Abstract
In the mammalian testis, the germ line stem cells are a small subpopulation of type A spermatogonia that proliferate and ultimately differentiate into sperm under the control of both endocrine and paracrine factors. To study the early phases of spermatogenesis at the molecular level, an in vitro system must be devised whereby germ line stem cells can be either cultured for a prolonged period of time or expanded as cell lines. In the study reported here, we chose to immortalize type A spermatogonia using the Simian virus large T-antigen gene (LTAg) under the control of an ecdysone-inducible promoter. While the cells escaped the hormonal control after a finite number of generations and expressed the LTAg constitutively, their growth remained slow and the cells exhibited morphological features typical of spermatogonia at the light microscopic level. Moreover, the cells expressed detectable levels of protein markers specific for germ cells such as Dazl, and specific for germ line stem cells such as Oct-4, a transcription factor, and GFRα-1, the receptor for glial cell line–derived neurotrophic factor (GDNF). Further analysis confirmed the spermatogonial phenotype and also revealed the expression of markers expressed in stem cells such as Piwi12 and Prame11. Since the cells respond to GDNF by a marked increase in their rate of proliferation, this cell line represents a good in vitro model for studying aspects of mouse germ line stem cell biology.
Keywords: Type A spermatogonia, SV40, Large T antigen, Testis Germ line stem cell, GFRα-1, Glial cell line–derived neurotrophic factor (GDNF)
Introduction
Stem cells are unique cell populations that are able to undergo both self-renewal and differentiation and are found in the embryo, as well as in the adult animal. In the early mammalian embryo, pluripotent embryonic stem cells are derived from the blastocyst stage and have the ability to form any fully differentiated cell of the body. As the embryo develops, stem cells become restricted in their ability to form different lineages (multipotent stem cells). Multipotent stem cells are also found in a wide variety of adult tissues such as bone marrow and brain. However, in the adult animal, the ability of certain stem cells to differentiate can be restricted to only one cell lineage (unipotent stem cells). Examples of mammalian unipotent stem cells include the stem cells residing in the gut epithelium, the skin, and the seminiferous epithelium of the testis.
A widely accepted model for spermatogonial development has been originally proposed by Huckins and Oakberg [1–3]. In this scheme, the Asingle (As) spermatogonia are the putative stem cells. They can self-renew or differentiate into Apaired (Apr) spermatogonia that remain connected by an intercellular bridge. The Apr spermatogonia further divide to form chains of 4, 8, or 16 Aaligned (Aal) spermatogonia. Further, the Aal cells will differentiate into type A1 spermatogonia. The A1 spermatogonia resume division to form A2 to A4 spermatogonia. Next, A4 cells divide to form intermediate (In) spermatogonia, and In spermatogonia divide to produce type B spermatogonia. Finally, type B spermatogonia divide to form primary spermatocytes that will enter meiosis. Spermatogonial stem cells and their progeny are contained in the basal part of the germinal epithelium, in contact with the basement membrane, and they are in close association with the somatic Sertoli cells [4]. Regulatory mechanisms mediated by growth factors produced by the Sertoli cells induce or inhibit the proliferation, differentiation, and further development of the germ cells [5, 6].
Spermatogonial stem cells can generate spermatogenesis when transplanted into the seminiferous tubules of an infertile male [7, 8]. Currently, there is a limited understanding of the molecular mechanisms that control the development of these cells into mature sperm. Spermatogonial stem cells exhibit a distinct phenotype such as the high expression of β-1 and α-6 integrins [9–11] and specific light-scattering properties [12]. While the stem cell identity of the As spermatogonia has not yet been rigorously demonstrated, their morphology and location in the seminiferous epithelium make them good candidates for being stem cells [3]. As spermatogonia express GFRα-1, the receptor for glial cell line–derived neurotrophic factor (GDNF) [13, 14]. As the As differentiate into Apr and Aal spermatogonia, they start expressing the surface receptor c-kit, which binds to stem cell growth factor (SCF) produced by Sertoli cells [15–17].
The ability to isolate, culture, and manipulate the germ line stem cell in vitro would allow us to unravel the molecular mechanisms that drive the first steps of spermatogenesis and to characterize the signaling pathways that induce spermatogonial differentiation versus self-renewal. In turn, this could help us understand the origin of certain testicular neoplasias and the causes of male infertility. To look at these issues, an in vitro system in which these cells could be maintained in long-term cultures would be ideal. In the study reported here, we attempted to establish a mouse spermatogonial stem cell line using the large T antigen under the control of an inducible promoter. The resulting immortalized cells express detectable levels of protein and RNA specific for As and Apr spermatogonia such as the GFRα-1 membrane receptor. Further analysis revealed the expression of additional markers accounting for a stem cell phenotype such as the genes oct-4, piwi12, and prame11 that have a role in stem cell maintenance and renewal in the germ line and other tissues.
Materials and Methods
Construction of pIND-LTAg
The plasmid pIND-LTAg was constructed from the pIND vector (Invitrogen, Carlsbad, CA, http://www.invitrogen.com), which contains the neor gene and an ecdysone-inducible promoter upstream of the multiple cloning site [18]. Ponasterone A, an analog of the Drosophila hormone ecdysone (Invitrogen), served as the inducer. pIND-LTAg was derived from the plasmid pSV3-neo (American Type Culture Collection [ATCC] no. 37150) and pIND by excising the 3.3 kb LTAg sequence out of pSV3-neo and ligating it into the BamHI site of pIND. To check if the pIND-LTAg was functional, the plasmid was cotransfected into MDA-231 breast carcinoma cells with the plasmid pVgRXR (Invitrogen) that codes for the ecdysone receptor and the zeocinr gene.
