Abstract
Moxifloxacin has been suggested as an option for monotherapy of intra-abdominal infections. Recent data support the use of a once-daily metronidazole regimen. The purpose of this study was to investigate the activity of levofloxacin (750 mg every 24 h [q24h]) plus metronidazole (1,500 mg q24h) compared with that of moxifloxacin (400 mg q24h) monotherapy in a mixed-infection model. By using an in vitro pharmacodynamic model in duplicate, Escherichia coli and Bacteroides fragilis were exposed to peak concentrations of 8.5 mg of levofloxacin/liter q24h, 32 mg of metronidazole/liter q24h, and 2 mg for moxifloxacin/liter q24h for 24 h. The activities of levofloxacin, metronidazole, moxifloxacin, and levofloxacin plus metronidazole were evaluated against E. coli, B. fragilis, and E. coli plus B. fragilis. The targeted half-lives of levofloxacin, metronidazole, and moxifloxacin were 8, 8, and 12 h, respectively. Time-kill curves were analyzed for time to 3-log killing, slope, and regrowth. Pre- and postexposure MICs were determined. The preexposure levofloxacin, metronidazole, and moxifloxacin MICs for E. coli and B. fragilis were 0.5 and 1, >64 and 0.5, and 1 and 0.25 mg/liter, respectively. Levofloxacin and moxifloxacin achieved a 3-log killing against E. coli and B. fragilis in all experiments, as did metronidazole against B. fragilis. Metronidazole did not decrease the starting inoculum of E. coli. The area under the concentration-time curve/MIC ratios for E. coli and B. fragilis were 171.7 and 85.9, respectively, for levofloxacin and 26 and 103.9, respectively, for moxifloxacin. Levofloxacin plus metronidazole exhibited the fastest rates of killing. The levofloxacin and moxifloxacin MICs for B. fragilis increased 8- to 16-fold after the organism was exposed to moxifloxacin. No other changes in the postexposure MICs were found. Levofloxacin plus metronidazole administered once daily exhibited activity similar to that of moxifloxacin against the mixed E. coli and B. fragilis infection. A once-daily regimen of levofloxacin plus metronidazole looks promising for the treatment of intra-abdominal infections.
Intra-abdominal infections include peritonitis, appendicitis, and abscesses, among a wide variety of other infections. These infections are commonly caused by a mixture of aerobic and anaerobic bacteria, which creates an especially problematic situation. Not only do anaerobes act synergistically with facultative bacteria, but facultative organisms also facilitate the infectious process by promoting the growth of anaerobes (4).
Escherichia coli and Bacteroides fragilis are the organisms most commonly isolated from intra-abdominal abscesses (12). B. fragilis is known to be the most important anaerobic pathogen but accounts for only 0.5% of the normal colonic flora (4). A capsular polysaccharide, adherence potential, piliation, and toxin production all contribute to the virulence of B. fragilis (4).
The polymicrobial nature of intra-abdominal infections requires the use of either dual therapy or broad-spectrum monotherapy. Fluoroquinolones have typically been avoided as monotherapy for mixed infections because of their marginal activities against anaerobes. However, moxifloxacin has been suggested for monotherapy of intra-abdominal infections and polymicrobial surgical infections due to its broad spectrum of activity against anaerobes in vitro (1, 3). Conversely, metronidazole is commonly used in combination regimens because of its excellent coverage against anaerobic organisms. Recently, a combination regimen of levofloxacin plus metronidazole once daily has been suggested as a potential treatment for mixed infections (K. A. Sprandel, G. L. Drusano, D. W. Hecht, J. C. Rotschafer, L. H. Danziger, and K. A. Rodvold, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-15, 2003; K. A. Sprandel, C. A. Schriever, S. L. Pendland, J. P. Quinn, M. H. Gotfried, L. H. Danziger, and K. A. Rodvold, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-1154, 2003).
The purpose of this study was to compare the activity of combination therapy with levofloxacin plus metronidazole once daily versus that of moxifloxacin monotherapy in an in vitro mixed-infection model with E. coli and B. fragilis.
