Abstract
Rat CD39, a membrane-bound ectonucleoside triphosphate diphosphohydrolase that hydrolyzes extracellular nucleoside tri- and diphosphates, has seven potential N-glycosylation sites at asparagine residues 73, 226, 291, 333, 375, 429, and 458. To determine their roles in the structure and function of CD39, we mutated these sites individually or in combination by replacing asparagine with serine or glutamine and analyzed the surface expression and the enzymatic activity of the mutants. The results indicate that rat CD39 can be glycosylated at all seven sites when expressed in COS7 cells. Glycosylation sites 73 at the N terminus, 333 in the middle, and 429 and 458 at the C terminus were principally required for cell surface appearance of enzymatically active CD39. Whereas deletion of these sites individually had modest effects on surface ATPase activity, some double deletions of these sites had major effects on both surface activity and expression. The importance of these N-glycosylation sites is recognizable in other members of the ectonucleoside triphosphate diphosphohydrolase family.
INTRODUCTION
One of the principal functions of extracellular nucleotides is signaling through purinergic receptors. A variety of ectoenzymes have evolved to modify extracellular nucleotides so as to terminate their signaling function. They include the glycosylphosphatidylinositol-anchored ectoalkaline phosphatase, the ectonucleotide pyrophosphatase/phosphodiesterase, and the ectoapyrase families. Ectoalkaline phosphatases catalyze the release of inorganic phosphate (Pi) from a variety of organic phosphate compounds. The members of the ectonucleotide pyrophosphatase/phosphodiesterase family catalyze the hydrolysis of phosphodiester and pyrophosphate bonds of nucleotides and nucleotide sugars. The ectoapyrases of vertebrates are now called ectonucleoside triphosphate diphosphohydrolases (E-NTP-Dases). The active site of the E-NTPDases is extracytoplasmic, either on the cell surface or in the lumen of intracellular membrane compartments, and it catalyzes the hydrolysis of phosphoanhydride bonds of nucleoside tri- and diphosphates. They are distinguishable from other membrane-bound ATPases by their broad substrate specificity, divalent cation (Ca2+ or Mg2+) requirements, and insensitivity to P- and V-type ATPase inhibitors (for review, see Plesner, 1995). The existence of nucleotidase activities on the outer surface of the plasma membrane of animal cells has been known since 1953 (Rothstein et al., 1953; Ziganshin et al., 1994). There are eight members of the E-NTPDase family, six with two transmembrane domains (Bigonnesse et al., 2004). NTPDases 1, 2, 3, and 8 are located in the plasma membrane, whereas NTPDase 4, also known as UDPase (Wang and Guidotti, 1998) and LALP70 (Biederbick et al., 1999), is in the Golgi membranes and lysosomal vacuoles; NTPDase 7 is present in intracellular membranes (Shi et al., 2001). NTPDase 5 has been shown to be a secreted enzyme (Mulero et al., 1999), and NTPDase 6 has been reported to be partially membrane bound to Golgi membranes, presumably through the uncleaved signal sequence (Braun et al., 2000); both have higher activity for nucleoside diphosphates than for nucleoside triphosphates.
CD39, NTPDase1, originally identified on the surface of B lymphocytes, was the first vertebrate ectoapyrase identified and cloned (Wang and Guidotti, 1996; Kaczmarek et al., 1996; Vanduffel and Lemmens, 2000). Because CD39 is present principally on the surface of endothelial cells, and it converts ATP directly into AMP without releasing ADP, its major function seems to be the inhibition of ADP-induced platelet aggregation when ATP is released in the circulatory system (Zimmermann, 1999). In fact, administration of a soluble form of CD39 reduces platelet aggregation (Gayle et al., 1998). Curiously, targeted gene disruption of CD39 in mice causes prolonged bleeding times, presumably through down-regulation of the platelet P2Y receptor involved in clot formation (Enjyoji et al., 1999). Based on its primary structure, CD39 is composed of two transmembrane domains near the N- and C-terminal ends, short cytoplasmic N- and C-terminal segments, and a large extracellular domain containing the active site (Maliszewski et al., 1994; Wang and Guidotti, 1996). In the plasma membrane and in some detergent solutions, CD39 forms oligomers that are essential for its enzymatic activity (Wang et al., 1998). Members of the ectoapyrase family are characterized by five highly conserved regions called apyrase conserved region (ACR)1–5. ACR1 and ACR4 are similar to the β- and γ-phosphate binding domains of a family of cytoplasmic ATPases, including actin, hsp70, and hexokinase (Handa and Guidotti, 1996). Mutation of conserved residues in the ACRs in general results in loss of activity of the enzyme (Schulte am Esch et al., 1999; Yang et al., 2001) whereas mutations of other amino acids in the ACRs can change the nucleotide specificity of the isoform (Smith et al., 1999; Grinthal and Guidotti, 2000).
Rat CD39 has seven potential glycosylation sites and is extensively glycosylated judging from the diffuse protein band observed on SDS-PAGE. N-glycosylation has a role in posttranslational maturation and localization of the protein. CD39 was retained in intracellular compartments and was inactive when COS7 cells were treated with tunicamycin, an inhibitor of N-linked glycosylation (Zhong et al., 2001). However, NTPDase6 expressed in Escherichia coli had enzymatic activity, challenging the generality of the importance of N-glycosylation for NTPDase activity (Murphy et al., 2003). Mutation of individual glycosylation sites also has suggested their involvement in the formation of the active structure. Mutation of Asn 81 in NTPDase3 to Asp or Glu, or of Thr83 to Ala (from the N-glycosylation consensus sequence N81NT83), resulted in a loss of 60% of the Ca2+-stimulated enzymatic activity, whereas the localization of the mutant at the cell surface was similar to that of wild-type NTPDase3 (Murphy and Kirley, 2003). When the highly conserved Asn 443 was mutated to Asp, the enzymatic activity of NTPDase2 was decreased to 7% of the wild-type value, but most of the protein remained in the endoplasmic reticulum (Mateo et al., 2003).
To elucidate the contribution of individual N-glycosylation sites to the structure and enzymatic activity of rat CD39, we mutated the seven potential N-glycosylation sites individually and in combination and analyzed the surface expression and activity of the mutants in COS7 cells. Our results suggest all the seven sites can be involved in glycosylation and that CD39 structure and enzymatic activity depend on the glycosylation of a subset of these sites.
MATERIALS AND METHODS
Materials
DMEM, LipofectAMINE, and fetal bovine serum (FBS) were from Invitrogen (Carlsbad, CA). Nucleotides, N-ethylmaleimide, protease inhibitor cocktail, and monoclonal anti-hemagglutinin (HA) Agarose were from Sigma-Aldrich (St. Louis, MO). QuikChange multisite-directed mutagenesis kit was from Stratagene (La Jolla, CA). Protran nitrocellulose was from Schleicher & Schuell (Keene, NH). Peptide N-glycosidase F (PNGase F) and endoglycosidase H (Endo H) were from New England Biolabs (Beverly, MA). Anti-CD39 antibody was generated in this laboratory (Wang et al., 1997). Monoclonal anti-HA antibody HA.11 was from Babco (Richmond, CA). Rabbit anti-HA antibody was from Bethyl Laboratories (Montgomery, TX). Enhanced chemiluminescence ECL streptavidin-horseradish peroxidase (HRP) and blocking reagent were from Amersham Biosciences UK (Little Chalfont, Buckinghamshire, England). EZ-link Sulfo-NHS-LC-Biotin and SuperSignal West Pico Chemiluminescent Substrate were from Pierce Chemical (Rockford, IL). Protein concentration was determined with the protein assay kit (Bio-Rad, Hercules, CA).