Cell Isolation, Transfection, and Subcloning
Animal investigations were conducted according to the NCR (National Research Council, National Academy Press) Guide for Care and Use of Laboratory Animals. Type A spermatogonia were isolated from 6-day-old Balb/c mice testes using the STAPUT method that utilizes gravity sedimentation on a 2%–4% bovine serum albumin (BSA) gradient [19]. Briefly, the testes from 60 pups were decapsulated and minced. Leydig cells and peritubular myoid cells were eliminated by a two-step enzymatic digestion using collagenase (1 mg/ml), hyaluronidase (1.5 mg/ml), and trypsin (1 mg/ml). The remaining cell suspension, containing mainly germ cells and Sertoli cells, was subjected to gravity sedimentation for 2.5 hours on a 2%–4% BSA gradient to separate the germ cells from the Sertoli cells. Cells were collected using a fraction collector. After STAPUT separation, the fractions containing only type A spermatogonia were pooled and plated for 2 hours on fetal calf serum (FCS)–coated plates to eliminate possible remaining Sertoli cells by adherence. Six-day-old mice were chosen since at this age only As, Apr, and some Aal spermatogonia are found in the seminiferous epithelium. This isolation method allows us to isolate populations of type A spermatogonia with a purity exceeding 95%. Cotransfection with the pIND-LTAg plasmid and the pVgRXR plasmid was performed with Lipofectin (Invitrogen), and the transfected cells were selected with the antibiotics zeocin (100 μg/ml) and G418 (100 μg/ml).
Cell Lines and Tissues
Several cell lines were tested in this study: the putative germ cell line C18-4, the Sertoli cell line 15P1 [20], the Sertoli cell line SF7 [21], and the NIH 3T3 fibroblast cell line (ATCC no. CRL-1658). The cell lines were grown in Dulbecco’s modified Eagle’s medium containing 1 mM sodium pyruvate, 50 U/ml penicillin-streptomycin, 100 mM nonessential amino acids, and 2 mM L-glutamine (Atlanta Biologicals, Norcross, GA, http://www.atlantabio.com) with 5% FCS (Atlanta Biologicals). The Sertoli cell lines and NIH 3T3 cells were used as negative controls. In addition, freshly isolated Sertoli cells, whole testis, and brain and kidney extracts were tested as positive and negative controls.
Immunocytochemistry
Cells were grown on FCS-coated coverslips until 80% confluency, then fixed with ice-cold methanol for 5 minutes. Reactions were performed according to standard protocols using the immunoperoxidase techniques. The antibodies used were a goat anti-mouse GFRα-1 and a goat anti-mouse c-kit from Santa Cruz Biotechnology (Santa Cruz, CA, http://www.scbt.com). We also used a rat anti-c-kit antibody (ACK45) from Pharmingen (San Diego, http://www.bdbiosciences.com/pharmingen), a goat anti-mouse Ret antibody from R&D Systems (Minneapolis, http://www.rndsystems.com), a goat anti-Oct-4 antibody from Santa Cruz Biotechnology, a rabbit anti-Oct-4 from Active Motif (Carlsbad, CA, http://www.activemotif.com), and two rabbit polyclonal anti-Dazl antisera [22].
Reverse Transcriptase Polymerase Chain Reaction
Total RNA was collected from the C18-4 cell line, 6-day-old mouse testis, the SF7 Sertoli cell line, and freshly isolated mouse Sertoli cells using TriReagent according to the manufacturer’s protocol (Molecular Research Center, Cincinnati, http://www.mrcgene.com). Total RNA samples were treated with 1 unit per 1 μg RNA of RQ1 RNase-free DNase (Promega Corp., Madison, WI, http://promega.com) to degrade any genomic DNA present. cDNA was synthesized from 5 μg of total RNA using SuperScript II reverse transcriptase (RT) and oligo(dT) primers to selectively reverse transcribe mRNA (Invitrogen). Two μ1 of the cDNA obtained from each sample was amplified for 35 cycles (denaturation at 94°C for 30 seconds, annealing at specific temperatures for 45 seconds, and elongation at 72°C for 45 seconds) using primers and conditions already described by others: c-kit [23]; LDH-C4: GenBank no. AF190799; α-actin [24]; and 3βHSD [25]. As a positive control and to check for the presence of contaminating genomic DNA, polymerase chain reaction (PCR) primers for cyclophilin, a housekeeping gene, were also used (GenBank no. 10090). As negative controls, PCR was performed on RT reaction products obtained without the use of RT.
The sequences of the primer pairs used for PCR amplification of each cDNA were devised by Wang et al. [26]. As a positive control, Gapd, a housekeeping gene, was used. As negative controls, and for each sample, PCR was performed on RT reaction products obtained without the use of RT. Each gene was tested in triplicate, using three different RNA preparations per cell line and tissue.
Immunoprecipitation and Western Blotting
Cells were cultivated in cell culture media completed as described above. The media were removed, and the cells were washed three times with ice-cold phosphate-buffered saline. Cells were disrupted with ice-cold immunoprecipitation buffer containing 150 mM NaCl, 15% v/v of mammalian protease inhibitors (Sigma-Aldrich, St. Louis, http://www.sigma-aldrich.com), and phenylmethyl sulfonyl fluoride at a final concentration of 1.0 μg/ml (Sigma-Aldrich). Proteins were recovered according to standard procedures, and their concentration was measured with the Bio-Rad DC assay (Bio-Rad Laboratories, Hercules, CA, http://www.bio-rad.com).
The GFRα-1 protein was immunoprecipitated using 10 μ1 of primary antibody (goat anti-mouse, Santa Cruz Biotechnology) and protein A agarose using standard methods. As positive controls, we used extracts of testis, brain, and kidney. As negative controls, we used protein extracts from the Sertoli cell line 15P1 and NIH3T3 fibroblasts. The concentrated proteins were run on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and transferred to a nitrocellulose membrane. The membrane was then probed with a 1:500 dilution of biotinylated rabbit anti-mouse GFRα-1 antibody (Pharmingen), followed by incubation with a 1:500 dilution of streptavidin-peroxidase (Vector Laboratories, Burlingame, CA, http://www.vectorlabs.com). Peroxidase activity was revealed with a solution containing 0.5 μg/ml 4-chloronapthol, 0.015% hydrogen peroxide, and 16.67% methanol in Tris-buffered saline at pH 7.5.
The Dazl and GDNF proteins were immunoprecipitated and probed using the same method. The antisera Dazl no. 149 and Dazl no. 150, kindly provided by Dr. Reijo-Pera [22], were used for the immunoprecipitation of Dazl and for the probing of the membrane after transfer. Similarly, a rabbit anti-mouse GDNF antibody (Santa Cruz Biotechnology) was used for the immunoprecipitation and probing of GDNF.
The Oct-4 protein was immunoprecipitated with a goat anti-Oct-4 antibody (Santa Cruz Biotechnology) and revealed on the nitrocellulose membrane with a rabbit anti-Oct-4 antibody (Active Motif).
