Abstract
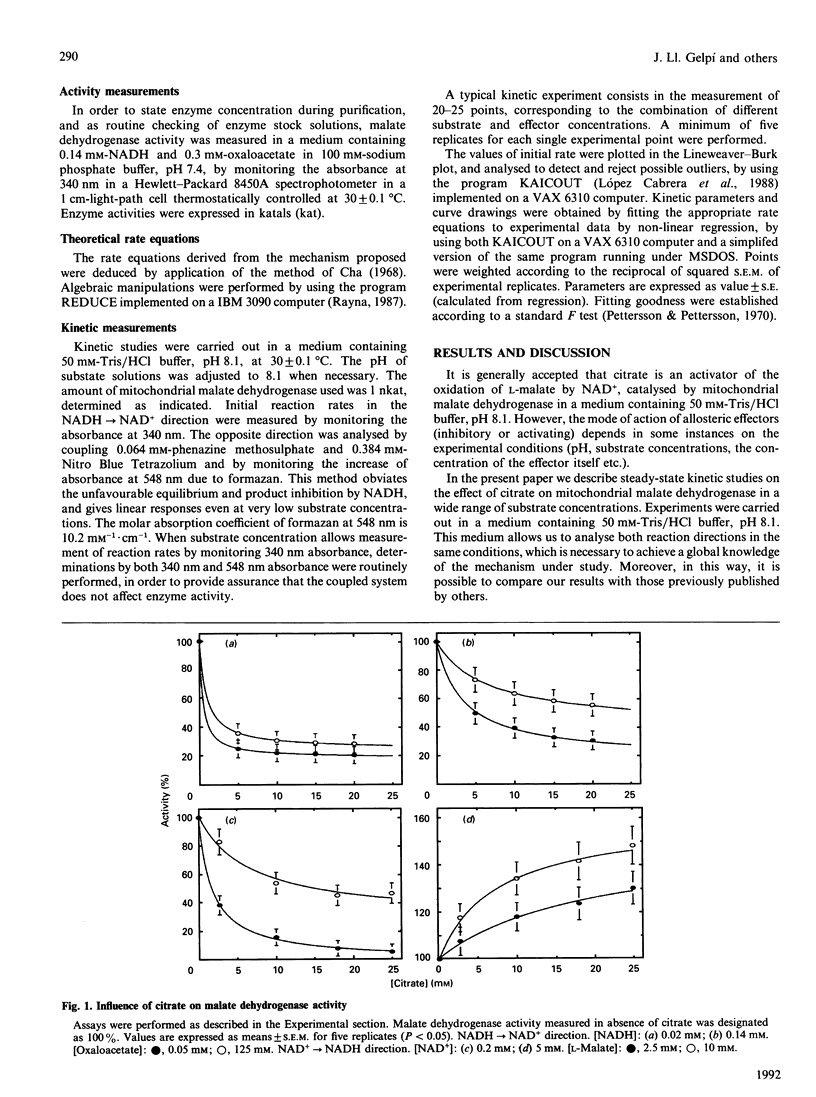
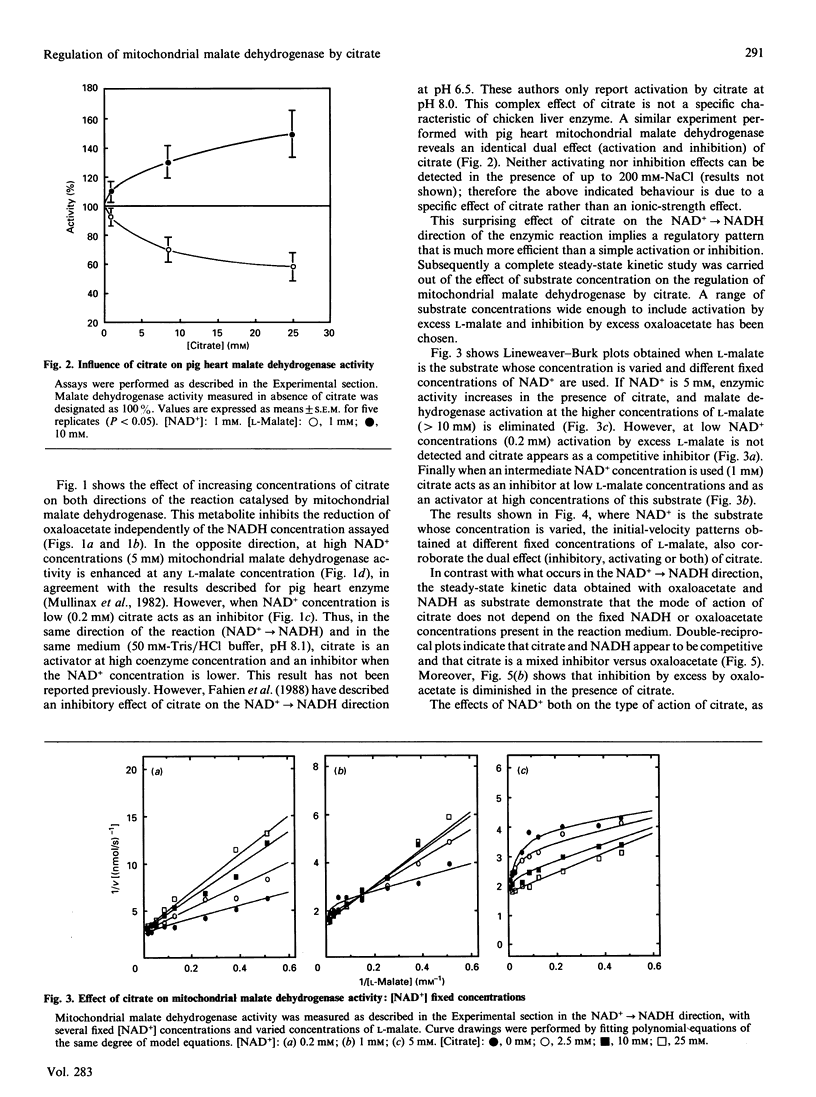
Mitochondrial malate dehydrogenase shows a complex regulation pattern in the presence of citrate. Previously published results indicate that this enzyme is activated by citrate in the NAD(+)----NADH direction and inhibited in the opposite direction. Moreover, high concentrations of L-malate or oxaloacetate produce deviations from the Michaelis-Menten behaviour. Results reported in this paper clearly show that citrate both activates and inhibits mitochondrial malate dehydrogenase in the same direction (NAD(+)----NADH), and in the same reaction medium, depending on substrate concentration. This surprising effect has made it necessary to propose a new kinetic mechanism that extends those previously suggested and allows us to explain both the citrate effect (activating or inhibitory) and the effect of high concentrations of L-malate and oxaloacetate.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein L. H., Grisham M. B., Cole K. D., Everse J. Substrate inhibition of the mitochondrial and cytoplasmic malate dehydrogenases. J Biol Chem. 1978 Dec 25;253(24):8697–8701. [PubMed] [Google Scholar]
- Cennamo C., Montecuccoli G., König G. Inhibition of mitochondrial malate dehydrogenase by citrate. Biochim Biophys Acta. 1967 Jul 11;139(2):514–516. doi: 10.1016/0005-2744(67)90057-5. [DOI] [PubMed] [Google Scholar]
- Cha S. A simple method for derivation of rate equations for enzyme-catalyzed reactions under the rapid equilibrium assumption or combined assumptions of equilibrium and steady state. J Biol Chem. 1968 Feb 25;243(4):820–825. [PubMed] [Google Scholar]
- Cha S. A simple method for derivation of rate equations for enzyme-catalyzed reactions under the rapid equilibrium assumption or combined assumptions of equilibrium and steady state. J Biol Chem. 1968 Feb 25;243(4):820–825. [PubMed] [Google Scholar]
- Dordal A., Mazo A., Gelpí J. L., Cortés A. Factors affecting L-malate activation of mitochondrial malate dehydrogenase from chicken liver. Biochem Int. 1990;20(1):177–182. [PubMed] [Google Scholar]