MATERIALS AND METHODS
Eleven concentration time-kill curve experiments with simulated regimens of moxifloxacin at 400 mg every 24 h (q24h), levofloxacin at 750 mg q24h, metronidazole at 1,500 mg q24h, or levofloxacin plus metronidazole against E. coli, B. fragilis, or a mixed-infection model with both organisms were performed. A growth control experiment was conducted in duplicate for E. coli alone, B. fragilis alone, and the mixed infection.
In vitro model.
The experiments were conducted by using a previously described in vitro model (14) placed in a Bactron IV anaerobic chamber (Sheldon Manufacturing, Cornelius, Oreg.). At the start of each experiment, an inoculum of the organism(s) was instilled into each chemostat, followed by a bolus injection of levofloxacin, moxifloxacin, metronidazole, or levofloxacin plus metronidazole to produce the desired initial antibiotic concentration. Each experiment, including those with the growth controls, was run in duplicate for 24 h. Antibiotic-free Anaerobe Broth, MIC (ABM; Becton Dickinson, Sparks, Md.), was pumped via a peristaltic pump into the chemostats at a predetermined rate. Simultaneously, an equal volume of drug-containing ABM was displaced from the chemostats into a waste reservoir. This simulated a monoexponential pharmacokinetic process that produces the desired half-lives of the antibiotics.
Bacteria.
Clinical isolates of E. coli (isolate 6857) and B. fragilis (isolate M97-117), kindly provided by Ortho-McNeil Pharmaceuticals (Raritan, N.J.) and Regions Hospital (St. Paul, Minn.), respectively, were studied. Prior to the concentration time-kill curve experiments, several colonies of each isolate were incubated anaerobically overnight in 50 ml of ABM. The overnight culture was then diluted 1:10 in fresh, warm ABM approximately 0.5 h prior to the experiment in order to allow the organisms to attain exponential growth. An appropriate amount of the culture, determined by comparison to a 0.5 McFarland equivalent turbidity standard, was added to each chemostat. The resultant starting bacterial inoculum was approximately 106 CFU/ml for all experiments. These procedures were followed for both organisms when the mixed-infection model experiments were conducted.
Susceptibility testing.
Testing for antibiotic susceptibilities was performed in duplicate or triplicate for each isolate and the control isolates (E. coli ATCC 25922 and B. fragilis ATCC 25285) prior to the concentration time-kill experiments and for all isolates, if they were present, at 24 h postexposure. Susceptibility testing was performed by the broth microdilution method in cation-adjusted Mueller-Hinton broth for E. coli and ABM for B. fragilis with an inoculum between 105 and 106 CFU/ml. The 96-well trays inoculated with E. coli were incubated for 16 to 20 h at 36°C in ambient air. The 96-well trays inoculated with B. fragilis were incubated for 46 to 48 h at 36°C under anaerobic conditions. MICs are reported as the concentration in the first clear well (no growth). Colonies present at 24 h were frozen at −80°C in sterile defibrinated sheep blood until they were needed and were then subcultured onto fresh agar plates for at least two consecutive days prior to susceptibility testing.
Antibiotics.
Stock solutions of levofloxacin (Ortho-McNeil Pharmaceuticals), moxifloxacin (Bayer, West Haven, Conn.), and metronidazole (Sigma-Aldrich, St. Louis, Mo.) were prepared according to the instructions of the manufacturer and were kept at −80°C until they were needed for individual experiments. Antibiotics were administered as bolus injections once in a 24-h period to simulate once-daily dosing.
Pharmacokinetics.
The respective half-lives simulated for levofloxacin, moxifloxacin, and metronidazole in each of the experiments were 8, 12, and 8 h. Targeted free peak drug concentrations (Cmax) for levofloxacin, moxifloxacin, and metronidazole were 8.5, 2.0, and 32 mg/liter, respectively. The Cmaxs, as well as the half-lives, were chosen because they represent clinically relevant free concentrations and half-lives in healthy volunteers after administration of the simulated intravenous doses shown in Table 1.