Growth Curves
The C18-4 cells were seeded at 10,000 cells per well in 24-well plates with complete cell culture media and 10% FCS. Experimental wells also contained 100 ng/ml recombinant rat GDNF or 100 ng/ml SCF (R&D Systems). Each day for the next 6 days, three of the experimental wells and three of the control wells were trypsinized and the cells were counted using trypan blue exclusion. The cell numbers are presented as mean ± standard deviation.
Transient Transfections
Transient transfections of the line C18-4 with the expression vector pTracer containing the green fluorescent protein (GFP; Invitrogen) were performed using the calcium phosphate method [27]. At 3–5 days after transfection, the cells were observed in situ using an inverted phase contrast/fluorescence microscope, and expression of GFP was visualized using an excitation wavelength of 490 nm.
Results
Cell Immortalization
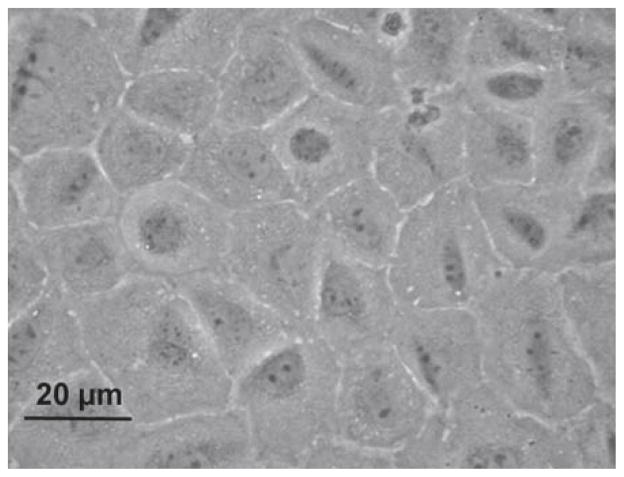
In our immortalization strategy, the ecdysone receptor from Drosophila was expressed from a vector called pVgRxR [18]. The ecdysone-responsive promoter, which drives the expression of the large T-antigen gene, is on a second vector called pIND-LTAg. Both vectors were stably cotransfected into freshly isolated type A spermatogonia that were induced to express the LTAg when treated with Ponasterone A, an analog of ecdysone. While the cells escaped the hormonal control after a finite number of generations and expressed the LTAg constitutively, their growth remained slow, with a doubling time of about 3 days. After several rounds of subcloning by limiting dilution, a clone was obtained that we called C18-4. The cells appeared round to polygonal, with a round nucleus, and a large nucleus-to-cytoplasm ratio in phase contrast microscopy (Fig. 1).
Figure 1.
Morphology of the C18-4 cells in phase contrast microscopy. The cells are round to polygonal, with a round nucleus, and a large nucleus-to-cytoplasm ratio.
Immunocytochemistry
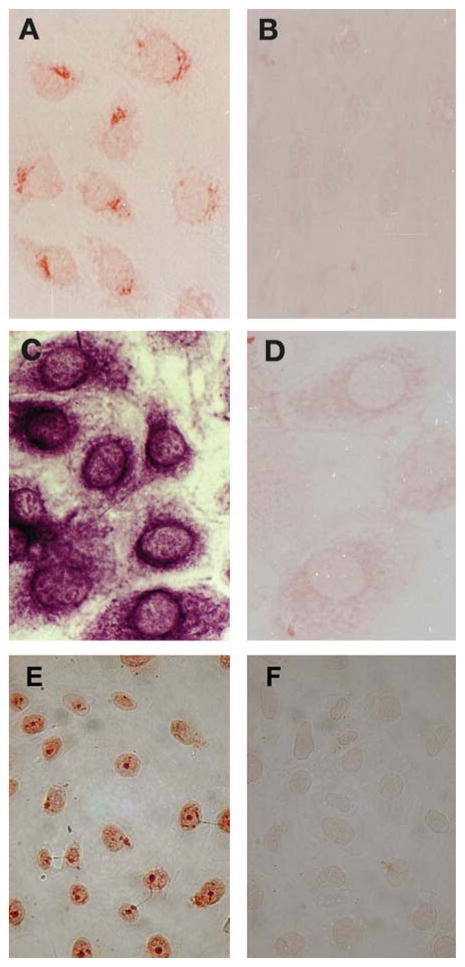
The purified C18-4 cells were stained by immunocytochemistry for the presence of the germ cell–specific Dazl protein using antisera nos. 149 and 150 [22]. Figure 2 shows that Dazl is expressed in the nucleus when it is stained with antiserum no. 149 (Fig. 2A), and it is expressed in the nucleus and the cytoplasm when it is stained with anti-serum no. 150 (Fig. 2C). The cells were also positive for GFRα-1, the coreceptor of Ret, and a marker for germ line stem cells (Fig. 3A). Further, they stained positive for the Ret transmembrane receptor, a marker for spermatogonia and spermatocytes (Fig. 3C). The cell nuclei, in particular the nucleoli, stained positive for Oct-4, an early marker for the germ line (Fig. 3E). The expression of the c-kit receptor could not be revealed using immunocytochemistry, but it was detected by RT-PCR in early passages only (Fig. 4).
Figure 2.
C18-4 cells, immunostained for the Dazl RNA-binding protein. (A): Staining for Dazl, a general marker for germ cells, revealed by immunocytochemistry using antiserum no. 149 and horseradish peroxidase. (B): Negative control without primary antibody. (C): Staining for Dazl, a general marker for germ cells, revealed by immunocytochemistry using antiserum no. 150 and horseradish peroxidase. (D): Negative control without primary antibody.
Figure 3.
C18-4 cells, immunostained for GFRα-1, the Ret transmembrane receptor, and the Oct-4 transcription factor. (A): Staining for GFRα-1, revealed by immunocytochemistry and horseradish peroxidase (×100). (B): Negative control without primary antibody (×100). (C): Staining for Ret, revealed by immunocytochemistry and horseradish peroxidase (×100). (D): Negative control without primary antibody (×100). (E): Staining for Oct-4, revealed by immunocytochemistry and horseradish peroxidase (×40). (F): Negative control without primary antibody (×40).
Figure 4.
c-kit gene expression in the C18-4 cells visualized by reverse transcription polymerase chain reaction. Lane A: size markers; lane B: adult testis; lane D: C18-4 cell line; lane F: freshly isolated Sertoli cell. Lanes C, E, and G: negative controls without reverse transcriptase.
