Abstract
Background
Side effects, including nausea and vomiting, sore mouth , diarrhoea, hepatotoxicity, myelosuppression, and immunosuppression , are commonly encountered in patients with colorectal cancer who are treated with chemotherapy. A variety of Chinese herbal medicines have been used for managing these adverse effects.
Objectives
To assess the effect of herbal medicines plus chemotherapy, compared with chemotherapy alone, on the side effects of chemotherapy on the quality of life, and on adverse events in patients with colorectal cancer.
Search methods
We searched the Cochrane Library, MEDLINE, EMBASE, CBM, and handsearched the relevant Chinese journals.
Selection criteria
Randomised trials comparing either chemotherapy only or chemotherapy plus anti‐emetics (tropisetron, sulpiride etc) with chemotherapy plus Chinese herbs.
Data collection and analysis
Trial quality was assessed independently by two reviewers. Data were extracted by one reviewer and checked by the second reviewer. Since the four included studies differed significantly in design, we could only perform limited meta‐analyses. We have therefore presented the majority of the data in narrative form.
Main results
We included four relevant trials. All of them were of low quality. All of studies used a decoction containing Huangqi compounds as the intervention with chemotherapy. The intervention groups of three studies were compared to a chemotherapy alone control group, the fourth study compared the decoction of Huangqi compounds with two other Chinese herbal interventions. None of the studies reported on primary outcome using Common Toxicity Criteria (CTC). There was a significant reduction in the proportion of patients who experienced nausea & vomiting when decoctions of Huangqi compounds were given in addition to chemotherapy. There was also a decrease in the rate of leucopenia (WBC <3 x 10^9 per L). Huangqi compounds were also associated with increases in the proportions of T‐lymphocyte subsets: CD3; CD4 and CD8. Huangqi decoctions had no significant effects on Immunoglobulins G, A or M.
Authors' conclusions
Despite the included studies being of low quality, the results suggest that decoctions of Huangqi compounds may stimulate immunocompetent cells and decrease side effects in patients treated with chemotherapy. Due to the methodological limitations of the studies, there is no robust demonstration of benefit. We found no evidence of harm arising from the use of Chinese herbs. We need high quality randomised controlled studies investigating the effects of decoctions of Chinese herbs, particularly Astragalus spp.(as in Huangqi), upon chemotherapy‐related side effects.
Keywords: Humans; Antineoplastic Agents; Antineoplastic Agents/adverse effects; Astragalus Plant; Astragalus propinquus; Colorectal Neoplasms; Colorectal Neoplasms/drug therapy; Drugs, Chinese Herbal; Drugs, Chinese Herbal/therapeutic use; Phytotherapy; Phytotherapy/methods; Randomized Controlled Trials as Topic
Plain language summary
Chinese medical herbs for chemotherapy side effects in colorectal cancer patients
Chinese herbal medicines are widely used to counteract the side‐effects of chemotherapy in patients being treated for cancer. As yet, there is no clear evidence that herbal medicines are effective in this role. We have performed a systematic review of the potential benefits of Chinese herbal medicines in patients being treated with chemotherapy for colorectal cancer. We identified four relevant studies, which included a total of 342 patients, with adequately reported data. We conclude that, from the limited information available, there is some evidence of benefit from decoctions of Huangqi compounds. Compared with patients treated by chemotherapy alone, patients treated with chemotherapy and Huangqi decoctions were less likely to experience nausea and vomiting or low white cell counts. There was some evidence to suggest that the decoctions also stimulated cells of the immune system, but did not affect the levels of antibodies in the blood. We could find no evidence of harm arising from the use of Huangqi decoctions. Our results suggest that further, larger‐scale, trials of the use of Huangqi decoctions in the prevention of chemotherapy‐related side‐effects are needed.
Background
(note: we have used the following abbreviations in this review: WBC ‐ white blood cell; CAM ‐ complementary and alternative medicine; TCM ‐ traditional Chinese medicines; CTC ‐ Common Toxicity Criteria; NK ‐ natural killer; NNT ‐ number needed to treat [ reciprocal of the absolute rate difference expressed as percentage]; WMD ‐ weighted mean difference; RD ‐ rate difference; MMC ‐ Mitomycin C; 5‐FU ‐ 5‐fluorouracil) Colorectal cancer is a predominantly disease of elderly people. In incidence, it ranks second behind carcinoma of the lung in men and the breast in women, and is a major cause of morbidity and mortality in United States and northern European countries (Beart 1992; Black 1997; Giovannucci 1999). Each year, there are 155,000 new cases of colorectal cancer in the United States, 50,000 of whom die from their disease (CDC 2003), and 33,000 new cases in the United Kingdom (NHS 2003). The rate is lowest in Asia and Africa (Giovannucci 1999). The incidence of colorectal cancer rises sharply with age, and is rarely diagnosed in those under age 40. Ninetythree percent of all cases are diagnosed in people over age 50, and 50% of patients are over 70 years old at the time of diagnosis (Giovannucci 1999; CDC 2003).
Environmental factors, particularly those related to lifestyle, including diet, obesity, physical inactivity, smoking and alcohol consumption are important in the aetiology of colorectal cancer (Shike 1999; Giovannucci 1999). Diet may contribute to the cause of up to 90% of colon cancers (Doll 1981). High fibre, fruit and vegetables, folate and methionine intake, are protective, whereas high animal fat, red meat, and alcohol consumption increase risk (Chen 1978; Fleiszer 1978;Giovannucci 1999). In Chinese and Japanese migrants living in the United States, the incidence of colorectal cancer approaches or exceeds the level of the American white population and is very much higher than amongst Chinese and Japanese living in China and Japan (Haenszel 1968; King 1980; Locke 1980).
TREATMENT
A high percentage of patients with colorectal cancer are curable if the disease is diagnosed and treated in early stages (Diaz‐Canton 1996; Winawer 1997; Winawer 2003). Surgery (colectomy) is the primary treatment for colorectal cancer (Labianca 1997). The effectiveness of surgery for colorectal cancer depends on it being carried out safely, and upon the rational selection of adjuvant therapy (CCCG 2000; Marvin 2002).