TABLE 1.
Values of the pharmacokinetic parameters for the study drugs
| Antimicrobial | Dose equivalent (mg)a | Cmax total (mg/liter) | % Protein binding | Cmax freeb (mg/liter) | Half-life (h) |
|---|---|---|---|---|---|
| Levofloxacinc | 750 | 12.1 | 30 | 8.5 | 8 |
| Moxifloxacin | 400 | 4.1 | 50 | 2.0 | 12 |
| Metronidazolec | 1,500 | 40 | 20 | 32 | 8 |
The doses were administered intravenously q24h.
Each antibiotic was dosed in the model to achieve peaks equal to those reported in humans following administration of the doses listed in the dose equivalent column with protein binding taken into account.
A combination regimen of levofloxacin plus metronidazole was also simulated.
HPLC.
The actual concentrations of levofloxacin, moxifloxacin, and metronidazole in batched samples stored in ABM (frozen at −80°C) were determined by a previously described (7, 13) high-performance liquid chromatography (HPLC; Scientific Research Consortium, Inc., St. Paul, Minn.) methodology, with slight modifications. The HPLC assay for metronidazole was linear over a range of 0.2 to 25 mg/liter (r2 ≥ 0.9998). The interday coefficient of variance was 8.9%. The HPLC assay for the fluoroquinolones was linear over a range of 0.1 to 20 mg/liter (r2 ≥ 0.9994). The interday and intraday coefficients of variance were 1.9 and 1.2%, respectively.
Pharmacodynamics.
At nine predetermined time points (0, 1, 2, 3, 4, 5.5, 7, 12, and 24 h), 1-ml samples were removed from the models for quantification of the bacterial density by serial saline dilution techniques. Bacterial counts were determined by 1:10 serial dilution of a 100-μl sample into saline that was plated onto Trypticase soy agar plus 5% sheep blood (Becton Dickinson, Cockeysville, Md.) for E. coli or CDC anaerobic blood agar (Becton Dickinson) for B. fragilis. Selective medium plates were used in the mixed infection experiments. MacConkey II agar (Becton Dickinson) was used for the selection of E. coli, while laked blood agar with kanamycin and vancomycin (LKV; Remel, Lenexa, Kans.) was used for the selection of B. fragilis colonies. Antibiotic carryover was addressed by using saline dilution techniques.
After aerobic incubation for 18 to 24 h at 37°C, the numbers of E. coli CFU on each plate were counted visually. The numbers of B. fragilis CFU were counted visually after anaerobic incubation for 48 h at 37°C. The theoretical lower limit of bacterial counting accuracy (LLA) was 300 CFU/ml. Time-kill curves were constructed by plotting the log10 CFU per milliliter versus time. The slopes of the time-kill curves were compared for a rate-of-killing analysis by using a linear model with GraphPad Prism software (version 4.0; GraphPad Software, Inc., San Diego, Calif.).
RESULTS
Susceptibility testing.
The preexposure MICs of levofloxacin, moxifloxacin, and metronidazole for the E. coli isolate were 0.5, 1, and > 64 mg/liter, respectively. Previous metronidazole susceptibility testing of B. fragilis in our laboratory, done with inocula of 105 and 107 CFU/ml, failed to demonstrate an inoculum effect (unpublished data). Likewise, a recently published study (5) reported that fluoroquinolones have no inoculum effect against B. fragilis. The preexposure MICs of levofloxacin, moxifloxacin, and metronidazole for the B. fragilis isolate were 1, 0.25, and 0.5 mg/liter, respectively. No changes in postexposure MICs were noted, with the exception of the MICs of moxifloxacin for B. fragilis in one of the models during the experiment. In one of the two models during this particular experiment, moxifloxacin exposure produced 8- to 16-fold increases in the moxifloxacin and levofloxacin MICs.
Pharmacokinetics.
