E) Generation of RT-PCR products from lariat or linear template RNA. An RT-PCR product could be generated by crossover PCR: 1) cDNA synthesis; 2) first-round PCR—annealing of the sense primer followed by extension; 3) second-round PCR—annealing of the first-round PCR product to the opposite end of the cDNA molecule due to complementarity between LTR regions (arrowheads), followed by extension; 4) third-round PCR—annealing of the antisense primer and extension to generate double-stranded PCR product. Similar crossover reaction products can in principle be obtained even in the absence of a full-length cDNA as illustrated in step 1. (F) Assessment of crossover PCR. RT-PCR was performed using in vitro transcribed full-length (FL; Ty1 nucleotides 241–5918) or 3′-truncated (5′ only; Ty1 nucleotides 241–5463) Ty1 RNAs, exactly as described in D.
5′ RACE
An intriguing observation made in the recent study by Cheng and Menees (2004) suggested that the 5′ end structure of Ty1 RNA from wild-type and dbr1 mutant VLPs is differentially accessible to amplification by RNA ligase-mediated 5′ rapid amplification of cDNA ends (RLM-RACE). Since we were unable to find evidence in favor of a branched Ty1 RNA in our previous assays, we performed RLM-RACE using our wild-type and dbr1 mutant VLP RNAs. During RLM-RACE, pretreatment of RNA with phosphatase and pyrophosphatase prior to annealing of the RNA oligo enables amplification of only capped 5′ RNA ends, whereas no pretreatment enables amplification of uncapped or non-mRNAs with just a single 5′ phosphate group. Using both wild-type and dbr1 mutant VLP RNA, we could readily amplify the 5′ end of the Ty1 RNA from both phosphatase treated and untreated samples (Fig. 3B ▶). Furthermore, RT-PCR analysis performed semiquantitatively revealed no obvious difference in amplification between wild-type and dbr1 mutant samples (Fig. 3C ▶). Sequencing of the major RACE product obtained indicates that the 5′ end of the Ty1 RNA is at nucleotide 241, consistent with previously published data (Mules et al. 1998; Cheng and Menees 2004). In summary, unlike Cheng and Menees (2004) who were unable to amplify the 5′ end of the Ty1 RNA from dbr1 mutant cells, we were able to easily amplify capped 5′ ends and were unable to observe a difference in 5′ end accessibility to amplification between wild-type and dbr1 cells with this assay.
RT-PCR across the putative lariat branchpoints
Reverse transcription-polymerase chain reaction (RT-PCR) has previously been used in a number of studies to determine the branched nucleotides of intron lariats (Vogel et al. 1997; Salgia et al. 2003; Odom et al. 2004). Like Cheng and Menees (2004), we are readily able to detect an RT-PCR product from Ty1 RNA that is consistent with a branch-point located precisely at the U3/R junction (Fig. 3D ▶). This product is consistent with the proposed lariat but could also be readily produced from linear RNA by “crossover PCR”. Crossover PCR from a linear template is possible due to the 50-nt region of near sequence identity (LTR region R) present at both the 5′ and 3′ ends of the Ty1 RNA (arrowheads, Fig. 3E ▶). To assess whether we could detect a difference in the quantity of putative lariat RNA present in wild-type and dbr1 mutant cells, we performed semiquantitative RT-PCR across the branchpoint. Using this assay, we were unable to observe any difference in the quantity of PCR product detected in wild-type and dbr1 mutant VLPs (Fig. 3D ▶). To address the possibility of crossover PCR generating this product, we performed RT-PCR using in vitro transcribed but uncapped linear Ty1 RNA. As predicted for a crossover product, an RT-PCR product was amplified from a full-length linear in vitro transcribed Ty1 RNA (Ty1 nucleotides 241–5918) but not from a truncated linear RNA missing the 3′ LTR (Ty1 nucleotides 241–5463) (Fig. 3F ▶). RT-PCR products were sequenced to verify correct amplification. These data suggest that RT-PCR is not a reliable technique for determination of the putative Ty1 lariat branchpoint structure, due to the presence of the direct repeats.