Adjuvant chemotherapy after surgery aims to destroy cancer cells, it usually started at the end of the first month after surgery (Labianca 1997). Systematic reviews have suggested that, in general, adjuvant chemotherapy is beneficial for patients with Dukes C cancer of the colon (SIGN 2003). Fluoropyrimidines are the cornerstones of adjuvant chemotherapy. A recent study shows that Capecitabine, an orally active prodrug that is converted to an active fluoropyrimidine, is effective as adjuvant therapy in colon cancer (XACT ref) . The problem with the adjuvant approach is that, although all patients are exposed to the toxicity of chemotherapy, only about 5 to 10% of treated patients obtain any benefit from it. Put bluntly, over 90% of treatment is either futile or unnecessary. Against this background, it is self‐evident that any intervention that successfully mitigates toxicity associated with chemotherapy for colorectal cancer will have a major impact upon the health and well‐being of patients with colorectal cancer.
Standard treatment for metastatic colorectal cancer is chemotherapy, again based on fluropyrimidines. Other cytotoxic drugs are also used: irinotecan, oxaliplatin. Biological agents, targeting the tumour vasculature (bevacuzimab) and the epidermal growth factors receptor (cetuximab) also have activity in colorectal cancer (Veronese 2004) .
Radiotherapy, often delivered synchronously with chemotherapy, is used in both the adjuvant and the definitive treatment of rectal cancer. It has little role to play in colon cancer.
Possible side effects of the chemotherapeutic regimens used in colorectal cancer include nausea and vomiting, stomatitis, palmar‐plantar syndrome, fatigue, diarrhoea (Martenson 1997), liver damage (Moertel 1993), suppression of the bone marrow, and immunosuppression. A variety of agents are used to alleviate these side effects. These include: antiemetics (domperidone, tropisetron, ondansetron, aprepitant); anti‐diarrhoeals (codeine phosphate, loperamide); vitamins (B6, pyridoxine). Levamisole was introduced into chemotherapy regimens for colorectal cancer primarily to boost any immune response to the tumour. It is now of only historical interest, recent trials show that it adds nothing to the effectiveness of regimens based on 5‐FU (Quasar 2000).
APPLICATIONS OF HERBAL MEDICINE IN THE TREATMENT OF NEOPLASTIC DISEASE
Herbal medicines are commonly used for alleviating the side‐effects of chemotherapy or radiotherapy and for improving the quality of life in patients with cancer. A population‐based questionnaire investigation found that 42% colorectal cancer patients used herbal remedies (Tough 2002). An investigation conducted in New Zealand found a similar result: 49% of cancer patients used complementary and alternative medicine (CAM), with vitamins, antioxidants, alternative diets, and herbal therapies the most commonly used agents. Younger patients were particularly likely to use CAM. CAM was used by 47% to improve quality of life and by 30% in the hope of a cure of their cancer (Chrystal 2002). In Shumay's study, all participants used 3 or more types of CAM, most commonly herbal or nutritional supplements (Shumay 2001). These findings suggest that many cancer patients use herbal medicines. The most commonly cited reasons for seeking complementary medicine were the adverse side effects from, and the lack of effectiveness of, standard therapies (Hilsden 1999).
Herbal medicines have benefits in their own right. These include: increasing the patients appetite; boosting the immune system; facilitating general recovery. Herbal medicines may have direct anti‐cancer effects (Ye 2002): induction of apoptosis rectal cancers (Xin 2001); prevention of metastasis (Ohnishi 1998); direct palliation of symptoms, such as bleeding, diarrhoea, and pain (Lin 1996; Jin 2001). There are also claims for improvements in median survival time and Karnovsky status (Ohnishi 1998; Zhou 1999; Liu 2000a; Li 2000). Herbal medicine therapy may be used before or after surgery, or combined with chemotherapy or radiotherapy. Herbal medicines have demonstrable biological effects: for example, ShenMai Injections significantly reduced TNF‐alpha mRNA (P < 0.01) and increased the lifespan (P < 0.05) of scalded mice (Wang 2002), they also promoted the recovery of haemoglobin, and increased natural killer (NK) cell activity and increased the CD4/CD8 ratio (Wang 1999; Liu 2000b). Composite Xiansu Capsule has immuno‐regulating effect on T‐cells (Hua 1999). Oren‐gedoku‐to (OGT) may be useful for the chemoprevention of colorectal cancer. Oren (Coptidis rhizoma) and Ogon (Scutellariae radix), OGT and Sanshishi inhibit azoxymethane (AOM)‐induced aberrant crypt foci (ACF) formation. This effect may be mediated by inhibition of cyclooxygenase‐2 (COX‐2) (Fukutake 2000).
The herbal medicine have their own benefits including increasing the patients appetite, boosting the immune system, facilitating the recovery of the body, and prevention of tumour regeneration or metastases. It is effective for anti‐cancer (Ye 2002), inducing cell apoptosis in rectal cancer patients (Xin 2001), preventing cancer metastasis (Ohnishi 1998), and alleviation of symptoms is possible, such as bleeding, diarrhea, and pain (Lin 1996, Jin 2001). Improve median survival time, Karnovsky scoring and immune function (Ohnishi 1998, Zhou 1999, Liu 2000a, Li 2000). Herbal medicine therapy may be used before or after surgery, or combine with the patient regimens of chemotherapy or radiotherapy. For example, ShenMai Injection reduced TNF‐alpha mRNA obviously (P < 0.01) and increased liveability of scald mice markedly (P < 0.05) (Wang 2002), promoted the recovery of hemoglobin, and elevated activity of the natural killer (NK) cell and T lymphocyte ( CD4, CD4/CD8 ratio) (Wang 1999, Liu 2000b), composite Xiansu Capsule has immuno‐regulating effect on T‐cells (Hua 1999). Oren‐gedoku‐to (OGT) be considered may be useful for colon cancer chemoprevention in terms of efficacy and toxicity. Oren (Coptidis rhizoma) and Ogon (Scutellariae radix), OGT and Sanshishi inhibit azoxymethane (AOM)‐induced aberrant crypt foci (ACF) formation. The mechanism is that both OGT and Sanshishi inhibited cyclooxygenase‐2 (COX‐2) (Fukutake 2000).