Comparison of the drug concentrations attained in the model (determined by HPLC) to the expected concentrations allowed verification of the values for the simulated pharmacokinetic parameters (half-life and Cmax). For the experiments with metronidazole alone against E. coli, including the mixed-infection model experiments, the gas production by the bacteria caused sporadic medium displacement, which accelerated the monoexponential elimination of the drug. Thus, the actual metronidazole concentrations in these experiments ranged from 33.6 to 51.9% of the expected concentrations, with a mean of 42.4% and a standard deviation of 10%. The half-lives, calculated by using the actual concentrations, of metronidazole alone against E. coli and against the mixed infection were 3 h. The actual concentrations of metronidazole in the remaining experiments with metronidazole against B. fragilis alone and in all experiments with the combination regimen of metronidazole plus levofloxacin ranged from 64.3 to 90.8% of the expected concentrations, with a mean of 83.5% and a standard deviation of 10%. The half-lives in these experiments, calculated by using the actual concentrations, ranged from 7.9 to 9.9 h.
The actual moxifloxacin concentrations ranged from 96.9 to 101.1% of the expected concentrations, with a mean of 98.9% and a standard deviation of 1.5%. The half-lives of moxifloxacin, calculated by using the actual concentrations, ranged from 10.2 to 10.3 h. The actual levofloxacin concentrations ranged from 99.7 to 105.3%, with a mean of 101.8% and a standard deviation of 1.6%. The half-lives of levofloxacin, calculated by using the actual concentrations, ranged from 7.5 to 8.7 h.
Time-kill curves.
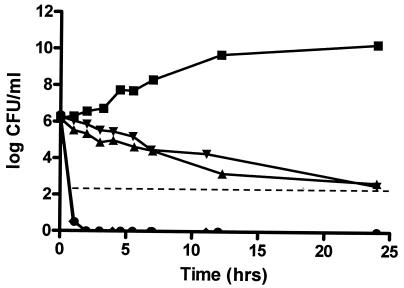
The time-kill curve for metronidazole, with an MIC of >64 mg/liter, against E. coli nicely followed the curve for the growth control. Levofloxacin, moxifloxacin, and levofloxacin plus metronidazole all produced a 3-log killing of E. coli, with a more rapid rate of killing by levofloxacin and levofloxacin plus metronidazole. Of note, significant regrowth, defined as an increase in the number of bacteria to quantifiable levels following a 3-log killing, occurred with moxifloxacin by the time that the sample at 5.5 h was obtained (Fig. 1). The area under the concentration-time curve (AUC)/MIC ratio for moxifloxacin was 26, while the AUC/MIC ratio for levofloxacin was 172. Thus, with the simulated clinical doses, the AUC/MIC ratio for moxifloxacin was below the typically recommended value of 100 to 125 for gram-negative bacteria, while that for levofloxacin was above this value.
FIG. 1.
Combined time-kill curve against E. coli. Growth control (squares), moxifloxacin (triangles), levofloxacin (upside-down triangles), metronidazole (diamonds), levofloxacin plus metronidazole (circles), and LLA (dashed horizontal line).
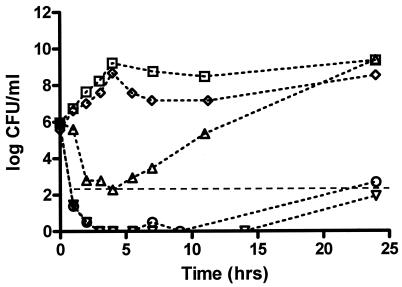
The time-kill curves for levofloxacin and moxifloxacin against B. fragilis alone were fairly similar, and both produced a slow 3-log killing. The AUC/MIC ratios for moxifloxacin and levofloxacin were 104 and 86, respectively, which are both above the recommended value of approximately 45 for anaerobes (10). Metronidazole and the combination of levofloxacin plus metronidazole produced very rapid 3-log killing (Fig. 2).
FIG. 2.
Combined time-kill curve against B. fragilis. Growth control (squares), moxifloxacin (triangles), levofloxacin (upside-down triangles), metronidazole (diamonds), levofloxacin plus metronidazole (circles), and LLA (dashed line).