RNase protection
As a final attempt to detect a Ty1 RNA branchpoint, we performed RNase T1 protection, a more sensitive technique used by Cheng and Menees to demonstrate that a Ty1 RNA species prepared from dbr1 mutant VLPs could robustly protect a branch-point-spanning probe (Cheng and Menees 2004). Using an RNA probe spanning the putative branch point (Ty1 nucleotides 389–241 joined to 5824–5475), we similarly observed a 499-nt fragment consistent with protection by a Ty1 lariat RNA. This species was, in our hands, completely protected in VLP RNA prepared from both wild-type and dbr1 mutant cells (Fig. 4A ▶) when RNase T1 was used; such products were not observed with RNase A. Additionally, in total RNA prepared from the same strains, the 499-nt fragment is protected along with two fragments of 399 nt and 149 nt that correspond to protection of the probe by a linear Ty1 RNA (Fig. 4A ▶). The protected lariat probe species is very similar to the one observed by Cheng and Menees (2004), although they reportedly detected it only in dbr1 mutant VLP RNA, and had difficulty observing it in total RNA or in RNA prepared from wild-type VLPs. These facts led us to consider the possibility that such T1 RNase protection assays can erroneously imply the existence of a branched structure when in fact there is none. For example, at high concentrations of Ty1 RNA (with respect to probe), as in VLP RNA preparations, most probes could span both ends of the same RNA (an intramolecular reaction), while at lower RNA concentrations (as in some total RNA preparations), the probe molecule concentration will exceed Ty1 RNA concentration and thus will more easily hybridize to both ends of linear RNA molecules, giving the predicted “linear” protection products. We note that the G residues (putative T1 substrates) nearest the branchpoint lie 2 nt and 9 nt from it and thus could easily be protected by base-pairing in such a pseudolariat. Thus by this hypothesis the “branch” detected by this method could be noncovalent and stabilized simply by Watson-Crick base pairing between the probe molecule and nonadjacent segments in the Ty1 RNA molecule (Fig. 4B ▶).
FIGURE 4.
Ribonuclease protection assays. (A) RNase T1 protection of Ty1 RNA from wild-type and dbr1 mutant cells. Schematics indicate the positions of the unique Ty1 RNA LTR sequences U5 and U3, and the repeated LTR R region (the direction of the R repeat is indicated by arrows). The predicted sizes of RNase protection fragments are shown. RNase protection reaction conditions are indicated above each lane. RNAs were prepared from either wild-type (WT) or dbr1 mutant strains expressing either wild-type (WT, lanes 3,4) or reverse transcriptase mutant (RT-, lanes 5–8) GAL-Ty1 expression plasmids. Ten percent of the no RNase T1 control was loaded in lane 1. To confirm complete digestion of the probe by RNase T1, and to control against false positive results due to probe self-protection, mouse liver RNA (M) was used as a nonspecific RNA target in lane 2. An in vitro transcribed sense RNA (corresponding to Ty1 nucleotides 5413–5824 joined to 241–486) was used as a positive control for protection by the putative lariat RNA structure (lane 9). The sequence of the RNA probe complementary to Ty1 corresponds to nucleotides 389–241 adjacent to 5824–5475. Sizes of RNase protection fragments detected are indicated. (B) Model for RNA probe protection by covalent and non-covalent “pseudolariats.” The positions of G residues (substrate for RNase T1 digestion) in the probe nearest to the proposed branch are indicated in bold type. A true covalent lariat structure of the type proposed would be predicted to protect a full-length complementary probe independent of template RNA concentration. At high template concentration (such as when template is present in molar excess of probe), the probe is predicted to artificially create a non-covalent pseudolariat structure resulting in detection of a full-length protection fragment. At low template concentration (when probe is present in molar excess of template), each linear template RNA molecule would likely have a probe hybridized at each end, preventing artificial lariat detection. (C) Titration of input RNAs. RNase protection was performed exactly as in A, except that the quantity of input template RNA was titrated over a single order of magnitude (VLP RNA dilutions: 1, 1/4, 1/10; total RNA dilutions: 1, 1/2.5, 1/10; in vitro transcribed RNA dilutions: 1, 1/25, 1/100). A larger linear probe fragment (450 nt) is protected by in vitro transcribed template (Ty1 nucleotides 241–5918), since the transcribed RNA includes the U3 region at the 3′ end of the RNA that is not normally present in endogenous Ty1 RNA samples. (D) Template VLP or total RNA was pre-incubated with of 0, 40, 120, or 240 ng purified Dbr1p (Nam and Boeke 1994) and then subjected to RNase protection exactly as described in C. (E) Verification of debranching by Dbr1p. An aliquot of each of the total RNAs used for RPA was separated on a 5% polyacrylamide/8 M urea gel, electroblotted to nylon membrane, and hybridized with an actin intron-specific probe. The position of branched and debranched intron lariat species are indicated.