The rationale for using herbal medicines in the treatment of colorectal cancer in traditional Chinese medicine is based upon what are believed to be quite specific properties of the various medicinal herbs. For example, for diarrhoea and "ribbon" or pencil‐shaped stools, Semen Coicis (Yi Yi Ren) and Fruetus Sehisandrae (Wu Wei Zi) ave been employed, or in cases of rectal bleeding, Red sage root (dan shen) and Radix Paeoniae Rubra (Chi Shao) have been used, Pericarpium Citri Reticulatae (Chen Pi) and Radix Vladimiriae (Guang Mu Xiang), etc. can be used for abdominal fullness, cramping and tenesmus (Jin 2001).
RATIONALE FOR UNDERTAKING THIS REVIEW
The evidence for use of herbal medicine for chemotherapy side effects and improving quality of life is mainly theoretical and anecdotal. Cancer patients may use multiple therapies, not just conventional supportive treatments, but also alternative approaches, and often without the knowledge of their oncologist (Chrystal 2002). As summarised above, there is some existing evidence to suggest plausible biological mechanisms whereby herbal medicines might mitigate the side effects of chemotherapy and improve quality of life in patients with colorectal cancer. However the evidence of the clinical value of herbal medicines in patients undergoing chemotherapy for colorectal cancer is limited and conflicting. There is also evidence to indicate that not all CAM is free from harm. There are concerns regarding adverse events, e.g. allergic reactions and Chinese herbal nephropathy (CHN) (Nortier 2000; Lord 2001; Lampert 2002). Despite these concerns, herbal medicines are widely used. We need to know, therefore, whether the available clinical evidence supports the use of Chinese herbal remedies for patients being treated for colorectal cancer: are there benefits and, if so, do these outweigh the potential harms?
Objectives
To assess, using evidence from RCTS, the effect of herbal medicines upon chemotherapy‐related side effects, quality of life, and objective measures of immune function in patients with colorectal cancer.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials, including cross‐over trials and within patient studies.
Types of participants
Any person diagnosed with colorectal cancer and treated with cytotoxic chemotherapy. The chemotherapy was graded according to its emetogenic potential: mildly emetogenic ‐ oral fluoropyrimidines (capecitabine, tegafur/UFT); moderately emetogenic ‐ IV 5FU +/‐ folinic acid; severely emetogenic ‐ IV irinotecan, oxaliplatin, etc (alone or in combination).
Types of interventions
Chinese herbal remedies (such as Shen Mai, Huangqi) known to be active against chemotherapy‐related toxicity. The comparisons were with either no other treatment or with another active anti‐emetic agent.
Types of outcome measures
PRIMARY OUTCOME
CTC >Grade 2 Nausea: oral intake significantly decreased
SECONDARY OUTCOMES
1. The proportion of people with nausea and/or vomiting, documented as a dichotomous variable with a 'yes'/'no' response 2. White blood cell (WBC) count 3. Peripheral blood counts of immunocompetent cells: NK cells and T lymphocytes 4. Quality of life
ADVERSE EVENTS
Any adverse event, arising as a result of treatment with Chinese herbal remedies, that causes (1) death, (2) life‐threatening illness, (3) significant toxicity.
Search methods for identification of studies
1. ELECTRONIC SEARCH:
Searches were conducted to identify all published and unpublished randomised controlled trials (RCTs). The search strategy identified studies in all languages and, when necessary, non‐English‐language papers were translated so that they could be fully assessed for potential inclusion in the review.
Trials were identified by searching the Cochrane Library (Issue 4, 2003), MEDLINE (1966 to 2003), EMBASE (1980 ‐ 2003) and the Chinese Biomedical Base, CBM (1982 ‐ 2003). The following search strategy was constructed by using a combination of subject headings and text words relating to the use of Chinese herbs in the treatment of patients with colorectal cancer receiving chemotherapy.
a) Search Strategy for MEDLINE (OVID): A. Search strategy to locate RCTs search terms 1‐29, as given in the Cochrane Handbook (Clarke 2002), appendix 5c.2
B. Search strategy to locate colorectal cancer: #30 colorectal neoplasm* #31 colorect* neoplasm* #32 colorect* cancer #33 colorectal Canc* #34 colorect* canc* #35 colorect* carcinoma #36 colorectal carcinom* #37 colorect* carcinom* #38 rect* neoplasms #38 rectal neoplasm* #39 rect* neoplasm* #40 rectal cancer #41 rect* cancer #42 rectal canc* #43 rect* canc* #44 rect* carcinoma #45 rectal carcinom* #46 rect* carcinom*)
C. Search Strategy to locate quality of life: #47 quality of life #48 qualit* of life #49 quality adjusted life years #50 qualit* adjusted life years #51 quality adjusted life year* #52 qualit* adjusted life year* #53 health status #54 mental health #55 well‐being #56 quality adjusted survival #57 qualit* adjusted survival
D. Search strategy to locate herbal medicine: #58 herbal medicine #59 herb* medic* #60 medic* herb* #61 Chinese herbal medicine #62 Chinese herb* medic* #63 Chinese medic* herb* #64 herbal #65 herb* #66 complementary #67 alternative medicine #68 comp* #69 alter* medic*
The above search are restricted by combining with 'and' and 'near': (#30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46) and (#47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57) near (#58 or #59 or #60 or #61 or #62 or #63 or #64 or #65 or #66 or #67 or #68 or #69)