Immunoprecipitation
The expression of GFRα-1 and Dazl in the C18-4 cell line was confirmed by immunoprecipitation (Fig. 5). To increase the specificity of detection, GFRα-1 was immunoprecipitated using a polyclonal antibody made in goat, then revealed after blotting using a polyclonal antibody made in rabbit. In Figure 5A, lane E, we show that the C18-4 cell line produces a protein exhibiting a molecular weight of 42 kDA that corresponds to the molecular weight of GFRα-1. As positive controls, we used protein extracts from developing brain, kidney, and testis (lanes B–D, respectively). As negative controls, we used NIH 3T3 cells and the Sertoli cell line 15P1 (lanes F and G) [20].
Figure 5.
Immunoprecipitation of GFRα-1, Dazl, and Oct- 4 using C18-4 protein extracts. (A): Immunoprecipitation of GFRα-1. Lane A: molecular weight markers; lane B: adult kidney; lane C: developing brain (6-day-old); lane D: adult testis; lane E: C18-4 cell line; lane F: 15P1 Sertoli cell line; lane G: NIH 3T3 cell line. (B): Immunoprecipitation of Dazl. Lane A: molecular weight markers; lane B: adult testis; lane C: C18-4 cell line. (C): Immunoprecipitation of Oct-4. Lane A: C18-4 cell line; lane B: adult testis; lane C: molecular weight markers.
The Dazl protein was immunoprecipitated and revealed after blotting using antiserum no. 149 [22]. As seen in Figure 5B, a strong band with a molecular weight of approximately 33 kDa was revealed in the testis extract, corresponding to the molecular weight of Dazl [22]. The same band was observed using C18-4 extracts (Fig. 5B). Similarly, the Oct-4 protein was revealed after immunoprecipitation (Fig. 5C).
Finally, immunoprecipitation failed to detect the expression of GDNF in the purified cells, indicating that the C18-4 cells are not contaminated by Sertoli cells.
Reverse Transcriptase Polymerase Chain Reaction
Neither 3βHSD, a marker for Leydig cells, nor α-actin, a marker for peritubular myoid cells, could be found by RT-PCR, further indicating that the cells are of germ cell origin. The absence of LDHC4 expression indicated that the cells are not spermatocytes.
To further confirm the spermatogonial origin of the C18- 4 cell line, we investigated by RT-PCR the expression of 36 spermatogonial-specific genes, which were previously identified by Wang and colleagues [26] in the 6-day-old mouse testis. There was a RT-PCR product for the housekeeping gene Gapd in both cell lines and in the neonatal testis samples, indicating that the RNA isolated contained intact transcripts (data not shown). RT-PCR further revealed that all 36 spermatogonial-specific genes tested were expressed in the 6-day-old testis, as expected. None of these genes were expressed in the SF7 Sertoli cell line, confirming the specificity of the transcripts. The expression of 10 spermatogonial- specific genes was detected in the C18-4 cell line. The results of these 10 are reported in Table 1.
Table 1.
Spermatogonial genes expressed in the C18-4 cell line, arranged in alphabetical order; 6-day-old testes were used as positive control, and the Sertoli cell line SF7 as negative control
| No. | Gene | SF7 (negative control) | Testis (positive control) | C18-4 | Protein function |
|---|---|---|---|---|---|
| Gapd | + | + | + | Glyceraldehyde-3-phosphate dehydrogenase (housekeeping gene) | |
| 1 | Fth117 | − | + | + | Testis-specific ferritin (iron metabolism) |
| 2 | Ott | − | + | + | Putative meiotic gene, ovary and testis |
| 3 | Piwi12 | − | + | + | Stem cell maintenance and renewal |
| 4 | Prame11 | − | + | + | Expressed in testis and melanoma cells |
| 5 | Sycp1 | − | + | + | Synaptonemal complex protein, transverse filaments |
| 6 | Tex13 | − | + | + | Novel 186-residues protein |
| 7 | Tex14 | − | + | + | Novel protein containing two protein kinase domains |
| 8 | Tex15 | − | + | + | Novel 2,785-residues protein |
| 9 | Tex16 | − | + | + | Novel 1,139-residues protein, rich in serine |
| 10 | Tex19 | − | + | + | Novel 351-residues protein with a coiled-coil region |
gene expressed; −, gene not expressed.
One of the genes expressed by the C18-4 cells was the Ott gene (Fig. 6A), which is transcribed in vivo in the germ cells of testis and ovary [28]. The primers used in this study can detect the presence of two bands by RT-PCR, corresponding to two isoforms, with sizes of 551 and 503 bp, respectively [28]. As shown in Figure 6A, two bands of the correct size are expressed by the C18-4 cells (lane B). However, only one band (551 bp) was detectable in the neonatal testis sample (lane D). Sertoli cells do not express the Ott gene (lane F). Figure 6B shows that the C18-4 cell line also expresses Sycp1, a gene coding for a protein of the synaptonemal complex, which is already expressed in spermatogonia [29]. The band obtained is of 311 bp, as expected (lane B). The same band is visible for the 6-day-old testis sample (lane D), but not in Sertoli cells (lane F). Figure 6C represents the expression of the gene Fth117 that codes for a testis-specific ferritin-binding protein [26]. Fth117 is expressed in the C18-4 cells, giving the expected band of 292 bp (lane B) by RT-PCR. Fth117 expression is found in the 6-day-old testis but is not found in Sertoli cells (lanes D and F, respectively). In addition to spermatogonial-specific genes with a known function, the C18-4 cell line expressed five novel genes that belong to the Tex (testis-expressed) gene family (Fig. 6D) [26]. None of those genes were expressed by the Sertoli cell line.
Figure 6.