To determine whether the RNase T1 protection assay (RPA) indeed detects a branched Ty1 RNA, we titrated the RPA input RNA concentration to determine whether protection of the full-length 499-nt probe fragment remains constant despite a change in the molar ratio between template and probe RNA, as would be predicted for a true lariat. In both VLP and total RNA samples, reducing the concentration of input RNA results in a progressive shift from protection of the 499-nt (branched RNA) probe fragment to protection of the 399-nt and 149-nt fragments corresponding to linear Ty1 RNA termini (Fig. 4C ▶). Furthermore, RPA using an in vitro synthesized linear Ty1 RNA shows similar titratable protection of the branched RNA probe fragment (Fig. 4C ▶). In contrast to this, titration of a positive control in vitro transcribed sense RNA complementary to the probe (Ty1 nucleotides 5413–5824 adjacent to 241–486) does not show a change in protection profile with increasing template dilution (data not shown). We additionally observed protection of the full-length probe 499 nt using separately transcribed synthetic short linear RNAs corresponding to the 5′ and 3′ ends of the probe (data not shown).
As a final assessment of the viability of this technique for detecting a lariat RNA structure, we pretreated dbr1 mutant VLP and total RNA preparations with highly purified Dbr1p protein (Nam et al. 1994), and then performed RPA using these samples (Fig. 4D ▶). While we can demonstrate by Northern blotting that debranching of the actin intron lariat has reached completion (Fig. 4E ▶) (Chapman and Boeke 1991), we could observe no change in the Ty1 RNase protection pattern of either VLP or total RNA samples (Fig. 4D ▶). In summary, these data suggest that RNase T1 protection is not a reliable method for determining branchpoint structure at high template RNA concentrations, and much care needs to be taken in the interpretation of results obtained in such experiments.
Conclusions
A role for Dbr1 protein in Ty1 replication has been known for over a decade, with transposition of Ty1 reduced by nearly tenfold in dbr1 mutants (Chapman and Boeke 1991). Cheng and Menees (2004) provided an exciting model for a possible role of the Dbr1 protein in Ty1 retrotransposition. Regrettably, we find no evidence consistent with the proposed Ty1 lariat in our extensive studies attempting to follow up on their findings. While our study does not exclude the possibility that Ty1 RNA might form an alternative perhaps unstable or transient branched structure not processed by Dbr1p, we conclude that the role of Dbr1 in retrotransposition thus remains enigmatic.
MATERIALS AND METHODS
Yeast strains
All strains used were descendants of strain JB224 (MATα ura3-167 his3Δ200), which carries the wild-type GAL-Ty1 plasmid pJEF724. YKN127 is a dbr1::HIS3 derivative of this strain. Strains YCEC44 and YCEC50 are wild-type and dbr1 derivatives of the above strains containing plasmid pJEF724D211E, which is identical to pJEF724 except that it contains the D211E mutation in Ty1 RT (Uzun and Gabriel 2001). Since Ty1 reverse transcription is accompanied by RNase H degradation of template RNA, it was suggested that use of the Ty1 RT mutant might increase detection of RNA branches (Cheng and Menees 2004). In the present study, the Ty1 RT mutant strain was analyzed in RNaseH digestion, RNase protection, RT-PCR, and RACE experiments. In no case was a significant difference observed between wild-type and RT mutant VLP RNAs. The Dbr− phenotype of the dbr1 strains used was confirmed by Northern analysis of the actin intron.