Cell Culture and Transient Transfection
COS7 cells were maintained in DMEM with 10% FBS at 37°C in 5% CO2. To transiently express CD39 and its mutants, subconfluent cells were transfected with plasmid DNA and LipofectAMINE according to the manufacturer's instructions. The ratio of DNA to LipofectAMINE was 1:3.
Construction of Plasmids Expressing CD39 Mutants
A 1.4-kb fragment of EcoRI digested pCI-neo-CD39-HA (constructed by Ting Fang Wang in this laboratory), which encodes an HA-tagged CD39, was inserted into pBluescript II SK(+), to make the plasmid pLC3. The QuikChange multisite-directed mutagenesis kit was used to mutate the seven potential N-glycosylation sites with pLC3 as the template by using the mutagenesis primers described in Table 1. The positive mutations were selected, verified, and introduced back into the corresponding region of pCI-neo-CD39-HA. All the CD39 constructs have an HA tag at the C-terminal end.
Table 1.
Individual N-glycosylaton mutants of CD39
| Mutation | Mutation primers | Amino acid changed |
|---|---|---|
| CD39Δ1 | CGGCTGAGAAGGAGAgTGATACAGGAGTacTGCAGCTG | Asn73-Ser, Val77-Leu |
| CD39Δ2 (N226Q) | GTCACCTTCGTGCCCCTgcAgCAGACTCTAGAGGCC | Asn226-Gln |
| CD39Δ2 (N226S) | GTCACCTTCGTGCCCCTAAgTCAGACTCTcGAGGCC | Asn226-Ser |
| CD39Δ3 | GAAGGTTGTGAgTGTAAGCGAgCTCTATGGCACTCCC | Asn291-Ser |
| CD39Δ4 | GCATCCTCAAGTTCTTCtcgAgCAGCCACTGCCCTTACTCC | Asn333-Ser, Asn334-Ser |
| CD39Δ5 | AAGAAGATGGCtAgCGACAGTGTCTCCTCTCAG | Asn375-Ser |
| CD39Δ6 | CCTTCTGCAAGGaTATcAgTTCACGGGAACCTCCTGGG | Asn429-Gln |
| CD39Δ7 | GGGCTACATGCTaAgCTTGACCAACATGATCCCAGC | Asn458-Ser |
The mutated nucleotides are shown in lowercase bold. Novel restriction sites are underlined. The label CD39Δn indicates the N-glycosylation site according to the arrangement shown in Figure 1.
Crude Membrane Preparation, SDS-PAGE, and Immunoblotting
Crude membranes were prepared as described previously (Wu and Guidotti, 2004). The membranes were frozen quickly with liquid nitrogen and kept at –70°C. SDS-PAGE and immunoblotting were performed as described previously (Wu and Guidotti, 2002). The antibodies are specified in the related figure legends.
Sucrose Density Gradient Sedimentation
Crude membranes of COS7 cells were incubated with 1% digitonin on ice for 1 h and centrifuged for 30 min at 46,000 rpm (∼150,000 × g) in a Beckman 70.1Ti rotor at 4°C. The supernatant was layered on 4.8 ml of a linear 5–20% (wt/vol) sucrose gradient (containing 0.2% digitonin, 50 mM HEPES, pH 7.4) and centrifuged for 14 h at 40,000 rpm (∼150,000 × g) at 4°C in a Beckman SW50.1 rotor. Fractions of 200 μl were collected from the top of the tubes. To localize the presence of CD39, 50-μl samples from each fraction were taken to determine calcium-dependent ATPase activity.
Deglycosylation of CD39
Deglycosylation of CD39 was carried out with crude membranes and with whole COS7 cells expressing wild-type and mutant CD39. Crude membranes or COS7 cells were solubilized with glycoprotein denaturing buffer (New England Biolabs) and incubated at 100°C for 10 min. Equal amounts of the solutions were treated with Endo H, PNGase F, or without glycosidase at 37°C overnight in 50 mM sodium citrate, pH 5.5, for the Endo H digestion or 50 mM sodium phosphate, pH 7.5, 1% NP-40 for the PNGase F digestion. The deglycosylated proteins were subjected to SDS-PAGE and immunoblotting with anti-HA antibody.
Cell Surface Biotinylation
Forty-eight hours after transfection, COS7 cells, expressing wild-type or mutant CD39, in 100-mm plates were washed three times with ice-cold phosphate-buffered saline (PBS) buffer, pH 7.8, and incubated with 1 mg/ml EZ-link Sulfo-NHS-Biotin (in PBS buffer with 1 mM MgCl2) at 4°C for 1 h. After removing the biotinylation buffer, cells were incubated in 100 mM glycine (in PBS buffer with 1 mM MgCl2) at 4°C for 20 min, washed three times with PBS buffer, and lysed for 1 h at 4°C with 1 ml of RIPA buffer (50 mM HEPES, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, and 0.1% SDS, pH 7.4) supplemented with a protease inhibitor cocktail. The solution was collected and centrifuged at 13,000 rpm at 4°C for 30 min. The supernatant (200 μl) was incubated with 40 μl of monoclonal anti-HA agarose in RIPA buffer at 4°C for 2–4 h, and then the agarose was washed three times with the same buffer. The proteins were released from the beads in Laemmli buffer at 95°C for 5 min, subjected to SDS-PAGE, and transferred to a nitrocellulose membrane. The biotinylated CD39 in the nitrocellulose membrane was detected with ECL streptavidin-HRP. The cell lysate supernatants also were directly subjected to SDS-PAGE, transferred to a nitrocellulose membrane, and immunoblotted with an anti-HA antibody.
Assay of E-NTPDase Activity
Forty-eight hours after transfection, COS7 cells with plasmids containing the various CD39 constructs were washed with an assay buffer containing 20 mM HEPES-Tris, pH 7.4, 120 mM NaCl, 5 mM KCl, 1 mM EGTA, 0.5 mM NaVO4, and 1 mM NaN3, and then 500 μl of the same buffer containing 2 mM ATP with 5 mM CaCl2 was added to start the reactions. The mixture was incubated at room temperature for 20 min. An aliquot of 30–50 μl of the solution was assayed for Pi (Ames, 1966).
The Ca2+ dependence of the ATPase activity of crude membranes from COS7 cells expressing wild-type and mutant CD39 was determined as described previously (Grinthal and Guidotti, 2000). The protein concentrations were determined with the Bio-Rad protein assay kit.
Immunofluorescence Staining
Chinese hamster ovary (CHO) cells transfected with plasmids containing wild-type or mutant CD39 cDNA were grown on coverslips for 48 h. The cells then were fixed with 4% paraformaldehyde for 10 min and permeabilized with ice-cold methanol for 2 min. After washing with 0.1% Triton X-100/phosphate-buffered saline three times, the coverslips were blocked with 10% goat serum/phosphate-buffered saline for 30 min, stained with rabbit anti-HA antibody followed by incubation with fluorescein isothiocyanate (FITC)-conjugated anti-rabbit IgG secondary antibodies for 30 min. At the same time, DNA was stained with 4,6-diamidino-2-phenylindole (DAPI) to localize the nucleus. The secondary antibody and DAPI were removed from the coverslips by washing with 0.1% Triton X-100/phosphate-buffered saline. The coverslips were observed under a Zeiss LSM510 confocal microscope.
RESULTS
Two questions concerning membrane protein glycosylation are investigated in this study: are all the potential glycosylation sites of CD39 capable of being glycosylated and is glycosylation of specific sites required for expression and activity?