b) A similar strategy will be used for EMBASE. #1 colorectal #2 neoplasms #3 colorectal neoplasms #4 colorect* #5 neoplasms #6colorect* neoplasms #7 colorectal #8 neoplasm* #9 colorectal neoplasm* #10 colorect* #11 neoplasm* #12 colorect* neoplasm* #13 colorectal #14 cancer #15 colorectal cancer #16 colorect* #17 cancer #18 colorect* cancer #19 colorectal #20 canc* #21 colorectal canc* #22 colorect* #23 canc* #24 colorect* canc* #25 colorectal #26 tumor #27 colorectal tumour #28 colorect* #29 tumour #30 colorect* tumour #31 rectal #32 neoplasms #33 rectal neoplasms #34 rect* #35 neoplasms #36 rect* neoplasms #37 rectal #38 neoplasm* #39 rectal neoplasm* #40 rect* #41 neoplasm* #42 rect* neoplasm* #43 rectal #44 cancer #45 rectal cancer #46 rect* #47 cancer #48 rect* cancer #49 rectal #50 canc* #51 rectal canc* #52 rect* #53canc* #54 rect* canc* #55 rectal #56 tumor #57 rectal tumour #58 rect* #59 tumour #60 rect* tumour #61 quality #62 of #63 life #64 quality of life #65 qualit* #66 of #67 life #68 qualit* of life #69 quality #70 adjusted #71 life #72 years #73 quality adjusted life years #74 qualit* #75 adjusted #76 life #77 years #78 qualit* adjusted life years #79 quality #80 adjusted #81 life #82 year* #83 quality adjusted life year* #84 health #85 status #86 health status #87 mental #88 health #89 mental health #90 wellbeing #91 quality #92 adjusted #93 survival #94 quality adjusted survival (#3 or #6 or #9 or #12 or #15 or #18 or #21 or #24 or #27 or #30 or #33 or #36 or #39 or #42 or #45 or #48 or #51 or #54 or #57 or #60) and (#64 or #68 or #73 or #78 or #83 or #86 or #89 or #90 or #94)
The above EMBASE search is following restricted to study design as below (combine with 'AND'): #1 explode 'clinical‐trial' / all subheadings #2 explode 'controlled‐study' / all subheadings #3 'randomization‐' / all subheadings #4 'case‐control‐study' / all subheadings #5 #2 NOT #4 #6 random* or clin* #7 #1 OR #3 OR #5 OR #6
c) A search strategy in the Cochrane Library: #1 COLONIC‐NEOPLASMS*:ME #2 RECTAL‐NEOPLASMS*:ME #3 (#1 or #2) #4 ((((COLORECT* near CANCER) or NEOPLASM*) OR CARCINOM*) OR ADENOM*) #5 ((((COLO* near CANCER) or NEOPLASM*) OR CARCINOM*) OR ADENOM*) #6 ((((RECT* near CANCER) or NEOPLASM*) OR CARCINOM*) OR ADENOM*) #7 ((#4 or #5) or #6) #8 (#3 or #7) #9 QUALITY‐OF‐LIFE*:ME #10 (HEALTH and STATUS) #11 (MENTAL and HEALTH) #12 WELL‐BEING #13 ((QUALITY and ADJUSTED) and SURVIVAL) #14 (((QUALITY and ADJUSTED) and LIFE) and YEARS) #15 (((((#9 or #10) or #11) or #12) or #13) or #14) #16 (#8 and #15)
d) A search strategy in the Chinese Biomedical Base (CBM Base) #1 colorectal cancer #2 colon cancer #3 rectal cancer #4 bowel cancer #5 Chinese traditional medicine #6 Chinese herbal medicine #7 herb* medicine #8 medic* herbs #9 medic* herb* #10 traditional medicine #11 (#1 or #2 or #3 or #4) and (#5 or #6 or #7 or #8 or #9 or #10)
e) We also searched for ongoing trials in:
National Research Register
Meta‐register of Controlled Trials
Medical Research Council Clinical Trials Directory.
2. HANDSEARCHES: We hand‐searched the following Chinese Journals: Chinese Journal of Integrated Traditional and Western Medicine, Journal of Traditional Chinese Medicine, Modern Traditional Chinese Medicine, New Journal of Traditional Chinese Medicine, LiaoNing Journal of Traditional Chinese Medicine, Chinese Journal of Integrated Traditional and Western Surgery, etc.
3. REFERENCES FROM PUBLISHED STUDIES We checked references from published studies for further trials.
4. UNPUBLISHED LITERATURE We could not identify any unpublished or on‐going trials.
5. CONFERENCE PROCEEDINGS There were no colorectal cancer conference proceedings and/or poster abstracts dealing with this topic during the past five years.
6. ADVERSE EFFECTS We could identify no published work on the adverse effects of Chinese herbal remedies in patients being treated with chemotherapy for colorectal cancer.
Data collection and analysis
STUDY SELECTION
Wu Taixiang (WT) scanned the results of the search strategy for potentially relevant trials, and retrieved the full articles for all potentially relevant trials. We scrutinised each trial report for multiple publications from the same data set. WT and Liu Guanjian (LG) independently assessed each of these trials for inclusion in the review using an eligibility form based on the contents of the section 'Criteria for Inclusion'. There were no recorded disagreements. We excluded studies that did not meet the inclusion criteria and have stated the reasons in 'Characteristics of excluded studies'.
ASSESSMENT OF METHODOLOGICAL QUALITY
WT and LG assessed methodological quality of each trial in terms of generation of allocation sequence, allocation concealment, blinding, and loss to follow up. For each trial, we classed each quality component as 'adequate', 'inadequate', 'unclear' or 'not used' according to Juni (Juni 2001). After including all eligible studies in the primary analysis, we intended to conduct sensitivity analyses for each of the quality factors using the subgroups adequate, inadequate, or unclear
DATA EXTRACTION
WT and LG independently extracted data using a piloted data extraction form. We extracted data on study characteristics including methods, participants, interventions, and outcomes. We resolved any disagreements by referring to the trial report and through discussion. If data from the trial reports were insufficient or missing, we contacted the authors for additional information. If the author could not be contacted, we allocated the study to the category "Studies awaiting assessment".
Where possible, we extracted data to allow an intention‐to‐treat analysis (the analysis should include all the participants in the groups to which they were originally randomly assigned). If the number randomised and the numbers analysed were inconsistent, we calculated the percentage loss‐to‐follow‐up and reported this information separately. For binary outcomes, we recorded the number of participants experiencing the event in each group of the trial. For continuous outcomes, for each group we extracted the arithmetic means and standard deviations.
Extraction was undertaken by one reviewer and checked by a second. Data entry into RevMan was double‐checked.