In vitro RNA circularization
Ty1 RNA was in vitro transcribed from the SP6 promoter of linearized template plasmids pCEC24 (Ty1 nucleotides 241–5463) or pCEC25 (Ty1 nucleotides 241–478 joined to 3946–5463) using the MAXIscript in vitro transcription kit (Ambion). Transcription was performed in the presence of a tenfold excess of guanosine monophosphate (5 mM GMP) in addition to the triphosphates (500 μM ATP, CTP, GTP, UTP). Template DNA was removed by DNase digestion, and RNAs were purified using the RNeasy mini kit (QIAGEN). Circularization reactions were performed essentially as described by Chen and Sarnow (1995, 1998). Briefly, linear RNAs were annealed with branching oligonucleotides JB7675 (CAGAATATACTAGAAGTTCTCCTCNGTATAAATTATTACCT GATACTTC) and JB7676 (CAGAATATACTAGAAGTTCTCCTC GTATAAATTATTACCTGATACTTC) in a 300 μL reaction containing 100 mM NaCl and 1 mM EDTA. Annealing reactions were heated to 90°C for 3 min, cooled slowly to room temperature, ethanol precipitated, and resuspended in RNase-free water. Ligation was performed using T4 DNA ligase (New England Biolabs) at 16°C for 16 h. Reactions were stopped by phenol:chloroform extraction, followed by ethanol precipitation and resuspension of RNA in RNase-free water.
To assess RNA circularization, DNA splint oligonucleotides were first digested with Turbo DNase (Ambion) to prevent their subsequent labeling; samples were ethanol precipitated and resuspended in RNase-free water. RNAs were dephosphorylated with calf intestinal phosphatase (Invitrogen) at 50°C for 1 h, and then purified using the QIAGEN RNeasy mini kit. Dephosphorylated RNA was rephosphorylated with either [γ-32P] ATP (Amersham Biosciences) or unlabeled ATP using T4 polynucleotide kinase (New England Bio-labs). Labeled RNAs were ethanol precipitated and resuspended in RNase-free water.
Preparation of VLP and total RNAs
Yeast cells harboring either GAL-Ty1 expression plasmid pJEF724 (Boeke et al. 1985) or RT catalytic mutant pJEF724 D211E (Uzun and Gabriel 2001) were grown first in 2% raffinose containing medium at 30°C for 6 h and then in 2% galactose-containing medium at 22°C for ~24 h as described previously (Eichinger and Boeke 1988). Cell pellets from 500 mL culture were processed for VLP isolation (Eichinger and Boeke 1988), and RNA was prepared according to the method described by Fu and Rein (1993). Yeast cell pellets from 10 mL of the same culture were used for total RNA isolation. Total RNA was prepared from cell pellets by acid phenol extraction (Collart and Oliveiro 1993). RNA integrity and concentration were assessed by gel electrophoresis and absorbance at 260 nm.
Northern blot analysis
RNAs were fractionated by denaturing gel electrophoresis through either 1.3%–2% agarose or 2.5%–5% polyacrylamide/8 M urea gels. RNA was transferred to a Gene Screen Plus charged nylon membrane (NEN Life Sciences) either by passive transfer using 10× SSC, or by electroblotting in 1× MOPS in accordance with manufacturer’s instructions, and then fixed by UV crosslinking. Membranes were hybridized with antisense riboprobes synthesized using the MAXIscript in vitro transcription kit (Ambion), or oligonucleotide probes end-labeled using T4 polynucleotide kinase (New England Biolabs). The Ty1-specific riboprobe described in Figure 1 ▶ was synthesized from the T7 promoter of linearized plasmid pCEC28 containing Ty1 nucleotides 4232–4356. The actin intron-specific riboprobe detailed in Figure 4 ▶ was synthesized from the T7 promoter of linearized plasmid pCEC29 containing nucleotides 54630–54685 adjacent to 54421–54494 of sequence accession number D50617 (GI: 2804269). Oligonucleotide probe sequences are described below. Filters were exposed either directly to X-ray film or to a Fuji Phosphorimager screen.