N-Glycosylation Sites of CD39
Rat CD39 contains seven potential N-glycosylation consensus sequences (Asn-X-Ser/Thr) where glycans can attach to the amide nitrogen of the asparagine residue. Figure 1A illustrates their location in the primary sequence. To abolish N-glycosylation at a particular site, we mutated the asparagine codon to a serine or glutamine codon in the cDNA for rat CD39 (Table 1).
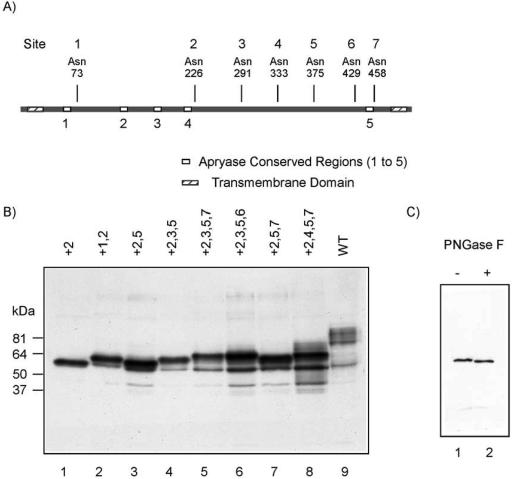
Figure 1.
Electrophoretic mobility of CD39 mutants with single and multiple N-glycosylation sites. (A) Schematic structure of rat CD39. The potential N-glycosylation sites are indicated above the protein. The transmembrane domains are shown as lined boxes. The apyrase conserved regions are shown as empty boxes. (B) Crude membranes (10 μg of protein) from COS7 cells expressing different CD39 mutants were examined by SDS-PAGE, and the gels were immunoblotted with an anti-HA antibody. The symbol “+” indicates the presence of the indicated N-glycosylation sites, whereas all the other sites have been removed. The numbering scheme is shown in A. (C) Crude membranes (10 μg of protein) from COS7 cells expressing mutant +2 containing only the second N-glycosylation site were treated with PNGase F, followed by SDS-PAGE and immunoblotting with an anti-HA antibody.
To elucidate which potential N-glycosylation sites are actually glycosylated during protein synthesis, we examined the electrophoretic mobilities of various CD39 mutants differing in the number of potential N-glycosylation sites. The idea is that a slower mobility of the protein containing an additional glycosylation site is an indication that the site is glycosylated. Figure 1B shows the mobility of CD39 mutants containing the glycosylation sites indicated by the numbers above the lane (glycosylation sites are numbered as shown in Figure 1A). Proceeding from lane 1 on, it is evident that glycosylation takes place at site 1 (lane 2 vs. lane 1), site 5 (lane 3 vs. lane 1), site 3 (lane 4 vs. lane 3 and lane 5 vs. lane 7), site 7 (lane 5 vs. lane 4 and lane 7 vs. lane 3), site 6 (lane 6 vs. lane 4), and site 4 (lane 8 vs. lane 7). Glycosylation at site 2 was revealed by treatment of the CD39 mutant containing only the site 2 asparagine with peptide N-glycosidase F. As show in Figure 1C, there is a slightly but obvious mobility shift for the protein after deglycosylation, indicating that site 2 also is involved in N-glycosylation.
We conclude that all the putative N-glycosylation sites can be glycosylated and are likely to be so in the native molecule.
The Effect of Single N-Glycosylation Site Mutations on CD39 Expression and Enzymatic Activity
The ATPase activities of intact COS7 cells transfected with mutated CD39 cDNAs were measured and compared with that of the cells expressing wild-type CD39 (Figure 2A). The results are presented as a percentage of the wild-type activity; the Δn label indicates which glycosylation site (Figure 1A) has been mutated. It is evident that mutation of the asparagine residues at sites 3, 5, and 6 did not affect the enzymatic activity. On the other hand, mutations at sites 1, 4, and 7 brought about a loss of 50, 30, and 70% of the ATPase activity, respectively. The mutated proteins also were examined by immunoblotting. Although the overall expression level of the Δ1 mutant (Figure 2B, lane 1) was significantly lower than that of the wild type (lane 9), the amount of the other mutants (Δ3 to Δ7) was comparable with that of the wild type. The mutation of site 7 was associated with the appearance of a band moving even more slowly than wild-type CD39 (Figure 2B, lane 7), suggesting an effect on glycosylation or on other protein modification in the absence of Asn 458. To investigate the state of glycosylation of the mutants, we deglycosylated the proteins with Endo H and PNGase F. Figure 3A shows that after Endo H treatment (lane H), a small fraction, ∼20%, of both wild-type and single site mutant proteins has complex glycans; this fraction is represented by the small band traveling more slowly that the fully deglycosylated protein (lane F) and more rapidly than the untreated protein (lane C). This surprising result suggests that a substantial fraction of the N-glycosylation sites of all the protein species is of the high mannose form. The only exception is the Δ7 mutant in which the mobility of a fraction of the protein is not affected by treatment with Endo H, indicating that all the N-glycosylation sites are of the complex glycan form.
Figure 2.
Expression and enzymatic activity of CD39 mutants lacking a single N-glycosylation site. (A) Intact COS7 cells expressing CD39 mutants were incubated with ATP in the presence of calcium; the released phosphate was measured and compared with that of COS7 cells expressing wild-type CD39. (B) Crude membranes (10 μg of protein) of COS7 cells transfected with plasmids containing wild-type and mutant CD39 cDNA were analyzed with SDS-PAGE and immunoblotting with an anti-HA antibody. The symbol “Δ” indicates the mutated N-glycosylation site. Δ2 is the N226Q mutation. WT is wild-type CD39. (C) COS7 cells expressing wild-type, Δ2(N226S), ΔAll(N226S) (Δ1,2,3,4,5,6,7), and + 4,7 CD39 were solubilized with Laemmli buffer, and an aliquot of the solution was examined by SDS-PAGE. The proteins were then transferred to a nitrocellulose membrane and immunoblotted with an anti-HA antibody.
Figure 3.
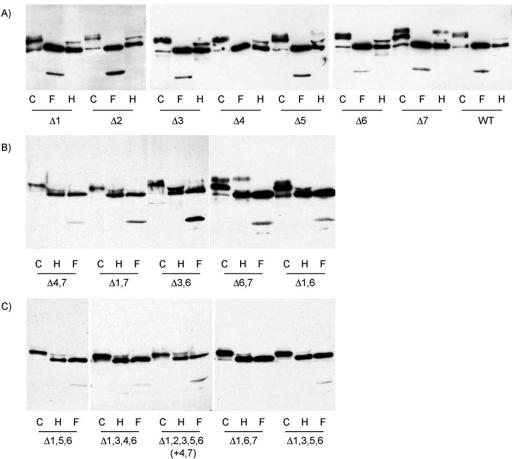
Characterization of the glycans of wild-type and mutant CD39. COS7 cells expressing wild-type or mutant CD39 were solubilized with glycoprotein denaturing buffer (New England Biolabs) and incubated at 100°C for 10 min. Equal amounts of cell lysate were treated with Endo H, PNGase F, or without glycosidase at 37°C overnight. After heating at 95°C for 5 min, the lysates were subjected to electrophoresis and immunoblotting with anti-HA antibody. (A) Wild-type CD39 and single site mutants. (B) Double site mutants. (C) Multiple site mutants. C, control without glycosidase treatment; H, treated with Endo H; F, treated with PNGase F.