Expression of spermatogonia-specific genes in the C18-4 cell line, visualized by RT-PCR. RNA samples were isolated from the C18-4 cells, from 6-day-old testes as positive control, and from the SF7 Sertoli cell line as negative control. After reverse transcription, the cDNA samples were amplified using the primers described by Wang et al. [26]. (A): RT-PCR for Ott (PCR product = 551 and 503 bp). (B): RT-PCR for Sycp1 (PCR product = 311 bp). (C): RT-PCR for Fth117 (PCR product = 292 bp). (D): RT-PCR for Tex13 (PCR product = 220 bp), Tex14 (PCR product = 635 bp), Tex15 (PCR product = 411 bp), Tex16 (PCR product = 660 bp), and Tex19 (PCR product = 184 bp). (E): RT-PCR for Piwi1 (PCR product = 241 bp). (F): RT-PCR for Prame11 (PCR product = 448 bp). Lanes A: size markers (100 bp ladder); B: C18-4 cell line; C: negative control for C18-4; D: 6-day-old testis; E: negative control for 6-day-old testis; F: SF7 Sertoli cells; G: negative control for SF7 Sertoli cells. Abbreviation: RT-PCR, reverse transcriptase polymerase chain reaction.
Interestingly, the C18-4 spermatogonial cell line also shows the expression of the piwi12 (piwi-like 2) gene (Fig. 6E). Piwi belongs to a family of genes that is involved in stem cell maintenance and renewal in Drosophila [30]. Two mouse homologues of piwi have recently been cloned: miwi (mouse piwi) and mili (miwi-like) [31–33]. The primers used in this study are derived from the Drosophila sequence and give a band of 241 bp. As expected, Piwi12 was also detected in the neonatal testis but not in Sertoli cells. Additionally, the C18-4 cells express a PRAME-like gene (Fig. 6F). PRAME is a protein expressed in the testis, as well as in several carcinomas, in acute myeloid leukemias, and in CD34+ hematopoietic stem cells [34–37].
Influence of GDNF and SCF on the Behavior of the C18-4 Cells
To determine if the C18-4 cell line responds to growth factors, we cultivated the cells in the presence or absence of 100 ng/ml GDNF in the culture medium. When GDNF is present, the rate of proliferation of the cells increases significantly (p < .005) (Fig. 7A). No influence on cell morphology or expression of the c-kit receptor was observed. Interestingly, the growth effect of GDNF is detected only when the culture medium also contains 10% FCS. No change in the rate of proliferation can be detected when FCS is replaced by a defined serum (Nu serum), which contains a minimal amount of cytokines. Further, addition of SCF to the culture medium did not stimulate the proliferation of the cells (Fig. 7B). SCF did not induce the cells to differentiate since RT-PCR analysis did not reveal the expression of LDHC4, a germ cell–specific isozyme that is detected at the earliest in preleptotene spermatocytes [38, 39].
Figure 7.
Rates of proliferation of the C18-4 cells with GDNF or SCF. (A): In the presence of GDNF, the rate of proliferation of the C18-4 cells increases significantly in comparison with the control cultures. (B): No changes could be observed when the cells are cultured with SCF. Abbreviations: GDNF, glial cell line–derived neurotrophic factor; SCF, stem cell factor.
Discussion
One of the major problems encountered when working with type A spermatogonia and germ line stem cells is the limited number of cells available. As a consequence, little is known about the biology of these cells and the cellular and molecular pathways underlying the first steps of spermatogenesis. We have now established a cell line from the 6-day-old mouse testis, which shows morphological features of type A spermatogonia at the light microscopic level and expresses germ line stem cell–specific genes. To ascertain that this cell line is of germ cell origin, we investigated the expression of the proteins Dazl and Ret by immunocytochemistry and immunoprecipitation.
In the mouse, Dazl is an autosomally located gene that has a predominant role in spermatogenesis and oogenesis [40–42]. Previous studies have revealed that, in the mouse testis, the protein is predominantly found in the nucleus and the cytoplasm of fetal gonocytes. In the adult animal, the protein is mainly present in the nucleus of spermatogonia [41]. Ret is a transmembrane receptor activated through the binding of its coreceptor, GFRα-1, to GDNF [43]. Ret is expressed by all premeiotic germ cells in the testis, including pachytene spermatocytes [44]. We show here that the established cell line expresses both Dazl and Ret proteins, indicating that the cells are of germ cell origin. Further, the pattern of expression of Dazl (in the nucleus only with antiserum 149, and in both the nucleus and the cytoplasm with antiserum 150) indicates that the cells derive from early spermatogonia. In the cytoplasm, Dazl formed dot-like structures as previously described by Ruggiu et al. [42, 45], which could be due in part to oligomerization of the protein with itself. Since the cells do not express markers specific for spermatocytes such as LDHC4, we conclude that the cell line is truly of spermatogonial origin. The germ cell origin is further confirmed by the fact that the Oct-4 nuclear protein is expressed and that no marker specific for somatic cells was found, by either immunoprecipitation or RT-PCR. The expression of Oct- 4 is weak in the nucleoplasm but strong in nucleoli and other granular structures in the nucleus, and this pattern is consistent with the findings of Parfenov et al. [46] in active oocytes.
To determine if the C18-4 line is derived from a germ line stem cell, we probed the cells for GFRα-1, a surface receptor that is specifically expressed by As and Apr spermatogonia in the testis [14]. GFRα-1 is the receptor for GDNF, which is produced by Sertoli cells [13, 47, 48]. GDNF is a pleiotropic factor that influences proliferation, survival, and differentiation in a number of target tissues during development [49, 50]. GDNF interacts with the GFRα-1 receptor, which, in turn, mediates stimulation of the Ret receptor tyrosine kinase [43]. The C18-4 cell line was positive for GFRα-1 using immunocytochemistry and immunoprecipitation, indicating that they are As or Apr spermatogonia. The cells responded to GDNF by significantly increasing their rate of proliferation. This behavior is similar to the behavior of As spermatogonia of transgenic mice overexpressing GDNF [47]. We also probed the cells for their expression of c-kit, the receptor for SCF. In germ cells, c-kit seems to be expressed by all spermatogonia except for As, which are the putative stem cells [23, 51]. Thus, c-kit is considered to be a marker of more differentiated spermatogonia. While c-kit could not easily be detected by immunocytochemistry, the transcript was revealed by RT-PCR in early passages, then disappeared. Addition of SCF to the culture medium did not increase proliferation or stimulate differentiation. Further, GDNF could not increase the expression of the c-kit receptor. Therefore, a more comprehensive approach such as micro-array analysis might be useful to assess the potential of the C18-4 cells to differentiate in the presence of either GDNF or SCF, or both. In addition, transplantation of the C18-4 cells into an infertile testis might provide the optimal environment for their differentiation.