RLM-RACE
5′ RACE was performed using the Invitrogen Gene Racer system. Briefly, VLP RNA samples were either treated (Phos +) or not treated (Phos −) with calf intestinal phosphatase (to remove the 5′ phosphate from uncapped RNAs) and tobacco acid pyrophosphatase (to remove the mRNA cap) prior to ligation of an RNA oligo (5′-CGACUGGAGCACGAGGACACUGACAUGGACUGAAGGA GUAGAAA-3′). To determine the 5′ end of the Ty1 RNA, ligated RNAs were reverse transcribed using random hexamer primers and PCR-amplified using an anchored forward primer specific to the annealed RNA oligo (CGACTGGAGCACGAGGACACTGA) and a Ty1 gene-specific reverse primer (CGGATCTTGATTTGT GTGGA). Internal control RT-PCR amplifications used primers 6668 (CTTACGACTCAGGCATGC) and 6669 (TGCATACAAAT ATGCCGAAGA). All PCRs were performed using QIAGEN Hotstar Taq DNA polymerase with touchdown cycling parameters: 95°C for 15 min; 94°C for 30 sec, 70°C for 45 sec × four cycles; 94°C for 30 sec, 65°C for 45 sec × four cycles; 94°C for 30 sec, 58°C for 30 sec, 72°C for 30 sec × 15–25 cycles. RT-PCR products were cloned using the TOPO TA cloning kit (Invitrogen) and sequenced for verification.
RNase T1 protection
RNase T1 protection assays (RPA) were performed using the Ambion RPA III kit following the manufacturer’s protocol. The RNA probe for RPA was synthesized from the T7 promoter of linearized plasmid pCEC26 (cloned branchpoint spanning RT-PCR product corresponding to Ty1 nucleotides 389–241 joined to 5824–5475) using the Ambion MAXIscript in vitro transcription kit. Standard RPA reactions were performed using 0.5 μg VLP RNA or 5 μg total RNA. Positive control sense RNA complementary to the probe was synthesized from the T7 promoter of linearized plasmid pCEC27 (cloned branchpoint spanning RT-PCR product corresponding to Ty1 nucleotides 5413–5824 adjacent to 241–486). For input RNA titration experiments, RPA was performed using 3–30 ng VLP RNA and 0.7–7 μg total RNA. Full-length Ty1 sense control RNA was synthesized from the SP6 promoter of linearized plasmid pCEC24 (Ty1 nucleotides 241–5463) using the Ambion MAXI-script in vitro transcription kit. For RPA, 0.08–8 ng transcribed RNA was used. RPA products were separated on denaturing 4% polyacrylamide/8 M urea gels. For imaging, gels were exposed directly to X-ray film at -80°C.
Primer extension
Extension primers JB6673 (CGGATCTTGATTTGTGTGGA), JB6715 (TTTTTTTTTTTTGATAAAGGCT) and JB6716 (TTTTTT TTTTTTCCATTGTTGA), which span the two major Ty1 polyadenylation sites (Elder et al. 1983) were labeled using T4 polynucleotide kinase (New England Biolabs) and annealed to 100 ng VLP RNA in a reaction containing 150 mM KCl, 10 mM Tris-HCl (pH 8.3), and 1mM EDTA. Polymerization was performed using 5U AMV reverse transcriptase (New England Biolabs) at 42°C for 1 h under the manufacturer’s conditions in the presence of 0.14 mg/mL actinomycin D. Template RNA was digested with RNase A, and extension products were phenol:chloroform extracted, ethanol precipitated, and resuspended in gel loading dye (0.05% bromophenol blue and xylene cyanol, 20 mM EDTA, 95% for-mamide). Samples were separated on a denaturing 9% polyacrylamide/7 M urea gel and exposed directly to X-ray film at −80°C.
RT-PCR across the putative branchpoint
Reverse transcription was performed from 50 ng VLP RNA using the Superscript 1st strand cDNA synthesis kit from Invitrogen following the manufacturer’s protocol. RT-PCR primers 6672 (CGAGACCAAGAAGAACATTGC) and 6673 (CGGATCTT GATTTGTGTGGA) were used to amplify across the branchpoint. PCRs were performed using QIAGEN Hotstar Taq DNA polymerase with cycling parameters: 95°C for 15 min; 94°C for 30 sec, 58°C for 30 sec, 72°C for 1 min × 15–25 cycles. RT-PCR products were cloned using the TOPO TA cloning kit (Invitrogen) and sequenced for verification.