With the Δ2 mutant (N226Q), there was no enzymatic activity and no visible protein by using anti-HA antibody (Figure 2, A and B, lane 2). Because the other mutations were substitutions of Ser for Asn, except for N429Q, we wondered whether the N226Q mutation might be the cause of the degradation of the protein. When Asn 226 was changed to Ser and the mutant cDNA expressed in COS7 cells, there was no loss of ATPase activity and no loss of protein (Figure 2, A and C). Apparently, the presence of Gln at position 226 changes the conformation of CD39 so that it is in large part destroyed.
Two possible situations can account for the decreased enzymatic activities of the mutants lacking the first, fourth, and seventh glycosylation site. One is that there are fewer mutant molecules at the cell surface compared with the wild type; the other is that the number of molecules is the same, but the kinetic parameters of the mutant molecules are different from that of the wild-type CD39. To answer this question, we sought to determine the number of CD39 molecules at the cell surface and the kinetic properties of the mutant proteins.
Intact COS7 cells expressing wild-type or mutant CD39 were biotinylated with a membrane-impermeable biotin derivative, EZ-link-Sulfo-NHS-LC-Biotin. After solubilization of the cells, CD39 was immunoprecipitated with anti-HA agarose to collect the CD39 molecules and the precipitate was separated by SDS-PAGE followed by blotting with HRP-conjugated streptavidin to visualize the CD39 molecules at the cell surface. Figure 4 shows the results of these experiments. Relative amounts of mutant proteins are determined in comparison with the amount of wild-type CD39 in each panel; comparisons between panels are not valid because of differences in exposure times. First, it is evident that the CD39 molecules at the cell surface (Figure 4A, top) consisted of the slowest moving molecules in the diffuse mixture present in the total lysate (Figure 4A, bottom). This result agrees with the previous one from this laboratory indicating that only the proteins with the highest level of glycosylation are at the cell surface (Zhong et al., 2001). Second, the amount of protein at the cell surface was similar for the wild type and the Δ1, Δ4, and Δ7 mutants (Figure 4A, lanes 1–4, top, and Table 2, group 1), whereas the total amount of the Δ1 mutant was less than that of the wild type and the other mutants (Figure 4A, lane 2, bottom). The surface expression of the Δ1 and Δ4 mutants observed by immunofluorescence was similar to that of wild-type CD39 (Figure 5). We conclude that the Δ1, Δ4, and Δ7 mutations do not affect the ability of the mutant proteins to move to the cell surface because the number of copies at the cell surface is similar to that of wild-type CD39. Thus, the decrease in surface enzymatic activity for these mutants is due to a change in the specific activity of the mutant proteins.
Figure 4.
Surface expression of the N-glycosylation site mutants of CD39. (A and B) COS7 cells expressing wild-type or mutant CD39 were biotinylated with EZ-link Sulfo-NHS-Biotin and then solubilized with RIPA buffer. Top, solutions were incubated with monoclonal anti-HA agarose to collect CD39 molecules. The beads were incubated with Laemmli buffer, and the released proteins were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and immunoblotted with ECL streptavidin-HRP. Bottom, solutions were subjected to SDS-PAGE, and the proteins were transferred to a nitrocellulose membrane and immunoblotted with anti-HA antibody. Relative amounts of mutant proteins are determined in comparison with the amount of wild-type CD39 in each panel; comparisons between panels are not valid because of differences in exposure times.
Table 2.
Cell surface ATPase activity and expression of CD39 mutants lacking one, two, three, four, five, or six N-glycosylation sites
| Mutant | Activity (SD) (% of WT) | Expression on cell surface (% of WT) | Specific activity (activity/expression) |
|---|---|---|---|
| Group1 | |||
| Δ1 | 50.60 (10.20) | 101.7 | 0.5 |
| Δ2 | 98.17 (2.53) | ||
| Δ3 | 110.80 (7.30) | ||
| Δ4 | 73.00 (3.80) | 89.7 | 0.8 |
| Δ5 | 103.00 (8.50) | 104.6 | 1.0 |
| Δ6 | 108.10 (8.90) | ||
| Δ7 | 31.00 (3.20) | 92.8 | 0.34 |
| Group 2 | |||
| Δ1,4 | 5.31 (0.63) | 33.3 | 0.15 |
| Δ4,7 | 22.00 (1.03) | ||
| Δ1,7 | 42.24 (2.84) | ||
| Δ1,6 | 95.00 (5.53) | 95.1 | 1.0 |
| Δ3,4 | 53.90 (4.83) | ||
| Δ4,6 | 52.11 (3.82) | ||
| Δ6,7 | 9.48 (0.64) | 88.8 | 0.11 |
| Δ1,3 | 56.70 (3.45) | ||
| Δ3,6 | 65.00 (3.98) | 97.8 | 0.66 |
| Group 3 | |||
| Δ1,4,7 | 3.06 (0.43) | ||
| Δ1,4,6 | 2.28 (0.27) | ||
| Δ1,3,4 | 3.25 (0.28) | ||
| Δ4,6,7 | 2.25 (0.28) | ||
| Δ3,6,7 | 10.36 (1.02) | ||
| Δ1,6,7 | 23.49 (1.05) | 30.9 | 0.73 |
| Δ1,5,6 | 87.46 (4.59) | ||
| Δ1,3,6 | 60.40 (4.35) | ||
| Δ3,5,6 | 67.04 (3.25) | ||
| Δ3,4,6 | 18.00 (2.57) | ||
| Group 4 | |||
| Δ3,4,6,7 | 1.19 (0.32) | ||
| Δ1,4,6,7 | 1.68 (0.25) | ||
| Δ1,3,4,6 | 11.50 (2.48) | 5.1 | 2.3 |
| Δ1,3,6,7 | 13.15 (0.53) | ||
| Δ1,3,5,6 | 45.30 (1.15) | 39.5 | 1.1 |
| Group 5 | |||
| Δ3,4,5,6,7 | 1.82 (0.39) | ||
| Δ1,3,4,6,7 | 1.90 (0.38) | ||
| Δ1,3,5,6,7 | 3.80 (0.22) | ||
| Δ1,2,3,5,6 | 45.73 (0.53) | 23.2 | 1.97 |
| Group 6 | |||
| Δ1,3,4,5,6,7 | 2.10 (0.43) | ||
| Δ1,2,3,4,5,6,7 | 0.50 (0.03) |
The activity is expressed as percentage of wild type. The numbers in parentheses are SDs, which were calculated from at least three independent experiments. The expression of mutants on the cell surface was determined by biotinylation; band intensity was analyzed with a Bio-Rad imager and Quantity One software. The results are the mean of at least two independent experiments.
Figure 5.
Immunofluorescence photographs of CHO cells expressing CD39 wild-type and CD39 mutants. CHO cells expressing wild-type CD39 and the Δ1, Δ4, and +2 mutants were grown for 48 h on coverslips. After fixation and permeabilization, the cells were stained with rabbit anti-HA antibody and FITC-conjugated anti-rabbit IgG secondary antibody. The nucleus was stained with DAPI. The cells were visualized with a Zeiss LSM510 confocal microscope.
To investigate whether removal of N-glycosylation sites changes the catalytic properties of the proteins, the dependence of the ATPase activity on the Ca2+ concentration was measured as described previously (Grinthal and Guidotti, 2000). The results for the Δ1, Δ4, Δ5, and Δ7 mutants are shown in Figure 6 and Table 3. First, the Δ5 mutant had kinetics indistinguishable from those of the wild-type CD39, with a biphasic Ca2+ activation and inhibition curve (Figure 6) and similar values for Km and Vmax (Table 3). The Δ1, Δ4, and Δ7 mutants with lower amounts of surface ATPase activity compared with wild-type CD39 (Figure 2 and Table 2) all had lower values of Vmax compared with wild-type CD39 (roughly 60% of the activity) (Table 3) and comparable values of Km, except for the Δ7 mutant whose Km value was approximately one-fourth of that of the wild type. These results indicate that the Δ1, Δ4, and Δ7 mutants have a decreased catalytic turnover compared with the wild-type CD39.