In this study, we also examined the expression profile of 36 spermatogonial-specific genes that have been previously identified by Wang and colleagues [26]. Ten of these genes were expressed in the C18-4 germ cells. The loss of expression of many of those genes may be the consequence of immortalization, or there may be a lack of specific external stimuli, such as growth factors produced by Sertoli cells, that are important for the expression of these particular genes. However, the overall pattern of gene expression indicates that the C18-4 cell line exhibits general properties of type A spermatogonia. Interestingly, the C18-4 cells express a gene belonging to the piwi gene family (piwi12, or piwi-like 2). This family defines a novel class of genes that are conserved during evolution and seem to be required for stem cell renewal, gametogenesis, and RNA interference. In Drosophila, the germ line stem cells of piwi mutants (male and female) differentiate without producing self-renewing divisions. This situation eventually leads to gonads lacking germ cells [30, 52]. Homologues of piwi have been identified in C aenorhabditis elegans (rde-1) [53], in human (hiwi) [54], and in the mouse (miwi and mili) [31–33]. Moreover, the C18-4 cells express another gene that is specific for undifferentiated progenitors such as PRAME-like. The PRAME protein belongs to the cancer/testis antigens group, a category of tumor antigens normally expressed in male germ cells of the testis but not in somatic cells. However, the gene is overexpressed in a number of malignancies, including acute myeloid leukemias, and is associated with tumor progression [55]. PRAME is also expressed in normal, CD34+ bone marrow cells [36] and is associated with stem cell proliferation. Thus, the expression of piwi-like and PRAME-like genes indicates that the C18-4 cell line has a stem cell component.
We recently reported the establishment of another mouse spermatogonial cell line, which was immortalized using the mTert gene [56]. This cell line, named S4, does not express much of the GFRα-1 receptor, but it does express c-kit and responds to SCF by differentiating in vitro. Therefore, we believe that the C18-4 cell line is derived from As spermatogonia, while the S4 line is derived from Apr or Aal spermatogonia. The C18-4 cell line will be useful to dissect the molecular mechanisms underlying germline stem cell proliferation, while the S4 cell line will be useful to understand differentiation and the molecular mechanisms that lead to meiosis. In addition to our cell lines, a cell line derived from rat spermatogonia with stem cell characteristics has also recently been produced [57]. Thus, testicular stem cell lines from two major model organisms are now available for further developmental studies.
In summary, based on the data presented here, our new germ cell line has characteristics of germ line stem cells. The lack of specific markers available to distinguish the As from the Apr spermatogonia impairs our ability to characterize these cells more precisely. However, to date, this is the only germ cell line expressing the GFRα-1 and Ret receptors. Further, this cell line responds to GDNF by proliferating at a rate that is significantly higher than the control without growth factor. Interestingly, no response can be detected when the medium contains GDNF and a defined serum such as NU serum instead of FCS. Since NU serum contains no or very few cytokines, this finding confirms the observation that GDNF often acts in synergy with other growth factors that are likely present in FCS [58, 59]. Although the cells are unable to differentiate to meiotic spermatocytes thus far in vitro, probably due to their expression of the large T antigen, we believe that they will be helpful for promoter studies of genes specifically expressed in the early phases of spermatogenesis. They also provide a good model to understand how Dazl regulates its target mRNAs, to unravel signaling pathways triggered by GDNF, and, possibly, to study the function of novel genes identified through expression arrays of primary spermatogonial stem cells. As shown in Figure 8, the C18-4 cells are transfectable since they can express, at least transiently, the GFP protein from an expression vector.
Figure 8.

Transient expression of green fluorescent protein in the C18-4 cells.
Acknowledgments
We thank Dr. Renee Reijo-Pera, Department of Obstetrics, Gynecology and Reproductive Sciences, University of California, San Francisco, for providing us with the Dazl no. 149 and 150 antibodies. We also thank Ms. Kathy van der Wee for valuable technical assistance and Dr. Kathy Beal for statistical analysis. This work was supported by the National Institutes of Health grant RO1-HD36483.
References
- 1.Huckins C. The spermatogonial stem cell population in adult rats. I: their morphology, proliferation and maturation. Anat Rec. 1971;169:533–557. doi: 10.1002/ar.1091690306. [DOI] [PubMed] [Google Scholar]
- 2.Oakberg EF. Spermatogonial stem-cell renewal in the mouse. Anat Rec. 1971;169:515–531. doi: 10.1002/ar.1091690305. [DOI] [PubMed] [Google Scholar]
- 3.de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21:776–798. [PubMed] [Google Scholar]
- 4.Dym M, Fawcett DW. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod. 1970;3:308–326. doi: 10.1093/biolreprod/3.3.308. [DOI] [PubMed] [Google Scholar]
- 5.Skinner MK. Cell-cell interactions in the testis. [Review] Endocrine Rev. 1991;12:45–77. doi: 10.1210/edrv-12-1-45. [DOI] [PubMed] [Google Scholar]
- 6.Jegou B. The Sertoli-germ cell communication network in mammals. Int Rev Cytol. 1993;147:25–96. [PubMed] [Google Scholar]