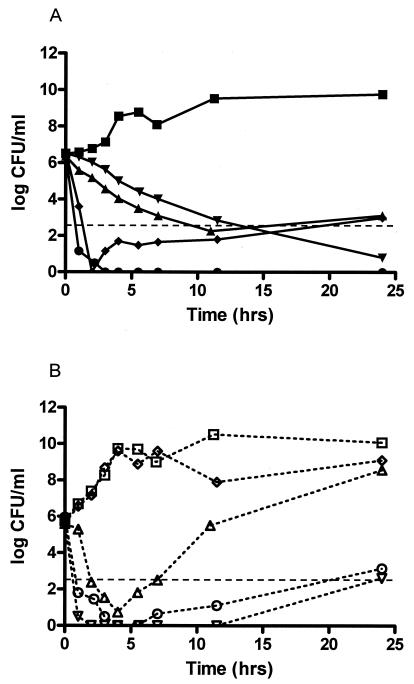
The time-kill curve for moxifloxacin against B. fragilis in the mixed-infection model looked much like the curve for moxifloxacin against B. fragilis alone. The killing, or the decrease in the numbers of CFU per milliliter, of E. coli by moxifloxacin in the mixed-infection model was 1.3 times greater than of E. coli alone, but significant regrowth still occurred. On the other hand, the time-kill curve for levofloxacin against E. coli in the mixed-infection model looked much like the curve against E. coli alone, while the killing of B. fragilis in the mixed-infection model was 1.5 times greater than that of B. fragilis alone. While these differences are interesting, they may not necessarily be significant on a log scale. Similar to the experiment with metronidazole against E. coli alone, the effect of metronidazole against E. coli in the mixed-infection model mimicked that against the growth control. Interestingly, unlike the experiment with B. fragilis alone, regrowth occurred with metronidazole against B. fragilis in the mixed-infection model. The level of regrowth was just above our LLA at 24 h. Finally, no changes occurred with the combination regimen of levofloxacin plus metronidazole. In other words, the time-kill curves for levofloxacin plus metronidazole against the mixed-infection model were almost identical to the curves for the combination regimen against each organism alone (Fig. 3).
FIG. 3.
Time-kill curves against mixed infections with of B. fragilis (A) and E. coli (B). (A) Results for the B. fragilis growth control (squares) and B. fragilis treated with moxifloxacin (triangles), levofloxacin (upside-down triangles), metronidazole (diamonds), and levofloxacin plus metronidazole (circles). Dashed line, LLA. (B) Results for the E. coli growth control (open squares) and E. coli treated with moxifloxacin (triangles), levofloxacin (upside-down triangles), metronidazole (diamonds), and levofloxacin plus metronidazole (circles). Dashed horizontal line, LLA.
Rate of killing.
Points were used for slope determination by linear regression either until they were below our LLA or until regrowth occurred, except as noted in Table 2. For the values noted in Table 2, the rate of killing was so rapid that the log CFU per milliliter was below our LLA by 1 h. Thus, the slope was calculated by using the data for the 0- and 1-h time points. All coefficients of determination in the linear regression analysis were greater than 0.93, with the exception of that for moxifloxacin against E. coli alone (r2 = 0.85), which had more of a nonlinear time-kill curve. The rate of killing by levofloxacin was slightly better in the mixed-infection model, while the rate of killing by metronidazole was slightly worse in the mixed-infection model. However, the rates of killing by the combination regimen of levofloxacin plus metronidazole against the organisms in the mixed-infection model and against each organism alone were almost identical.
TABLE 2.
Comparison of rates of killing
| Antibiotic | Rate of killing (CFU · h/ml)
|
||
|---|---|---|---|
| E. coli | B. fragilis | E. coli + B. fragilis | |
| Levofloxacin | −4.355a | −0.153 | −5.179a/−0.339 |
| Metronidazole | NAb | −5.669a | NA/−3.215 |
| Moxifloxacin | −1.075 | −0.403 | −1.61/−0.392 |
| Levofloxacin + metronidazole | −4.488a | −5.323a | −4.158a/−5.283a |
Below the limit of detection by 1 h, but the slope includes the data for the 0- and 1-h time points.