DATA ANALYSIS
We analysed the data using Review Manager (Version 4.3). We were only able to perform limited pooled analyses. We used random effects assumptions throughout. We have summarised dichotomous data as absolute rate difference in percent. We have reported continuous data as weighted mean difference (WMD). We have used 95% confidence intervals throughout.
Non‐randomised controlled studies have been listed but not discussed further.
Results
Description of studies
Of 19 studies identified, 4 met the predefined criteria for inclusion. See table of 'Characteristics of included studies'.
We tried to contact the authors of studies by Ran 1999 for information about missing data and by Wu 1999 to identify whether or not it was a randomised controlled trial. However we were unsuccessful, and so we have indexed the two studies as 'Studies awaiting assessment'.
The traditional Chinese medicines used in all of the included trials were decoctions of Huangqi compounds. In the studies by Xiao 1998 and Guo 1999, interventions were chemotherapy plus decoctions of Huangqi compounds versus chemotherapy alone. The study by He 1995 compared three different herbal remedies in patients treated with chemotherapy: decoction of Huangqi compounds versus oral Shenbaibao versus oral Berbamine Hydrochloride. The study by Li 2000 was a three‐way comparison of Huangqi compounds plus chemotherapy versus chemotherapy alone versus Huangqi compounds alone.
Ten studies were excluded, see table of 'Characteristics of excluded studies'.
Risk of bias in included studies
All included studies were of poor methodological quality. There was a high risk of selection bias and detection bias in these studies. See 'Table: Characteristics of included studies', particularly the 'Methods' 'Notes', and 'Allocation concealment' sections.
In all four studies, allocation of participants was described as 'random'. One study (Guo 1999) used a random number table to allocate participants, but the published data were based on only 67% of randomised participants (25/38 and 21/31 respectively in the two groups) and a further five patients were subsequently lost to follow up (2/25 and 3/21 respectively). The other publications did not describe the randomisation procedure in detail. Concealment of allocation prior to enrolment was not mentioned in any study
None of the four included studies described blinding of participants and/or assessors.
There are thus multiple methodological shortcomings in these studies.
Only two studies (Guo 1999; Li 2000), including 94 participants, contributed data on T‐lymphocyte subset counts and immunoglobulin levels, two studies (Xiao 1998; Guo 1999), including 154 participants, provided data on the proportion of people with nausea and/or vomiting. One study (Li 2000), including 48 participants, contributed data on NK cell counts, and three studies (He 1995; Xiao 1998; Guo 1999) including 294 participants provided data on WBC counts
Effects of interventions
Since the data were heterogeneous and of poor quality, we decided to concentrate on a descriptive summary of results and perform only limited meta‐analyses. There were no reported data on our selected Primary Outcome: CTC nausea grade 2 or above.
T‐lymphocyte subsets
Compared to patients treated with chemotherapy alone, Guo 1999 reported an increase of 6.4% (95% c.i. 5.1% to 7.7%) in the proportion of CD3+ cells; of 5.8% (95% c.i. 4.6% to 7.0%) in the proportion of CD4+ cells; and of 2.3% (95% c.i. 0.5% to 4.1%) in CD8+ cells four months after treatment in patients treated with decoctions of Huangqi compounds in addition to their chemotherapy.
The smaller study by Li (Li 2000) showed no significant changes in lymphocyte subsets in patients randomised to Huangqi decoctions (Table 1).
1. The effects of Chinese medical herbs on the adverse effects of chemotherapy.
| Studies | CD3+ (%) | CD4+ (%) | CD8+ (%) | WBC | Side effects | NK cell (%) | IgG (g/L) | IgA (g/L) | IgM (g/L) |
| Xiao 1998 | Number of patients with WBC less than 3.0 x 10^9 per L: TCM + chemo group: 7/50 (14%) chemo alone group: 14/25 (56%) Rate difference 42% (95% c.i. 20 to 60%) | TCM + chemo group: reduced appetite 9/50 (18%), nausea with vomiting 11/50 (22%), hair loss 8/50 (16%). chemo alone group: reduced appetite 13/25 (52%), nausea with vomiting 14/25 (56%), hair loss 15/25 (60%) Rate differences: Reduced appetite 34% (95% c.i. 12% to 54%) Nausea and Vomiting 34% (95% c.i. 11% to 54%) Hair Loss 44% (95% c.i. 21% to 62%) | |||||||
| He 1995 | Huangqi compounds decoction group: 4400/ml (95% c.i. 4144 to 4655) Shenbaibao group: 4100/ml (95% c.i. 3841 to 4358) Berbamine Hydrochloride group: 3590/ml (95% c.i. 3320 to 3859) | ||||||||
| Guo 1999 | Huangqi compounds decoction + chemo group: 49.3 (95% c.i. 48.4 to 50.2) Chemo alone group: 42.9 (95% c.i. 41.9 to 43.9). Difference (decoction +chemo vs. chemo alone) 6.4% (95% c.i.: 5.1% to 7.7%) | Huangqi compounds decoction + chemo group: 44.6 (95% c.i. 43.6 to 45.6) Chemo alone group: 38.8 (95% c.i. 38.1 to 39.5) Difference (decoction +chemo vs. chemo alone) 5.8 (95% c.i. 4.6 to 7.0) | Huangqi compounds decoction + chemo group: 26.9 (95% c.i. 25.4 to 28.4) Chemo alone group: 24.6 (95% c.i. 23.7 to 25.5) Difference (decoction+chemo vs. chemo alone) 2.3 (0.5 to 4.1) | Number of patients with WBC less than 3.0 x 10^9 per L: Huangqi compounds decoction + chemo group: 7/38 (18%) Chemo alone group 15/31 (48%) Rate difference: 30% (95% c.i. 7% to 49%) | Huangqi compounds decoction + chemo group: nausea 16/38 (42%), vomiting10/38 (26%), fatigue 14/38 (37%) Chemo alone group: nausea 25/31 (81%), vomiting 18/31 (58%), fatigue 21/31 (68%) Rate differences: Nausea 39% (95% c.i. 16% to 56%) Vomiting 32% (95% c.i. 8% to 51%) Fatigue 31% (95% c.i. 7% to 50%) | Huangqi compounds decoction + chemo group: 6.32 (95% c.i. 5.1 to 7.5) Chemo alone group: 4.32 (95% c.i. 2.6 to 6.0) Difference 2.0 (95% c.i. 0 to 4.0) | Huangqi compounds decoction + chemo group: 0.67 (95 % c.i. 0 to 1.4) Chemo alone group: 0.51 (95% c.i. 0 to 2.6) | Huangqi compounds decoction + chemo group: 0.71 (95% c.i. 0 to 1.5) Chemo alone group: 0.38 (95% c.i. 0 to 1.4) | |