- 7.Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation [see comments] Proc Natl Acad Sci U S A. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation [see comments] Proc Natl Acad Sci U S A. 1994;91:11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palombi F, Salanova M, Tarone G, et al. Distribution of beta 1 integrin subunit in rat seminiferous epithelium. Biol Reprod. 1992;47:1173–1182. doi: 10.1095/biolreprod47.6.1173. [DOI] [PubMed] [Google Scholar]
- 10.Schaller J, Glander HJ, Dethloff J. Evidence of beta 1 integrins and fibronectin on spermatogenic cells in human testis. Hum Reprod. 1993;8:1873–1878. doi: 10.1093/oxfordjournals.humrep.a137952. [DOI] [PubMed] [Google Scholar]
- 11.Shinohara T, Avarbock MR, Brinster RL. β1- and α6-integrin are surface markers on mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 1999;96:5504–5509. doi: 10.1073/pnas.96.10.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shinohara T, Orwig KE, Avarbock MR, et al. Spermatogonial stem cell enrichment by multiparameter selection of mouse testis cells. Proc Natl Acad Sci U S A. 2000;97:8346–8351. doi: 10.1073/pnas.97.15.8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viglietto G, Dolci S, Bruni P, et al. Glial cell line-derived neutrotrophic factor and neurturin can act as paracrine growth factors stimulating DNA synthesis of Ret-expressing spermatogonia. Int J Oncol. 2000;16:689–694. doi: 10.3892/ijo.16.4.689. [DOI] [PubMed] [Google Scholar]
- 14.Dettin L, Ravindranath N, Hofmann MC, et al. Morphological characterization of the spermatogonial subtypes in the neonatal mouse testis. Biol Reprod. 2003;69:1565–1571. doi: 10.1095/biolreprod.103.016394. [DOI] [PubMed] [Google Scholar]
- 15.Yoshinaga K, Nishikawa S, Ogawa M, et al. Role of c-kit in mouse spermatogenesis: identification of spermatogonia as a specific site of c-kit expression and function. Development. 1991;113:689–699. doi: 10.1242/dev.113.2.689. [DOI] [PubMed] [Google Scholar]
- 16.Sorrentino V, Giorgi M, Geremia R, et al. Expression of the c-kit proto-oncogene in the murine male germ cells. Oncogene. 1991;6:149–151. [PubMed] [Google Scholar]
- 17.Besmer P, Manova K, Duttlinger R, et al. The kit-ligand (steel factor) and its receptor c-kit/W: pleiotropic roles in gametogenesis and melanogenesis. Development (Suppl) 1993:125–137. [PubMed] [Google Scholar]
- 18.No D, Yao TP, Evans RM. Ecdysone-inducible gene expression in mammalian cells and transgenic mice. Proc Natl Acad Sci U S A. 1996;93:3346–3351. doi: 10.1073/pnas.93.8.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dym M, Jia MC, Dirami G, et al. Expression of c-kit receptor and its autophosphorylation in immature rat type A spermatogonia. Biol Reprod. 1995;52:8–19. doi: 10.1095/biolreprod52.1.8. [DOI] [PubMed] [Google Scholar]
- 20.Paquis-Flucklinger V, Michiels JF, Vidal F, et al. Expression in transgenic mice of the large T antigen of polyomavirus induces Sertoli cell tumours and allows the establishment of differentiated cell lines. Oncogene. 1993;8:2087–2094. [PubMed] [Google Scholar]
- 21.Hofmann MC, Narisawa S, Hess RA, et al. Immortalization of germ cells and somatic testicular cells using the SV40 large T antigen. Exp Cell Res. 1992;201:417–435. doi: 10.1016/0014-4827(92)90291-f. [DOI] [PubMed] [Google Scholar]
- 22.Reijo RA, Dorfman DM, Slee R, et al. DAZ family proteins exist throughout male germ cell development and transit from nucleus to cytoplasm at meiosis in humans and mice. Biol Reprod. 2000;63:1490–1496. doi: 10.1095/biolreprod63.5.1490. [DOI] [PubMed] [Google Scholar]
- 23.Schrans-Stassen BH, van de Kant HJ, de Rooij DG, et al. Differential expression of c-kit in mouse undifferentiated and differentiating type A spermatogonia. Endocrinology. 1999;140:5894–5900. doi: 10.1210/endo.140.12.7172. [DOI] [PubMed] [Google Scholar]
- 24.Min BH, Strauch AR, Foster DN. Nucleotide sequence of a mouse vascular smooth muscle α-actin cDNA. Nucleic Acids Res. 1988;16:10374. doi: 10.1093/nar/16.21.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baker PJ, Sha JA, McBride MW, et al. Expression of 3β-hydroxysteroid dehydrogenase type I and type VI isoforms in the mouse testis during development. Eur J Biochem. 1999;260:911–917. doi: 10.1046/j.1432-1327.1999.00245.x. [DOI] [PubMed] [Google Scholar]
- 26.Wang PJ, McCarrey JR, Yang F, et al. An abundance of X-linked genes expressed in spermatogonia. Nat Genet. 2001;27:422–426. doi: 10.1038/86927. [DOI] [PubMed] [Google Scholar]
- 27.Gorman CM, Moffat LF, Howard BH. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982;2:1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerr SM, Taggart MH, Lee M, et al. Ott, a mouse X-linked multigene family expressed specifically during meiosis. Hum Mol Genet. 1996;5:1139–1148. doi: 10.1093/hmg/5.8.1139. [DOI] [PubMed] [Google Scholar]
- 29.Sage J, Martin L, Meuwissen R, et al. Temporal and spatial control of the Sycp1 gene transcription in the mouse meiosis: regulatory elements active in the male are not sufficient for expression in the female gonad. Mech Dev. 1999;80:29–39. doi: 10.1016/s0925-4773(98)00191-9. [DOI] [PubMed] [Google Scholar]
- 30.Cox DN, Chao A, Baker J, et al. A novel class of evolutionarily conserved genes defined by piwi are essential for stem cell self-renewal. Genes Dev. 1998;12:3715–3727. doi: 10.1101/gad.12.23.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuramochi-Miyagawa S, Kimura T, Yomogida K, et al. Two mouse piwi-related genes: miwi and mili. Mech Dev. 2001;108:121–133. doi: 10.1016/s0925-4773(01)00499-3. [DOI] [PubMed] [Google Scholar]
- 32.Deng W, Lin H, Qiao D, et al. Miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev Cell. 2002;2:819–830. doi: 10.1016/s1534-5807(02)00165-x. [DOI] [PubMed] [Google Scholar]
- 33.Kuramochi-Miyagawa S, Kimura T, Ijiri TW, et al. Mili, a mammalian member of piwi family gene, is essential for spermatogenesis. Development. 2004;131:839–849. doi: 10.1242/dev.00973. [DOI] [PubMed] [Google Scholar]
- 34.Kirkin AF, Dzhandzhugazyan K, Zeuthen J. Melanomaassociated antigens recognized by cytotoxic T lymphocytes. APMIS. 1998;106:665–679. doi: 10.1111/j.1699-0463.1998.tb00210.x. [DOI] [PubMed] [Google Scholar]