- Fahien L. A., Kmiotek E. H., MacDonald M. J., Fibich B., Mandic M. Regulation of malate dehydrogenase activity by glutamate, citrate, alpha-ketoglutarate, and multienzyme interaction. J Biol Chem. 1988 Aug 5;263(22):10687–10697. [PubMed] [Google Scholar]
- Fahien L. A., Kmiotek E. Complexes between mitochondrial enzymes and either citrate synthase or glutamate dehydrogenase. Arch Biochem Biophys. 1983 Feb 1;220(2):386–397. doi: 10.1016/0003-9861(83)90428-9. [DOI] [PubMed] [Google Scholar]
- Foe L. G., Kemp R. G. Properties of phospho and dephospho forms of muscle phosphofructokinase. J Biol Chem. 1982 Jun 10;257(11):6368–6372. [PubMed] [Google Scholar]
- Gabriel J. L., Plaut G. W. Citrate activation of NAD-specific isocitrate dehydrogenase from bovine heart. J Biol Chem. 1984 Feb 10;259(3):1622–1628. [PubMed] [Google Scholar]
- Gregolin C., Ryder E., Warner R. C., Kleinschmidt A. K., Chang H. C., Lane M. D. Liver acetyl coenzyme A carboxylase. II. Further molecular characterization. J Biol Chem. 1968 Aug 25;243(16):4236–4245. [PubMed] [Google Scholar]
- Harada K., Wolfe R. G. Malic dehydrogenase. VII. The catalytic mechanism and possible role of identical protein subunits. J Biol Chem. 1968 Aug 10;243(15):4131–4137. [PubMed] [Google Scholar]
- Lopez-Cabrera A., Cabré F., Franco R., Canela E. I. Identification and rejection of outliers in enzyme kinetics. Int J Biomed Comput. 1988 Oct;23(1-2):9–20. doi: 10.1016/0020-7101(88)90059-1. [DOI] [PubMed] [Google Scholar]
- McEvily A. J., Mullinax T. R., Dulin D. R., Harrison J. H. Regulation of mitochondrial malate dehydrogenase: kinetic modulation independent of subunit interaction. Arch Biochem Biophys. 1985 Apr;238(1):229–236. doi: 10.1016/0003-9861(85)90160-2. [DOI] [PubMed] [Google Scholar]
- Miyanaga O., Evans C., Cottam G. L. Regulation of L-pyruvate kinase activity by insulin and glycolytic intermediates. Biochem Biophys Res Commun. 1984 Mar 15;119(2):671–676. doi: 10.1016/s0006-291x(84)80302-2. [DOI] [PubMed] [Google Scholar]
- Mullinax T. R., Mock J. N., McEvily A. J., Harrison J. H. Regulation of mitochondrial malate dehydrogenase. Evidence for an allosteric citrate-binding site. J Biol Chem. 1982 Nov 25;257(22):13233–13239. [PubMed] [Google Scholar]