| Li 2000 | Huangqi compounds decoction alone group: 63.3 (95% c.i. 59.5 to 67.1); Huangqi plus chemotherapy group: 59.4 (95% c.i. 55.3 to 63.5); Chemotherapy alone group: 57.2 (95% c.i. 51.8 to 62.6); Difference (chemo+decoction vs, chemo alone) 2.2% (95%c.i. ‐4.2% to +8.6%) | Huangqi compounds decoction alone group: 39.3 (95% c.i. 35.7 to 42.9); Huangqi plus chemotherapy group: 38.8 (95% c.i. 34.2 to 43.4); Chemotherapy alone group: 37.8 (95% c.i. 34.0 to 41.6); Difference (decoction+chemo vs. chemo alone) 1.0 (95% c.i. ‐4.7 to +6.7) | Huangqi compounds decoction alone goup: 32.2 (95% c.i. 28.8 to 35.6); Huangqi plus chemotherapy group: 33.4 (95% ci.i 28.4 to 38.4); Chemotherapy alone group: 30.5 (95% c.i.26.6 to 34.4); Difference (decoction+chemo vs. chemo alone) 2.9 (95% c.i. ‐3.1 to +8.9) | Huangqi compounds decoction alone group: 36.4 (95% c.i. 33.6 to 39.2) Huangqi plus chemotherapy group: 35.5 (95% c.i. 32.6 to 38.4) Chemotherapy alone group: 34.7 (95% c.i. 31.8 to 37.6) | Huangqi compounds decoction alone group: 18.11 (95% c.i. 14.5 to 21.7) Huangqi plus chemotherapy group: 17.35 (95% c.i. 14.5 to 20.2) Chemotherapy group: 15.74 (95% c.i. 14.3 to 17.2) | Huangqi compounds decoction alone group: 2.35 (95% c.i. 2.1 to 2.6) Huangqi plus chemotherapy group: 2.59 (95% c.i. 1.9 to 3.3) Chemotherapy group: 2.08 (95% c.i. 1.7 to 2.5) | Huangqi compounds decoction alone group: 1.70 (95% c.i. 1.3 to 2.1) Huangqi plus chemotherapy group: 1.65 (95% c.i. 1.3 to 2.0) Chemotherapy group: 1.55 (95% c.i. 1.2 to 1.9) |
The pooled data show significant increases in CD3 and CD8 lymphocyte subsets in patients treated with traditional Chinese medicines plus chemotherapy compared to patients treated with chemotherapy alone (see table of comparisons): WMD CD3, 4.37 (95% c.i. 0.26 to 8.48); WMD CD8 2.35 (95% c.i. 0.71 to 3.99). There was a trend towards an increase in the proportion of CD4 cells, but this was not significant (WMD 3.45: 95% c.i. ‐1.25 to 8.15)
NK cells The study by Li described an increase above baseline in the proportion of NK cells in all three groups during the first four months of treatment (Li 2000). The increase was steepest in the group treated without chemotherapy, that is with Huangqi decoctions alone. By the third month after treatment there was no difference in the proportions of NK cells between three groups (Table 1).
Immunoglobulins
None of the studies showed any significant change in serum immunoglobulin levels in patients treated with TCM plus chemotherapy compared to patients treated with chemotherapy alone. White blood cell counts (WBC)
Three studies (He 1995; Xiao 1998; Guo 1999) provided data on the effect of TCM on white cell counts. He 1995 reported absolute counts, Xiao 1998 and Guo 1999 provide data on the proportion of patients with significant leucopenia (WBC < 3.0 x 10^9 per L). Berbamine plus chemotherapy was associated with significantly lower WBC than Huangqi decoctions plus chemotherapy (He 1995): Berbamine group 3.59 (95% c.i. 3.32 to 3.85) per L; Huangqi group 4.40 (95% c.i. 4.14 to 4.65) per L.
Both the Xiao 1998 and Guo 1999 studies showed that the proportion of patients with leucopenia was significantly lower in the groups treated with Huangqi compounds in addition to chemotherapy (see additional table). The pooled estimate of absolute rate reduction was 36% (95% c.i. 21% to 51%): NNT 3, 95% c.i. 2 to 5. (see table of comparisons).
Proportion of patients with nausea and/or vomiting
The study by Xiao 1998 reported significant reductions in the proportion of patients experiencing nausea & vomiting, reduced appetite and hair loss in patients treated by chemotherapy and Huangqi compounds decoction compared with those treated by chemotherapy alone (see table of comparisons). The absolute rate reductions and NNT for each side effect were: nausea and vomiting 34% (95% c.i. 11% to 54%), NNT 3 (95% c.i. 2 to 10); reduced appetite 34% (95% c.i. 12% to 54%), NNT 3 (95% c.i. 2 to 10); hair loss 44% (95% c.i. 21% to 62%), NNT 2 (95% c.i. 2 to 5). Guo 1999 et al similarly reported significantly less nausea and vomiting (see table of comparisons). They also showed that, with Huangqi decoctions, a smaller proportion of patients complained of fatigue: rate difference 31% (95% c.i. 7% to 50%), NNT 3 (95% c.i. 2 to 14).
The pooled data from the studies by Xiao 1998 and Guo 1999 confirm that TCM plus chemotherapy, compared with chemotherapy alone, significantly reduces the proportion of patients who experience nausea and vomiting: rate reduction 33% (95% c.i. 17% to 49%, NNT 3 (95% c.i. 2 to 6). (see table of comparisons)
Quality of Life We could find no reported data for this endpoint. Adverse effects of Huangqi compounds
We could find no reports of any adverse effects related to the use of Huangqi compounds.