NA, not applicable.
DISCUSSION
This study demonstrates the comparable activities of levofloxacin plus metronidazole given once daily and moxifloxacin monotherapy in an in vitro pharmacodynamic model. Interestingly, the time-kill curve for the combination regimen against E. coli alone looked much like the curve for levofloxacin against E. coli, whereas the time-kill curve for the combination regimen against B. fragilis alone looked much like the curve for metronidazole against B. fragilis (Fig. 1 to 3). This suggests that levofloxacin accounts for the killing of E. coli, while metronidazole is responsible for the killing of B. fragilis. Although several studies have suggested that metronidazole possesses activity against E. coli in either the presence or the absence of B. fragilis, (6, 8), the results of this study do not support these findings and are similar to the findings of Pendland et al. (9).
The E. coli isolate used in this study, for which the fluoroquinolone MICs are higher than those typically reported for E. coli, was chosen for two reasons. First, the slightly higher MICs allow the visualization of any differences in the time-kill profiles between the fluoroquinolones. In other words, if a highly susceptible isolate had been used, the values on the time-kill curves would almost immediately have decreased below our LLA for both fluoroquinolones, preventing any differentiation. Second, the use of a less susceptible isolate represents a worst-case scenario and provides a conservative estimate of fluoroquinolone activity against the E. coli isolate that might typically be seen in intra-abdominal infections.
Moxifloxacin has been suggested as monotherapy for mixed infections (1, 3), although Snydman et al. (11) emphasize the importance of correct species identification of B. fragilis group species due to significant differences in in vitro activities. Additionally, the interpretation of in vitro susceptibility test results for moxifloxacin against B. fragilis cannot be performed because NCCLS has not yet adopted breakpoints for this antibiotic-bacterium combination. A study conducted by Brook (2) reports that the in vivo activities of fluoroquinolones against B. fragilis depend on the in vitro susceptibility testing results. However, in this study the activity of levofloxacin against B. fragilis in the in vitro pharmacodynamic model was almost identical to that of moxifloxacin, even though the moxifloxacin MIC was fourfold lower than that of levofloxacin for B. fragilis. Thus, the correlation between activity and in vitro susceptibility testing results reported by Brook (2) does not seem to apply to in vitro pharmacodynamic modeling.
The standard dose of metronidazole (500 to 1,000 mg every 6 to 8 h) was determined well before the science of pharmacodynamics surfaced. For metronidazole, the combination of the concentration-dependent bactericidal activity, significant postantibiotic effect, long half-life, and favorable safety profile enable manipulation of dosing regimens. Thus, the use of more convenient regimens of larger doses given less frequently seems feasible. A recent study evaluated the bactericidal activity of intravenous levofloxacin plus various doses of intravenous metronidazole, including a regimen of 1,500 mg q24h, against clinical isolates of B. fragilis, Bacteroides thetaiotaomicron, Porphyromonas asaccharolytica, and E. coli in healthy subjects (Sprandel et al., 43rd ICAAC, abstr. A-1154). The investigators found that the combination of levofloxacin plus the same total daily dose of metronidazole (500 mg every 8 h or 1,500 mg q24h) resulted in comparable bactericidal activities against all isolates tested. The results of our study further support the potential for a once-daily combination regimen of levofloxacin plus metronidazole for the treatment of intra-abdominal infections.
Acknowledgments
This study was supported by a grant from Ortho-McNeil Pharmaceuticals.