RNase H digestion
RNase H was targeted to digest either 500 ng VLP or 10 μg total RNA using oligonucleotides A (CAACATATGAAGCTAGACCA), B (GTATCAACTCATATGTCATG), C (TTGTCATCACATCAG CAATG), and D (ATGGGTGTCCGTAAAATGAC) as described by Green (Green and Noller 1996). Digestion fragments were separated on a 1.3% agarose denaturing gel and transferred to nylon membrane as described in the Northern blot analysis. Oligonucleotide probes 1 (CATTCTGTGGAGGTGGTACTGAAGCA) and 2 (TTGTCATCACATCAGCAATG) were labeled using T4 polynucleotide kinase (New England Biolabs) and hybridized to RNA immobilized on membranes. Blots were exposed to Fuji Phosphorimager screens.
Dbr1p digestion of VLP and total RNAs
First, 750 ng VLP RNA or 14μg total RNA was incubated for 1 h at 30°C with 0 ng, 40 ng, 120 ng, and 240 ng highly purified Dbr1p (Nam et al. 1984) in a 15-μL reaction containing 20 mM HEPES-KOH (pH7.6), 125 mM KCl, 0.5 mM MgCl2, 1 mM DTT, 10% glycerol. Digested RNAs were extracted with phenol:chloroform, precipitated with ethanol, and resuspended in RNase-free water.
Acknowledgments
This work was supported by NIH grant GM36481. We thank Rachel Green, Joan Curcio, and Henry Levin for helpful discussions and Robert Yarrington and Mattias Cardell for helpful comments on the manuscript.
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.7124405.
REFERENCES
- Boeke, J.D., Garfinkel, D.J., Styles, C.A., and Fink, G.R. 1985. Ty elements transpose through an RNA intermediate. Cell 40: 491–500. [DOI] [PubMed] [Google Scholar]
- Boeke, J.D., Eichinger, D., Castrillon, D., and Fink, G.R. 1988. The Saccharomyces cerevisiae genome contains functional and nonfunctional copies of transposon Ty1. Mol. Cell Biol. 8: 1432–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman, K.B. and Boeke, J.D. 1991. Isolation and characterization of the gene encoding yeast debranching enzyme. Cell 65: 483–492. [DOI] [PubMed] [Google Scholar]
- Chen, C. and Sarnow, P. 1995. Initiation of protein synthesis by the eukaryotic translational apparatus on circular RNAs. Science 268: 415–417. [DOI] [PubMed] [Google Scholar]
- ———. 1998. Internal ribosome entry sites tests with circular mRNAs. Methods Mol. Biol. 77: 355–363. [DOI] [PubMed] [Google Scholar]
- Cheng, Z. and Menees, T.M. 2004. RNA branching and debranching in the yeast retrovirus-like element Ty1. Science 303: 240–243. [DOI] [PubMed] [Google Scholar]
- Chu, V.T., Liu, Q., Podar, M., Perlman, P.S., and Pyle, A.M. 1998. More than one way to splice an RNA: Branching without a bulge and splicing without branching in group II introns. RNA 4: 1186–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collart, M.A. and Olivero, S. 1993. Preparation of yeast RNA. In Current protocols in molecular biology (eds. F.M. Ausbrel et al.), pp. 13.12.1–13.12.5. Wiley, New York. [DOI] [PubMed]
- Eichinger, D.J., and Boeke, J.D. 1988. The DNA intermediate in yeast Ty1 element transposition copurifies with virus-like particles: Cell-free Ty1 transposition. Cell 54: 955–966. [DOI] [PubMed] [Google Scholar]
- Elder, R.T., Loh, E.Y., and Davis, R.W. 1983. RNA from the yeast transposable element Ty1 has both ends in the direct repeats, a structure similar to retrovirus RNA. Proc. Natl. Acad. Sci. 80: 2432–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, W. and Rein, A. 1993. Maturation of dimeric viral RNA of Moloney murine leukemia virus. J. Virol. 67: 5443–5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel, D.J., Boeke, J.D., and Fink, G.R. 1985. Ty element transposition: Reverse transcriptase and virus-like particles. Cell 42: 507–517. [DOI] [PubMed] [Google Scholar]