Discussion
We identified nineteen prospective controlled trials relating to the question of whether or not Chinese herbal medicines could decrease the toxicity experienced by patients treated with chemotherapy for colorectal cancer. However, of these, only four randomised studies were suitable for inclusion. All four were of low quality. All of the studies used self prepared traditional Chinese herbs as the intervention. The active ingredient in these decoctions is Huangqi. This is prepared from the dried root of Astragalus membranaceus and Astragalus mongolicus ‐ both types of vetch. The main chemical components of the extracts are triterpene saponins and astragaloglucans. The four studies we were able to include ranged in size from 48 to 150 patients: the total number of patients was 342. It was disappointing to find that no study reported data on our primary outcome of interest: Grade 2 CTC toxicity.
We found evidence that extracts containing Huangqi have beneficial effects on white blood cells, both in terms of total counts and in specific subsets of immuno‐competent lymphocytes. There was also evidence that these compounds decreased the incidence of nausea and vomiting. There were no discernible effects on serum levels of immunoglobulins G, A or M.
Quality of life was not assessed in any of the included studies, nor were there any reports of adverse events related to the Chinese herbal medicines themselves.
LIMITATIONS OF THE REVIEW
This studies identified in this systematic review are few in number, small in size, and poor in quality. These factors seriously limit the robustness and applicability of any conclusions that can be drawn. The chemotherapy regimens used in the four studies are not typical of those currently used worldwide. The studies used interventions which, although primarily based on decoctions of Huangqi, differed from each other. Against this background, of herbal polypharmacy, it is difficult to decide which agents might be active, and which not. We could find no useful data on overall treatment outcome: the possibility that these interventions might decrease both the toxicity and the effectiveness of chemotherapy, though unlikely, has not been excluded. Nevertheless, we have found some evidence of biological effect for Chinese herbal medicines. The evidence is sufficient to warrant further studies of the question ‐ provided that such studies are properly designed and conducted.
Authors' conclusions
Implications for practice.
Despite most of the included studies being of low quality, the results suggest that traditional Chinese herbal medicines stimulated the production of white cells, decreased the proportion of patients who experienced significant nausea and vomiting, and increased the proportion of immuno‐competent lymphocytes (CD3 and CD8). The methodological shortcomings of the included studies mean that we have only weak evidence for these being genuine effects. The evidence is certainly inadequate to suggest that clinical practice should be changed on the basis of these results.
Implications for research.
Our results suggest that these complex compounds may have beneficial clinical and biological effects. We now need properly designed and conducted clinical trials to assess whether interventions based on traditional Chinese medicines have any useful role to play in the management of toxicity in patients treated with chemotherapy for colorectal cancer.
What's new
| Date | Event | Description |
|---|---|---|
| 23 July 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 4, 2003 Review first published: Issue 1, 2005
| Date | Event | Description |
|---|---|---|
| 9 November 2004 | New citation required and conclusions have changed | Substantive amendment |
Notes
Original title for the published protocol has been changed, leaving out quality of life in the published review
Acknowledgements
We thank Dr. Henning Keinke Andersen, Ph.D., Ina Fjeldmark, secretary, Cochrane Colorectal Cancer Group, Dr Hak Su Goh, for advice and encouragement in preparing this review, and the Danish Videns‐ og Forskningscenter for Alternativ Behandling for providing financial support for this review.
Data and analyses
Comparison 1. nausea and vomiting.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 rate of nausea and vomiting | 2 | 144 | Risk Difference (M‐H, Random, 95% CI) | ‐0.33 [‐0.49, ‐0.17] |
1.1. Analysis.
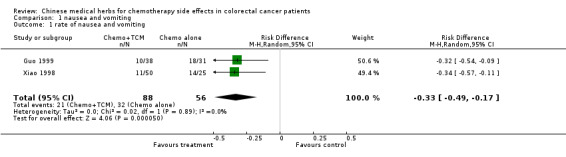
Comparison 1 nausea and vomiting, Outcome 1 rate of nausea and vomiting.
Comparison 2. leucopenia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 proportion of patients with WBC <3.0 x 10^9 per L | 2 | 144 | Risk Difference (M‐H, Random, 95% CI) | ‐0.36 [‐0.51, ‐0.21] |
2.1. Analysis.
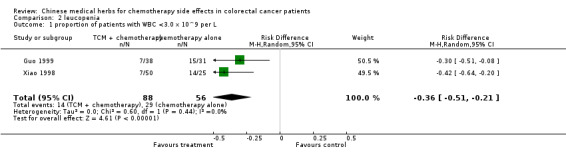
Comparison 2 leucopenia, Outcome 1 proportion of patients with WBC <3.0 x 10^9 per L.
Comparison 3. lymphocyte subsets.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 percentage CD3‐positive cells | 2 | 101 | Mean Difference (IV, Random, 95% CI) | 4.37 [0.26, 8.48] |
| 2 percentage CD4‐positive cells | 2 | 101 | Mean Difference (IV, Random, 95% CI) | 3.45 [‐1.25, 8.15] |
| 3 percentage CD8‐positive cells | 2 | 101 | Mean Difference (IV, Random, 95% CI) | 2.35 [0.71, 3.99] |
3.1. Analysis.
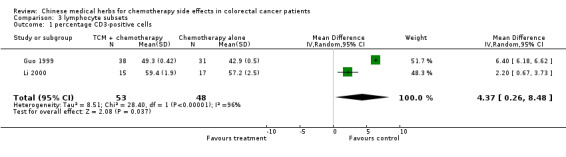
Comparison 3 lymphocyte subsets, Outcome 1 percentage CD3‐positive cells.
3.2. Analysis.
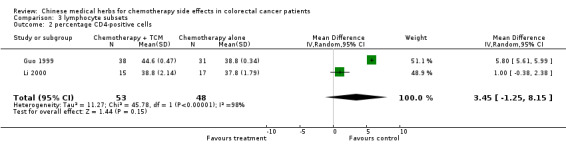
Comparison 3 lymphocyte subsets, Outcome 2 percentage CD4‐positive cells.