Figure 6.
Ca2+ dependence of the ATPase activity of wild-type CD39 and N-glycosylation site mutants. Crude membranes (10 μg of protein) of COS7 cells expressing wild-type or mutant CD39 were incubated with ATP in the presence of different concentrations of free calcium as described in Grinthal and Guidotti (2000). Released phosphate was assayed according to Ames (1966).
Table 3.
Ca2+-dependent kinetics of CD39 mutants
| Km | Vmax | |
|---|---|---|
| nm[Pi]/min/μg | ||
| WT | 2.91×10-5 | 2.91 |
| Δ1 | 4.62×10-5 | 1.54 |
| Δ4 | 3.51×10-5 | 1.75 |
| Δ5 | 2.72×10-5 | 2.72 |
| Δ7 | 7.72×10-6 | 1.93 |
| +4,7 | 2.35×10-5 | 0.71 |
Concentrations of free calcium were calculated using the MaxChelator program with calcium complex constants from Martell and Smith (1974). The graphing program IGOR was used to display calcium titration curves and to calculate Km values for free Ca2+.
Because the enzymatic activity of CD39 depends on the oligomeric state of the protein (Wang et al., 1998), we asked whether the mutants are dimeric when solubilized in digitonin, as is wild-type CD39. The result obtained with the Δ7 mutant is shown in Figure 7, which illustrates that the size of the mutant determined by sucrose density gradient centrifugation was identical to that of the wild-type CD39. Sedimentation analysis also was carried out with the Δ1 and Δ4 mutants revealing that their dimeric structure is not affected by the mutation (our unpublished data).
Figure 7.
Sucrose density gradient sedimentation of solubilized wild-type CD39 and the Δ7 mutant. Crude membranes prepared from COS7 cells expressing CD39 wild-type or the Δ7 mutant were solubilized with 1% digitonin, layered on a linear gradient 5–20% (wt/vol) sucrose, and centrifuged for 14 h at 150,000 × g. Fractions of 200 μl were collected from the top of centrifuge tubes. The presence of CD39 was determined by measuring the ATPase activity of the fractions (see Materials and Methods).
We conclude that the decreased activity of the Δ1, Δ4, and Δ7 mutants is not related to a change in the oligomerization state of the protein.
N-Glycosylation Affects CD39 Stability
The rationale for the experiment reported here is the observation that a fraction of CD39 molecules is cleaved into a N-terminal fragment of 27 kDa and a C-terminal fragment of 56 kDa by an endogenous proteolytic process that presumably cleaves the bond between Arg188 and Phe189. Exposure of cells expressing CD39 to trypsin increases the amount of the fragments (Schulte am Esch et al., 1999). In Figure 2B, which shows the results of immunoblotting with anti-HA antibody of the membranes containing the mutant CD39 molecules, one can observe various bands in the 50- to 55-kDa region of all the mutants except for the Δ4 mutant. We supposed that these bands were the C-terminal fragments of the cleaved CD39 molecules. This supposition was confirmed by examination with anti-HA antibody of the mutants deglycosylated with PNGase F (Figure 3A). The wild-type CD39 and the mutants, except for Δ4, show the presence of bands at 60 and 35 kDa, representing the deglycosylated intact polypeptide and the C-terminal fragment. The Δ4 mutant has the interesting phenotype that it is resistant to cleavage. Apparently, the lack of glycosylation at Asn 333 affects the conformation of the protein so that the cleavage site is not available.
The various mutants also were examined by immunoblotting with an anti-CD39 antibody that is specific for the N-terminal region of the molecule, whereas the anti-HA antibody recognizes the C terminus of CD39. The results of this analysis are shown in Figure 8. A 27-kDa band was clearly visible in wild-type CD39. In the Δ1 mutant, the cleavage band migrated faster (at ∼20 kDa) than the wild-type band, supporting the localization of the first N-glycosylation site on the small fragment. With the N226Q mutation at site 2, although no intact CD39 band was observed, a fragment of ∼35 kDa was visible, supporting the conclusion that the protein had been degraded. In the absence of glycans at site 4, there was no 27-kDa fragment, supporting the view that the cleavage site is occluded in this mutant. The Δ3, Δ5, Δ6, and Δ7 mutants all have bands in the 27-kDa region.
Figure 8.
Effect of N-glycosylation on CD39 posttranslational processing. Crude membranes (10 μg of protein) of COS7 cells expressing wild-type or mutant CD39 were subjected to SDS-PAGE, and the proteins, after transfer to nitrocellulose, were immunoblotted with an anti-CD39 antibody.
We conclude that glycosylation of site 4 has subtle effects on the structure of CD39.
Combinatorial Effects of N-Glycosylation on CD39 Enzymatic Activity
Because all seven potential N-glycosylation sites apparently are glycosylated in COS7 cells, we asked whether some glycosylation sites are essential for ATPase activity and what is the minimal number of glycosylation sites required for activity. Accordingly, we constructed a variety of CD39 mutants all containing site 2, to prevent the degradation of the protein with the N226Q mutation, and other N-glycosylation sites and measured their enzymatic activity, surface expression, and glycan composition in COS7 cells. The results are shown in Table 2, Figures 3 and 4.
Starting from the single mutants shown in Figure 2A and group 1 of Table 2, we constructed mutants lacking two, three, four, and five glycosylation sites to examine the cumulative effects of these deletions. As indicated above, deletions of sites 1, 4, and 7 individually brought about a loss of ATPase activity but no change in surface expression. In group 2 of Table 2 are shown in order double deletions of combinations of sites 1, 4 and 7, then of sites 1, 4, and 7 with sites whose single deletion had no effect, and then of combinations of sites whose single deletion had no effect. The Δ1,4 mutant had virtually no ATPase activity (5 vs. 35% expected), and its surface expression was decreased (Figure 4B, lane 3) suggesting that the absence of sites 1 and 4 is highly detrimental. The Δ4,7 mutant had the expected cumulative decrease (22 vs. 21% expected) and the Δ1,7 a higher than expected ATPase activity (42 vs. 15% expected); interestingly, in both mutants the complex glycan form of the site 7 mutation was changed to that of wild-type CD39 (Figure 3B). This suggests the deletion of sites 1 and 7 is advantageous compared with the absence of site 7 alone. The latter point is made forcefully by the result with the Δ1,6 mutant that regained full surface expression (Figure 4B, lane 6) and full ATPase activity (Table 2, group 2) compared with the Δ1 mutant; clearly, deletion of these two sites caused a gain of function. On the other hand, the Δ3,4, Δ4,6, and especially the Δ6,7 mutants had decreased or practically no enzymatic activity, indicating the negative effects of the deletions of these pairs of sites. Strikingly, the surface expression of the Δ6,7 mutant was similar to that of wild-type CD39 (Figure 4B, lane 5), and the complex glycan form of the site 7 mutation was retained (Figure 3B). It seems that this form of CD39 has not folded properly. The activities of the Δ1,6 and the Δ3,4, Δ4,6, and Δ6,7 mutants were distinctly different from that of the Δ1,3 mutant, which had the same activity as the Δ1 mutant, as expected from the lack of effect of the Δ3 mutation. On the other hand, the Δ3,6 mutant had only 65% of the activity of wild-type CD39 in spite of the fact that deletion of each site individually was without effect; also, a larger fraction of the protein was of the complex glycan form compared with wild-type CD39 (Figure 3B) and its surface expression was similar to that of wild-type CD39 (Figure 4A, lane 5). This result shows that the absence of both sites has a negative effect on the protein.