- Müller J. Binary and ternary complexes of malate dehydrogenase with substrates and substrate analogs. Biochim Biophys Acta. 1985 Jul 18;830(1):95–100. doi: 10.1016/0167-4838(85)90136-0. [DOI] [PubMed] [Google Scholar]
- Narabayashi H., Lawson J. W., Uyeda K. Regulation of phosphofructokinase in perfused rat heart. Requirement for fructose 2,6-bisphosphate and a covalent modification. J Biol Chem. 1985 Aug 15;260(17):9750–9758. [PubMed] [Google Scholar]
- Pettersson G., Pettersson I. Statistical methods for determination of empirical rate equations for enzyme reactions. Acta Chem Scand. 1970;24(4):1275–1286. doi: 10.3891/acta.chem.scand.24-1275. [DOI] [PubMed] [Google Scholar]
- Roderick S. L., Banaszak L. J. The three-dimensional structure of porcine heart mitochondrial malate dehydrogenase at 3.0-A resolution. J Biol Chem. 1986 Jul 15;261(20):9461–9464. [PubMed] [Google Scholar]
- Siess E. A., Brocks D. G., Lattke H. K., Wieland O. H. Effect of glucagon on metabolite compartmentation in isolated rat liver cells during gluconeogenesis from lactate. Biochem J. 1977 Aug 15;166(2):225–235. doi: 10.1042/bj1660225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telegdi M., Wolfe D. V., Wolfe R. G. Malate dehydrogenase. XII. Initial rate kinetic studies of substrate activation of porcine mitochondrial enzyme by malate. J Biol Chem. 1973 Sep 25;248(18):6484–6489. [PubMed] [Google Scholar]
- Tischler M. E., Friedrichs D., Coll K., Williamson J. R. Pyridine nucleotide distributions and enzyme mass action ratios in hepatocytes from fed and starved rats. Arch Biochem Biophys. 1977 Nov;184(1):222–236. doi: 10.1016/0003-9861(77)90346-0. [DOI] [PubMed] [Google Scholar]
- Watkins P. A., Tarlow D. M., Lane M. D. Mechanism for acute control of fatty acid synthesis by glucagon and 3':5'-cyclic AMP in the liver cell. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1497–1501. doi: 10.1073/pnas.74.4.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D. C., Jurgensen S. R., Geesin J. C., Harrison J. H. Subunit interactions in mitochondrial malate dehydrogenase. Kinetics and mechanism of reassociation. J Biol Chem. 1981 Mar 10;256(5):2377–2382. [PubMed] [Google Scholar]