- Green, R. and Noller, H.F. 1996. In vitro complementation analysis localizes 23S rRNA posttranscriptional modifications that are required for Escherichia coli 50S ribosomal subunit assembly and function. RNA 2: 1011–1021. [PMC free article] [PubMed] [Google Scholar]
- Michel, F. and Ferat, J.L. 1995. Structure and activities of group II introns. Annu. Rev. Biochem. 64: 435–461. [DOI] [PubMed] [Google Scholar]
- Mules, E.H., Uzun, O., and Gabriel, A. 1998. In vivo Ty1 reverse transcription can generate replication intermediates with untidy ends. J. Virol. 72: 6490–6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam, K., Hudson, R.H.E., Chapman, K.B., Ganeshan, K., Damha, M.J., and Boeke, J.D. 1994. Yeast lariat debranching enzyme: Substrate and sequence specificity. J. Biol. Chem. 269: 20613–20621. [PubMed] [Google Scholar]
- Odom, O.W., Schenkenberg, D.L., Garcia, J.A., and Herrin, D.L. 2004. A horizontally acquired group II intron in the chloroplast psbA gene of a psychrophilic Chlamydomonas: In vitro self-splicing and genetic evidence for maturase activity. RNA 10: 1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett, R.A., Konarska, M.M., Grabowski, P.J., Hardy, S.F., and Sharp, P.A. 1984. Lariat RNAs as intermediates and products in the splicing of messenger RNA precursors. Science 225: 898–903. [DOI] [PubMed] [Google Scholar]
- Padgett, R.A., Konarska, M.M., Aebi, M., Hornig, H., Weissman, C., and Sharp, P.A. 1985. Nonconsensus branch-site sequences in the in vitro splicing of transcripts of mutant rabbit β-globin genes. Proc. Natl. Acad. Sci. 82: 8349–8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman, P.S. and Boeke, J.D. 2004. Molecular biology. Ring around the retroelement. Science 303: 182–184. [DOI] [PubMed] [Google Scholar]
- Rodriguez, J.R., Pikielny, C.W., and Rosbach, M. 1984. In vivo characterization of yeast mRNA processing intermediates. Cell 39: 603–610. [DOI] [PubMed] [Google Scholar]
- Ruskin, B. and Green, M.R. 1985. An RNA processing activity that debranches RNA lariats. Science 229: 135–140. [DOI] [PubMed] [Google Scholar]
- Ruskin, B., Krainer, A.R., Maniatis, T., and Green, M.R. 1984. Excision of an intact intron as a novel larait structure during pre-mRNA splicing in vitro. Cell 38: 317–331. [DOI] [PubMed] [Google Scholar]
- Salgia, S.R., Singh, S.K., Gurha, P., and Gupta, R. 2003. Two reactions of Haloferax vocanii RNA splicing enzymes: Joining of exons and circularization of introns. RNA 9: 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuschl, T., Sharp, P.A., and Bartel, D.P. 2001. A ribozyme selected from variants of U6 snRNA promotes 2′-5′ branch formation. RNA 7: 29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzun, O. and Gabriel, A. 2001. A Ty1 reverse transcriptase active-site aspartate mutation blocks transposition but not polymerization. J. Virol. 75: 6337–6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel, J., Hess, W.R., and Borner, T. 1997. Precise branch point mapping and quantification of splicing intermediates. Nucleic Acids Res. 15: 2030–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. and Silverman, S.K. 2003. Deoxyribozymes that synthesize branched and lariat RNA. J. Am. Chem. Soc. 125: 6880–6881. [DOI] [PubMed] [Google Scholar]
- Winston, F., Durbin, K.J., and Fink, G.R. 1984. The SPT3 gene is required for normal transcription of Ty1 elements in S. cerevisiae. Cell 39: 675–682. [DOI] [PubMed] [Google Scholar]