Group 3 of Table 3 shows the results obtained with deletions of three glycosylation sites. The first point of interest is that all mutants containing the Δ1,4 or Δ6,7 mutation had practically no ATPase activity, irrespective of any additional mutation, indicating the importance of these four sites for the structure of CD39. The only exception was the Δ1,6,7 mutant with 23% activity, loss of the complex glycan form of the site 7 mutation (Figure 3C), and decreased surface expression compared with the Δ6,7 mutant (Figure 4B, lane 4); this result again shows the positive effect of the deletions of sites 1 and 6,7. This effect also is demonstrated by the Δ1,5,6 mutant that had 87% of the wild-type CD39 activity. On the other hand, the Δ1,3,6 and Δ3,5,6 mutants had 60 and 67% of the wild-type CD39 activity, as did the parent Δ3,6 mutant, indicating that the negative interaction of the site 3 and site 6 deletions is not overridden by the positive effect of site 1 and site 6 deletions and is not affected by the neutral site 5 deletion. Interestingly, deletion of site 4 in addition to sites 3 and 6 was highly deleterious for the Δ3,4,6 mutant.
The results in rows 4 and 5 of Table 2 show again that all the quadruple and quintuple mutants that lack sites 1 and 4 and/or sites 6 and 7 had virtually no ATPase activity. The Δ1,3,5,6 and Δ1,2,3,5,6 mutants had 45% of the wild-type activity, indicating that the presence of sites 4 and 7 alone endows CD39 with a decreased but substantial surface expression (Figure 4B, lane 7 and Figure 4A, lane 6) and enzymatic activity (Table 2, groups 4 and 5); the Δ1,3,5,6 mutant had very little complex glycan compared with the Δ1,2,3,5,6 mutant and wild-type CD39. Of the nine mutants with >40% of the enzymatic activity of wild-type CD39, seven had both site 4 and site 7 glycosylation sites, whereas two mutants had either site 4 or site 7. This result supports the generalization that the proteins containing glycosylation sites 4 and 7 had the greatest activity.
The following insights emerge from this information. There seems to be interactions between glycosylation sites 1, 3, 4, 6, and 7. Mutants lacking glycosylation sites 1 and 4, Δ1,4, had no or very low ATPase activity, whether or not there were other mutations. The deletion of glycosylation site 6 had various effects: by itself, there was no effect on ATPase activity; coupled with deletion of sites 3, 4, or 7, the double deletion mutants, especially Δ6,7, had a greater loss of activity than expected from the sum of the activities of the individual mutants. The same phenomenon was true for the Δ3,4 mutant. On the other hand, mutants lacking glycosylation sites 1 and either 6 or 7 or both 6 and 7, had an increase of ATPase activity compared with mutants that did not lack the combination of glycosylation sites.
The protein lacking all N-glycosylation sites was not visible, and there was no enzymatic activity at the cell surface (Figure 2C); in this case, the site 2 mutation was N226S to prevent degradation brought about by the N226Q mutation. If only site 2 was present, no protein was detected at the cell surface (Figure 4A, lane 7), and there was very little if any enzymatic activity (Table 2, group 6). The presence of this mutant principally in intracellular compartments rather than at the cell surface is evident from the immunofluorescent pictures shown in Figure 5.
DISCUSSION
N-linked glycans are required for the proper folding of many membrane and secreted proteins (Ronnett et al., 1984; Slieker et al., 1986; Konig et al., 1988). Glycoproteins that are not glycosylated are generally found in the endoplasmic reticulum (Williams and Enns, 1991; Marquardt and Helenius, 1992). As discussed by Taylor and Drickamer (Taylor and Drickamer, 2003), the effects of oligosaccharide chains on protein structure are diverse: from stabilization of the structure, to protection against proteolysis, to modulation of protein–protein interactions. In the context of these considerations, we have tried to decipher the logic of glycosylation of CD39.
Our results indicate that all seven potential N-glycosylation sites of rat CD39 can be used in vivo. N-glycans at these sites play different roles in protein expression, tertiary structure, and activity. Protein expression was decreased to 50% of that of wild-type CD39 when site 1 was mutated, even though presence of the protein at the cell surface was not affected. Removal of N-glycans at site 4 or site 7 affected protein tertiary structure, indicated by resistance to proteolytic cleavage (site 4) or a change in content of complex glycans and in electrophoretic mobility (site 7), but it did not change the amount of protein at the cell surface. In all three situations, the enzymatic activity at the plasma membrane was reduced, indicating a decrease in the specific activity of these CD39 mutants. In contrast, elimination of N-glycosylation sites 2, 3, 5, and 6 individually had no effect on the ATPase activity of these CD39 mutants or on the surface expression of the Δ5 mutant.
The absence of sets of N-glycans had several effects. It affected protein structure but not surface expression (decrease in specific activity) (Δ1, Δ4, Δ7, Δ6,7, and Δ3,6); surface expression but not structure (no change in specific activity) (Δ1,3,5,6); surface expression and structure (decrease or increase in specific activity) (Δ1,4, Δ1,6,7, Δ1,3,4,6, Δ1,2,3,5.6 and Δ1,3,4,5,6,7), and neither surface expression nor structure (no change in specific activity) (Δ5 and Δ1,6).
It was unexpected that only the site 7 mutation was associated with a change in the pattern of glycosylation of the protein. All the other mutants had approximately the same fraction of high mannose and complex glycan forms as wild-type CD39, with the possible exceptions of the Δ3,6 and the Δ1,3,5,6 mutants. This result suggests that the same fraction of the wild-type CD39 and of the mutants moved from the endoplasmic reticulum to the Golgi compartments.
The central result is that the presence of glycosylation sites 4 and 7 is necessary for the presence of active CD39 at the cell surface. All mutants that had >40% of the activity of wild-type CD39 contained glycosylation sites 4 or 7 or both. Furthermore, all mutants lacking sites 1 and 4 or sites 6 and 7 had virtually no ATPase activity, emphasizing the importance of these glycosylation sites for the production of native CD39. Examination of the structure of CD39 (Figures 1A and 9, top diagram) reveals that site 1 is located next to ACR1 and the first two cysteine residues; these form a disulfide bond because the N-terminal proteolytic fragment of CD39 is not linked to the C-terminal fragment by a disulfide linkage (Schulte am Esch et al., 1999). Site 4 is located in a region with many cysteine residues that all form disulfide bonds. Sites 6 and 7 are near ACR5.
Figure 9.
Schematic representation of the N-glycosylation sites and cysteine residues of NTPDases 1–6. The structures of NTPDases 1–6 are shown sequentially. The potential N-glycosylation sites are indicated by a black arrow on top of the rectangle; the cysteine residues by a line under the rectangle. NTPDases 1, 2, and 3 have similar arrangements of all 10 of the extracytoplasmic cysteine residues, whereas only two N-glycosylation sites are common to all three structures.