REFERENCES
- 1.Behra-Miellet, J., L. Dubreuil, and E. Jumas-Bilak. 2002. Antianaerobic activity of moxifloxacin compared with that of ofloxacin, ciprofloxacin, clindamycin, metronidazole and beta-lactams. Int. J. Antimicrob. Agents 20:366-374. [DOI] [PubMed] [Google Scholar]
- 2.Brook, I. 1993. In vivo efficacies of quinolones and clindamycin for treatment of infections with Bacteroides fragilis and/or Escherichia coli in mice: correlation with in vitro susceptibilities. Antimicrob. Agents Chemother. 37:997-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edmiston, C. E., C. J. Krepel, G. R. Seabrook, L. R. Somberg, A. Nakeeb, R. A. Cambria, and J. B. Towne. 2004. In vitro activities of moxifloxacin against 900 aerobic and anaerobic surgical isolates from patients with intra-abdominal and diabetic foot infections. Antimicrob. Agents Chemother. 48:1012-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein, E. J. 2002. Intra-abdominal anaerobic infections: bacteriology and therapeutic potential of newer antimicrobial carbapenem, fluoroquinolone, and desfluoroquinolone therapeutic agents. Clin. Infect. Dis. 35:S106-S111. [DOI] [PubMed] [Google Scholar]
- 5.Harnett, S. J., A. P. Fraise, J. M. Andrews, G. Jevons, N. P. Brenwald, and R. Wise. 2004. Comparative study of the in vitro activity of a new fluoroquinolone, ABT-492. J. Antimicrob. Chemother. 53:783-792. [DOI] [PubMed] [Google Scholar]
- 6.Ingham, H. R., C. J. Hall, P. R. Sisson, D. Tharagonnet, and J. B. Selkon. 1980. The activity of metronidazole against facultatively anaerobic bacteria. J. Antimicrob. Chemother. 6:343-347. [DOI] [PubMed] [Google Scholar]
- 7.Jessa, M. J., D. A. Barrett, P. N. Shaw, and R. C. Spiller. 1996. Rapid and selective high-performance liquid chromatographic method for the determination of metronidazole and its active metabolite in human plasma, saliva and gastric juice. J. Chromatogr. B Biomed. Appl. 677:374-379. [DOI] [PubMed] [Google Scholar]
- 8.Onderdonk, A. B., T. J. Louie, F. P. Tally, and J. G. Bartlett. 1979. Activity of metronidazole against Escherichia coli in experimental intra-abdominal sepsis. J. Antimicrob. Chemother. 5:201-210. [DOI] [PubMed] [Google Scholar]
- 9.Pendland, S. L., R. Jung, C. R. Messick, C. A. Schriever, and J. Patka. 2002. In vitro bactericidal activity of piperacillin, gentamicin, and metronidazole in a mixed model containing Escherichia coli, Enterococcus faecalis, and Bacteroides fragilis. Diagn. Microbiol. Infect. Dis. 43:149-156. [DOI] [PubMed] [Google Scholar]
- 10.Peterson, M. L., L. B. Hovde, D. H. Wright, G. H. Brown, A. D. Hoang, and J. C. Rotschafer. 2002. Pharmacodynamics of trovafloxacin and levofloxacin against Bacteroides fragilis in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 46:203-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snydman, D. R., N. V. Jacobus, L. A. McDermott, R. Ruthazer, E. Goldstein, S. Finegold, L. Harrell, D. W. Hecht, S. Jenkins, C. Pierson, R. Venezia, J. Rihs, and S. L. Gorbach. 2002. In vitro activities of newer quinolones against Bacteroides group organisms. Antimicrob. Agents Chemother. 46:3276-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stearne, L. E., C. Kooi, W. H. Goessens, I. A. Bakker-Woudenberg, and I. C. Gyssens. 2001. In vitro activity of trovafloxacin against Bacteroides fragilis in mixed culture with either Escherichia coli or a vancomycin- resistant strain of Enterococcus faecium determined by an anaerobic time-kill technique. Antimicrob. Agents Chemother. 45:243-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright, D. H., V. K. Herman, F. N. Konstantinides, and J. C. Rotschafer. 1998. Determination of quinolone antibiotics in growth media by reversed-phase high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 709:97-104. [DOI] [PubMed] [Google Scholar]
- 14.Zabinski, R. A., K. Vance-Bryan, A. J. Krinke, K. J. Walker, J. A. Moody, and J. C. Rotschafer. 1993. Evaluation of activity of temafloxacin against Bacteroides fragilis by an in vitro pharmacodynamic system. Antimicrob. Agents Chemother. 37:2454-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]