- 35.Pellat-Deceunynck C, Mellerin MP, Labarriere N, et al. The cancer germ-line genes MAGE-1, MAGE-3 and PRAME are commonly expressed by human myeloma cells. Eur J Immunol. 2000;30:803–809. doi: 10.1002/1521-4141(200003)30:3<803::AID-IMMU803>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 36.Steinbach D, Hermann J, Viehmann S, et al. Clinical implications of PRAME gene expression in childhood acute myeloid leukemia. Cancer Genet Cytogenet. 2002;133:118–123. doi: 10.1016/s0165-4608(01)00570-2. [DOI] [PubMed] [Google Scholar]
- 37.Li CM, Guo M, Borczuk A, et al. Gene expression in Wilms’ tumor mimics the earliest committed stage in the meta-nephric mesenchymal-epithelial transition. Am J Pathol. 2002;160:2181–2190. doi: 10.1016/S0002-9440(10)61166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li SS, O’Brien DA, Hou EW, et al. Differential activity and synthesis of lactate dehydrogenase isozymes A (muscle), B (heart), and C (testis) in mouse spermatogenic cells. Biol Reprod. 1989;40:173–180. doi: 10.1095/biolreprod40.1.173. [DOI] [PubMed] [Google Scholar]
- 39.Alcivar AA, Trasler JM, Hake LE, et al. DNA methylation and expression of the genes coding for lactate dehydrogenases A and C during rodent spermatogenesis. Biol Reprod. 1991;44:527–535. doi: 10.1095/biolreprod44.3.527. [DOI] [PubMed] [Google Scholar]
- 40.Cooke HJ, Lee M, Kerr S, et al. A murine homologue of the human DAZ gene is autosomal and expressed only in male and female gonads. Hum Mol Genet. 1996;5:513–516. doi: 10.1093/hmg/5.4.513. [DOI] [PubMed] [Google Scholar]
- 41.Reijo R, Seligman J, Dinulos MB, et al. Mouse autosomal homolog of DAZ, a candidate male sterility gene in humans, is expressed in male germ cells before and after puberty. Genomics. 1996;35:346–352. doi: 10.1006/geno.1996.0366. [DOI] [PubMed] [Google Scholar]
- 42.Ruggiu M, Speed R, Taggart M, et al. The mouse Dazla gene encodes a cytoplasmic protein essential for gametogenesis. Nature. 1997;389:73–77. doi: 10.1038/37987. [DOI] [PubMed] [Google Scholar]
- 43.Jing S, Wen D, Yu Y, et al. GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF. Cell. 1996;85:1113–1124. doi: 10.1016/s0092-8674(00)81311-2. [DOI] [PubMed] [Google Scholar]
- 44.Creemers LB, Meng X, den OK, et al. Transplantation of germ cells from glial cell line-derived neurotrophic factor-overexpressing mice to host testes depleted of endogenous spermatogenesis by fractionated irradiation. Biol Reprod. 2002;66:1579–1584. doi: 10.1095/biolreprod66.6.1579. [DOI] [PubMed] [Google Scholar]
- 45.Ruggiu M, Cooke HJ. In vivo and in vitro analysis of homodimerisation activity of the mouse Daz11 protein. Gene. 2000;252:119–126. doi: 10.1016/s0378-1119(00)00219-5. [DOI] [PubMed] [Google Scholar]
- 46.Parfenov VN, Pochukalina GN, Davis DS, et al. Nuclear distribution of Oct-4 transcription factor in transcriptionally active and inactive mouse oocytes and its relation to RNA polymerase II and splicing factors. J Cell Biochem. 2003;89:720–732. doi: 10.1002/jcb.10545. [DOI] [PubMed] [Google Scholar]
- 47.Meng X, Lindahl M, Hyvonen ME, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- 48.Meng X, de RDG, Westerdahl K, et al. Promotion of semi-nomatous tumors by targeted overexpression of glial cell line-derived neurotrophic factor in mouse testis. Cancer Res. 2001;61:3267–3271. [PubMed] [Google Scholar]
- 49.Trupp M, Ryden M, Jornvall H, et al. Peripheral expression and biological activities of GDNF, a new neurotrophic factor for avian and mammalian peripheral neurons. J Cell Biol. 1995;130:137–148. doi: 10.1083/jcb.130.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Golden JP, DeMaro JA, Osborne PA, et al. Expression of neurturin, GDNF, and GDNF family-receptor mRNA in the developing and mature mouse. Exp Neurol. 1999;158:504–528. doi: 10.1006/exnr.1999.7127. [DOI] [PubMed] [Google Scholar]
- 51.Ohta H, Yomogida K, Dohmae K, et al. Regulation of proliferation and differentiation in spermatogonial stem cells: the role of c-kit and its ligand SCF. Development. 2000;127:2125–2131. doi: 10.1242/dev.127.10.2125. [DOI] [PubMed] [Google Scholar]
- 52.Cox DN, Chao A, Lin H. Piwi encodes a nucleoplasmic factor whose activity modulates the number and division rate of germline stem cells. Development. 2000;127:503–514. doi: 10.1242/dev.127.3.503. [DOI] [PubMed] [Google Scholar]
- 53.Tabara H, Sarkissian M, Kelly WG, et al. The rde-1 gene, RNA interference, and transposon silencing in C. elegans. Cell. 1999;99:123–132. doi: 10.1016/s0092-8674(00)81644-x. [DOI] [PubMed] [Google Scholar]
- 54.Qiao D, Zeeman AM, Deng W, et al. Molecular characterization of hiwi, a human member of the piwi gene family whose overexpression is correlated to seminomas. Oncogene. 2002;21:3988–3999. doi: 10.1038/sj.onc.1205505. [DOI] [PubMed] [Google Scholar]
- 55.Scanlan MJ, Gure AO, Jungbluth AA, et al. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev. 2002;188:22–32. doi: 10.1034/j.1600-065x.2002.18803.x. [DOI] [PubMed] [Google Scholar]
- 56.Feng LX, Chen Y, Dettin L, et al. Generation and in vitro differentiation of a spermatogonial cell line. Science. 2002;297:392–395. doi: 10.1126/science.1073162. [DOI] [PubMed] [Google Scholar]
- 57.van Pelt AM, Roepers-Gajadien HL, Gademan IS, et al. Establishment of cell lines with rat spermatogonial stem cell characteristics. Endocrinology. 2002;143:1845–1850. doi: 10.1210/endo.143.5.8806. [DOI] [PubMed] [Google Scholar]
- 58.Krieglstein K, Henheik P, Farkas L, et al. Glial cell line-derived neurotrophic factor requires transforming growth factor-beta for exerting its full neurotrophic potential on peripheral and CNS neurons. J Neurosci. 1998;18:9822–9834. doi: 10.1523/JNEUROSCI.18-23-09822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kanatsu-Shinohara M, Ogonuki N, Inoue K, et al. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]