We propose the hypothesis that site 1 glycosylation is important for the proper arrangement of ACR1 with respect to the other components of the active site, whereas N-glycans at site 4 are required for proper folding of the region between ACR4 and ACR5 that contains eight cysteine residues that are disulfide bonded. Whereas individual mutations of site 1 and site 4 are slightly detrimental, removal of both site 1 and 4 leads basically to no active protein. It also can be deduced that sites 6 and 7 have a strong effect on the arrangement of ACR5 in the native protein, so that elimination of both sites leads to practically no native protein. This structural change is accompanied by an increase in the fraction of glycosylation sites that have complex glycans compared with the situation in wild-type CD39. The finding that the Δ1,6 mutant is completely active suggests that site 1 glycosylation is not required for folding of this region of CD39.
Is it possible to deduce these finding through examination of the glycosylation sites of the various isoforms of CD39? Rat, mouse, human, cow, pig, and chicken CD39 all have glycosylation sites 1, 4, and 7, whereas the other glycosylation sites are variable; for instance, human CD39 has site 5 but not site 6, mouse CD39 has site 6 and not site 5, and the rat isoform has both. This result supports the conclusion that sites 1, 4, and 7 are relatively essential for the structure of CD39 and suggests that site 5 may substitute for site 6 in human CD39. Comparison of CD39 with NTPDases2–6 (Figure 9) suggests the following generalizations. In the subset of proteins present at the plasma membrane, CD39, CD39L1, and CD39L3 (NTPDases1–3), all have glycosylation sites corresponding to site 1 and site 7, in agreement with the conclusion deduced from our results. Site 4 is present in CD39 and in the mouse and rat isoforms of CD39L1, not in human CD39L1 and in CD39L3, suggesting that in the latter the function of site 4 is taken over by the nearby sites. Presumably, the location of N-glycosylation sites does not have to be exactly identical. This is in sharp contrast to the rigorous conservation of the position of cysteine residues in all three plasma membrane enzymes, indicating that the disulfide bond arrangement is critical for proper structure. The subset of NTPDases that is secreted or in internal compartments (NTPDases4–6) differ from the previous set in having two to three glycosylation sites, instead of five to seven.
The importance of N-glycans for CD39 function has been addressed previously by our laboratory and those of others. We reported that COS7 cells capable of expressing a secreted form of CD39 retained inactive CD39 in intracellular compartments when treated with tunicamycin (Zhong et al., 2001). This result agrees with the observation that no CD39 protein is visible in COS7 cells containing a plasmid with CD39 cDNA lacking all the N-glycosylation sites (Figure 2C). In contrast, Smith and Kirley (1999) reported that human NTPDase3 was present at the plasma membrane, although inactive, upon treatment of COS cells with tunicamycin. The interpretation of the results obtained with tunicamycin is complicated by the fact that this compound affects glycosylation and function of all glycoproteins, including those involved in the secretory pathway, so it is difficult to attribute the effects observed with CD39 to the glycosylation state of this protein rather than that of the other glycoproteins.
The involvement of specific glycosylation sites of NTPDases in their function also has been reported. Mutation of Asn 81 in NTPDase3 to Asp or Glu or of Thr83 to Ala (from the N-glycosylation consensus sequence N81NT83) resulted in a loss of 60% of the Ca2+-stimulated enzymatic activity (Murphy and Kirley, 2003). This result is similar to that reported here (Figure 2). When the highly conserved Asn 443 was mutated to Asp, the enzymatic activity of NTPDase2 was decreased to 7% of the wild-type value (Mateo et al., 2003). This effect was more pronounced than that reported here with CD39 (NTPDase1) (Figure 2). It may represent variations in folding pathway or stability between the various members of the family, or it may be a consequence of the presence of the new amino acid rather than the lack of glycosylation. In view of the startling difference in the effect of a Ser versus a Gln substitution at Asn 226 (Figure 2), one should sort out whether a phenotype is due to a loss of glycosylation or the amino acid mutation.
Interestingly, an active fragment of NTPDase 6 has been obtained by expression in E. coli (Murphy et al., 2003). Although it was not reported whether mutation of the two N-glycosylation sites of NTPDase6 affected its expression in animal cells, NTPDase5 was secreted in a fully active form from COS7 cells treated with tunicamycin (Mulero et al., 2000). This result may indicate that glycosylation is not required for secreted or intracellular members of the NTPDase family. There remains the possibility that N-glycosylation is principally required for traffic through the endoplasmic reticulum and the Golgi compartments, and the results presented here are a report of the interaction of the N-glycosylated regions of the molecules with chaperones in the compartments. Accordingly, it is possible that most members of the NTPDase family remain active after removal of their N-glycans from the folded proteins (Helenius and Aebi, 2004).
The major conclusion of this analysis is that sites 1, 4, 6, and 7 are required for the proper folding and arrangement of the active site of CD39. Elimination of sites 1 and 4 or sites 6 and 7 is lethal, whereas elimination of the other binary combinations is still compatible with expression of some enzymatic activity.
Acknowledgments
We are grateful to Renate Hellmiss-Peralta for help with the figures. We thank Drs. Xiaoqi Liu, Alison Grinthal, Tianhua Zhou, Sari Paavilainen, Julie McGeoch, and Hanlin Wang and members of the Kleckner group for help with the experiments and during the preparations of this manuscript. This work was supported by grant HL-08893 from the National Institutes of Health (to G. G.).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-10-0886) on January 26, 2005.
References
- Ames, B. N. (1966). Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol 8, 115–117. [Google Scholar]
- Biederbick, A., Rose, S., and Elsasser, H. P. (1999). A human intracellular apyrase-like protein, LALP70, localizes to lysosomal/autophagic vacuoles. J. Cell Sci. 112, 2473–2484. [DOI] [PubMed] [Google Scholar]
- Bigonnesse, F., Levesque, S. A., Kukulski, F., Lecka, J., Robson, S. C., Fernandes, M. J., and Sevigny, J. (2004). Cloning and characterization of mouse nucleoside triphosphate diphosphohydrolase-8. Biochemistry 43, 5511–5519. [DOI] [PubMed] [Google Scholar]
- Braun, N., Sevigny, J., Robson, S. C., Enjyoji, K., Guckelberger, O., Hammer, K., Di Virgilio, F., and Zimmermann, H. (2000). Assignment of ecto-nucleoside triphosphate diphosphohydrolase-1/cd39 expression to microglia and vasculature of the brain. Eur. J. Neurosci. 12, 4357–4366. [PubMed] [Google Scholar]
- Enjyoji, K., et al. (1999). Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat. Med. 5, 1010–1017. [DOI] [PubMed] [Google Scholar]
- Gayle, R. B., 3rd, et al. (1998). Inhibition of platelet function by recombinant soluble ecto-ADPase/CD39. J. Clin. Investig. 101, 1851–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinthal, A., and Guidotti, G. (2000). Substitution of His59 converts CD39 apyrase into an ADPase in a quaternary structure dependent manner. Biochemistry 39, 9–16. [DOI] [PubMed] [Google Scholar]
- Handa, M., and Guidotti, G. (1996). Purification and cloning of a soluble ATP-diphosphohydrolase (apyrase) from potato tubers (Solanum tuberosum). Biochem. Biophys. Res. Commun. 218, 916–923. [DOI] [PubMed] [Google Scholar]
- Helenius, A., and Aebi, M. (2004). Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 73, 1019–1049. [DOI] [PubMed] [Google Scholar]
- Kaczmarek, E., Koziak, K., Sevigny, J., Siegel, J. B., Anrather, J., Beaudoin, A. R., Bach, F. H., and Robson, S. C. (1996). Identification and characterization of CD39/vascular ATP diphosphohydrolase. J. Biol. Chem. 271, 33116–33122. [DOI] [PubMed] [Google Scholar]
- Konig, R., Ashwell, G., and Hanover, J. A. (1988). Glycosylation of CD4. Tunicamycin inhibits surface expression. J. Biol. Chem. 263, 9502–9507. [PubMed] [Google Scholar]
- Maliszewski, C. R., et al. (1994). The CD39 lymphoid cell activation antigen. Molecular cloning and structural characterization. J. Immunol. 153, 3574–3583. [PubMed] [Google Scholar]
- Marquardt, T., and Helenius, A. (1992). Misfolding and aggregation of newly synthesized proteins in the endoplasmic reticulum. J. Cell Biol. 117, 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martell, A. E., and Smith, R. M. (1974). Critical Stability Constants, New York: Plenum Press.
- Mateo, J., Kreda, S., Henry, C. E., Harden, T. K., and Boyer, J. L. (2003). Requirement of Cys399 for processing of the human ecto-ATPase (NTPDase2) and its implications for determination of the activities of splice variants of the enzyme. J. Biol. Chem. 278, 39960–39968. [DOI] [PubMed] [Google Scholar]
- Mulero, J. J., Yeung, G., Nelken, S. T., Bright, J. M., McGowan, D. W., and Ford, J. E. (2000). Biochemical characterization of CD39L4. Biochemistry 39, 12924–12928. [DOI] [PubMed] [Google Scholar]
- Mulero, J. J., Yeung, G., Nelken, S. T., and Ford, J. E. (1999). CD39–L4 is a secreted human apyrase, specific for the hydrolysis of nucleoside diphosphates. J. Biol. Chem. 274, 20064–20067. [DOI] [PubMed] [Google Scholar]
- Murphy, D. M., Ivanenkov, V. V., and Kirley, T. L. (2003). Bacterial expression and characterization of a novel, soluble, calcium-binding, and calcium-activated human nucleotidase. Biochemistry 42, 2412–2421. [DOI] [PubMed] [Google Scholar]
- Murphy, D. M., and Kirley, T. L. (2003). Asparagine 81, an invariant glycosylation site near apyrase conserved region 1, is essential for full enzymatic activity of ecto-nucleoside triphosphate diphosphohydrolase 3. Arch. Biochem. Biophys. 413, 107–115. [DOI] [PubMed] [Google Scholar]
- Plesner, L. (1995). Ecto-ATPases: identities and functions. Int. Rev. Cytol. 158, 141–214. [DOI] [PubMed] [Google Scholar]
- Ronnett, G. V., Knutson, V. P., Kohanski, R. A., Simpson, T. L., and Lane, M. D. (1984). Role of glycosylation in the processing of newly translated insulin proreceptor in 3T3–L1 adipocytes. J. Biol. Chem. 259, 4566–4575. [PubMed] [Google Scholar]
- Rothstein, A., Meier, R. C., and Scharff, T. G. (1953). Relationship of cell surface to metabolism. IX. Digestion of phosphorylated compounds by enzymes located on surface of intestinal cell. Am. J. Physiol. 173, 41–46. [DOI] [PubMed] [Google Scholar]
- Schulte am Esch, J., 2nd, Sevigny, J., Kaczmarek, E., Siegel, J. B., Imai, M., Koziak, K., Beaudoin, A. R., and Robson, S. C. (1999). Structural elements and limited proteolysis of CD39 influence ATP diphosphohydrolase activity. Biochemistry 38, 2248–2258. [DOI] [PubMed] [Google Scholar]
- Shi, J. D., et al. (2001). Molecular cloning and characterization of a novel mammalian endo-apyrase (LALP1). J. Biol. Chem. 276, 17474–17478. [DOI] [PubMed] [Google Scholar]
- Slieker, L. J., Martensen, T. M., and Lane, M. D. (1986). Synthesis of epidermal growth factor receptor in human A431 cells. Glycosylation-dependent acquisition of ligand binding activity occurs post-translationally in the endoplasmic reticulum. J. Biol. Chem. 261, 15233–15241. [PubMed] [Google Scholar]
- Smith, T. M., and Kirley, T. L. (1999). Glycosylation is essential for functional expression of a human brain ecto-apyrase. Biochemistry 38, 1509–1516. [DOI] [PubMed] [Google Scholar]
- Smith, T. M., Lewis Carl, S. A., and Kirley, T. L. (1999). Mutagenesis of two conserved tryptophan residues of the E-type ATPases: inactivation and conversion of an ecto-apyrase to an ecto-NTPase. Biochemistry 38, 5849–5857. [DOI] [PubMed] [Google Scholar]
- Taylor, M. E., and Drickamer, K. (2003). Structure-function analysis of C-type animal lectins. Methods Enzymol. 363, 3–16. [DOI] [PubMed] [Google Scholar]
- Vanduffel, L., and Lemmens, R. (2000). Ecto-ATPases and Related Ectonucleotidases, Maastricht, The Netherlands: Shaker Publishing B.V.
- Wang, T. F., and Guidotti, G. (1996). CD39 is an ecto-(Ca2+,Mg2+)-apyrase. J. Biol. Chem. 271, 9898–9901. [PubMed] [Google Scholar]
- Wang, T. F., and Guidotti, G. (1998). Golgi localization and functional expression of human uridine diphosphatase. J. Biol. Chem. 273, 11392–11399. [DOI] [PubMed] [Google Scholar]
- Wang, T. F., Ou, Y., and Guidotti, G. (1998). The transmembrane domains of ectoapyrase (CD39) affect its enzymatic activity and quaternary structure. J. Biol. Chem. 273, 24814–24821. [DOI] [PubMed] [Google Scholar]
- Wang, T. F., Rosenberg, P. A., and Guidotti, G. (1997). Characterization of brain ecto-apyrase: evidence for only one ecto-apyrase (CD39) gene. Brain Res. Mol. Brain Res. 47, 295–302. [DOI] [PubMed] [Google Scholar]
- Williams, A. M., and Enns, C. A. (1991). A mutated transferrin receptor lacking asparagine-linked glycosylation sites shows reduced functionality and an association with binding immunoglobulin protein. J. Biol. Chem. 266, 17648–17654. [PubMed] [Google Scholar]
- Wu, J. J., and Guidotti, G. (2002). Construction and characterization of a monomeric insulin receptor. J. Biol. Chem. 277, 27809–27817. [DOI] [PubMed] [Google Scholar]
- Wu, J. J., and Guidotti, G. (2004). Proreceptor dimerization is required for insulin receptor post-translational processing. J. Biol. Chem. 279, 25765–25773. [DOI] [PubMed] [Google Scholar]
- Yang, F., Hicks-Berger, C. A., Smith, T. M., and Kirley, T. L. (2001). Site-directed mutagenesis of human nucleoside triphosphate diphosphohydrolase 3, the importance of residues in the apyrase conserved regions. Biochemistry 40, 3943–3950. [DOI] [PubMed] [Google Scholar]
- Zhong, X., Malhotra, R., Woodruff, R., and Guidotti, G. (2001). Mammalian plasma membrane ecto-nucleoside triphosphate diphosphohydrolase 1, CD39, is not active intracellularly. The N-glycosylation state of CD39 correlates with surface activity and localization. J. Biol. Chem. 276, 41518–41525. [DOI] [PubMed] [Google Scholar]
- Ziganshin, A. U., Hoyle, C. H., Ziganshina, L. E., and Burnstock, G. (1994). Effects of cyclopiazonic acid on contractility and ecto-ATPase activity in guinea-pig urinary bladder and vas deferens. Br. J. Pharmacol 113, 669–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann, H. (1999). Nucleotides and CD 39, principal modulatory players in hemostasis and thrombosis. Nat. Med. 5, 987–988. [DOI] [PubMed] [Google Scholar]