3.3. Analysis.
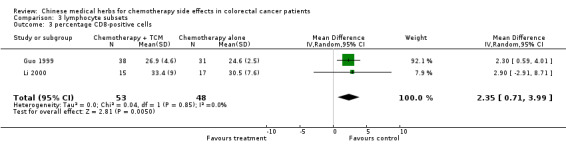
Comparison 3 lymphocyte subsets, Outcome 3 percentage CD8‐positive cells.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Guo 1999.
| Methods | Allocation using table of random numbers | |
| Participants | Intervention group: 38 (male 22, female 16, ages 33 to 65), Dukes' stages: B 8; C 15; D 15; adenocarcinoma 30; mucinous adenocarcinoma 6; undifferentiated carcinoma 2. Control group: 31 (male 17, female 14, ages 28 to 67), Dukes' stages: B 8; C 11; D 12; adenocarcinoma 23; mucinous adenocarcinoma 7; undifferentiated carcinoma 1. | |
| Interventions | Chemotherapy with Methyl CCNU+ 5‐FU+ Vincristine in both groups starting one month after operation. Four weeks per course. Four weeks interval between each course. Intervention group: chemotherapy plus oral FuZheng Anticancer Prescription daily, two months per course, for a total of two courses. Control group: chemotherapy only | |
| Outcomes | 1. Performance status (Karnofsky Score); 2. Median survival time; 3. Kaplan‐Meier survival rate; 4. Carcinoembryonic antigen (CEA); 5. Immunoglobulin and T‐lymphocyte subset counts 6. Adverse effect of herbal remedy | |
| Notes | Sichuan Academy of Traditional Chinese Medicine, Chengdu, China. The FuZheng Anticancer Prescription was prepared by the author himself. The contents of the decoction include YiRen (Semen Coicis) 60 g, RenShen (Radix Ginseng) 10 g, LinZhi 10 g, ShanQi (Radix Motoginseng 10 g), HuangQi (Radix Astragali) 15 g, BaiShu (Rhizoma Atractylodis Macrocephalae) 15 g, KuQiaoTou 15 g, WuHuaGuo 15 g, ZhuLing (Polyporus) 15 g, ShanCiGu (Pseudobulbus Cremastrae seu Pleiones) 15 g, ShanDouGeng (Radix Sophorae Tonkinensis) 15 g, DanShen (Radix Salviae miltiorrhizae) 30 g, BaijiangCao 30 g, The various ingredients were added to, or subtracted from the decoction, based upon the characteristics of each individual patient. | |
He 1995.
| Methods | Parallel design. Randomisation procedure unclear. Blinding not used. | |
| Participants | 150 postoperative patients with bowel cancer: Group A (n=50: 23 male; 27 female): 2 papillary adenocarcinoma; 21 moderately differentiated adenocarcinoma; 17 poorly differentiated adenocarcinoma; 6 mucinous adenocarcinoma; 4 signetring carcinoma; Dukes' B stage 14 cases, C stage 36 cases. Group B (n=50: 25 male; 25 female): 1 papillary adenocarcinoma; 23 moderately differentiated adenocarcinoma; 19 poorly differentiated adenocarcinoma; 2 mucinous adenocarcinoma; 5 signetring carcinoma; Dukes' B stage 16 cases, C stage 34 cases. Group C (50): 2 papillary adenocarcinoma; 22 moderately differentiated adenocarcinoma; 18 poorly differentiated adenocarcinoma; 6 mucinous adenocarcinoma; 2 signetring carcinoma. |
|
| Interventions | Chemotherapy with FMC regimen (5‐FU, cytosine arabinoside, Mitomycin) in all three groups. Additional treatment, given from first day of chemotherapy: Group A oral Danggui Huangbai decoction twice a day for six weeks; Group B oral Shengbaibao t.i.d for six weeks; Group C oral Berbamine Hydrochloride 112mg/time, t.i.d in six weeks. | |
| Outcomes | White blood cell count (WBC) at 2 weeks, 4 weeks and 6 weeks after the start of chemotherapy. | |
| Notes | DangGuiBuXueHuangBaiTang [HuangQi (Radix Astragali) 80 g and DangGui (Radix Angelicae Sinensis) 40 g, HuangBai (Cortex Phellodendri) 10 g] was a decoction prepared by the authors themselves, and ShengBaiBao was a commercial product manufactured by Zhengzhou Oriental Pharmaceutical Company. | |
Li 2000.
| Methods | Parallel design. Randomisation procedure unclear. Blinding not used. | |
| Participants | 48 postoperative patients with bowel cancer (stages Dukes' B and C): 16 in Group A, 17 in Group B and 15 in Group C. | |
| Interventions | Group A: oral Chang‐ai Kangfu decoction alone Group B: chemotherapy alone; 5‐FU (total 5g), MMC(total 20mg) Group C: chemotherapy with 5‐FU and MMC plus Changkangfutang decoction | |
| Outcomes | T‐lymphocyte subset analysis (including CD3+, CD4+, CD8+ counts), NK cell activity and immunoglobulin concentration at 1 week, 1 month, two months and 3 months after surgery. | |
| Notes | Professor Wang Pei of the Beijing Traditional Chinese Medical University prepared, and has previously used, Chang‐ai Kangfu decoction. The study was carried out at Guang'anmen Hospital, China academy of Traditional Chinese medicine, Taizhou City Hospital and the First Hospital of Guanxi Medical University. This study was co‐ordinated by Guangxi Medical University, China. The decoction contains 16 herbal medicines including HuangQi (Radix Astragali), Zhuning (Polyporus), Buguzhi (Fructus Psoraleae), Banzhilian (Herba Lobeliae Chinensis), and several other components which were not specified in detail. | |
Xiao 1998.
| Methods | Parallel design. Randomisation procedure unclear. Blinding not used. | |
| Participants | 75 postoperative patients with moderately advanced or advanced bowel cancer. 50 in Group A and 25 in Group B. Similar at base line in gender, age, general condition and pathological grading. | |
| Interventions | Both groups were treated with chemotherapy using 5‐FU and MMC Group A: chemotherapy plus oral Fuyuan decoction, 15 ml/time and 3 times daily for 3 months; three courses in the first year after operation and two courses in the second year and one course in the third year. Group B: chemotherapy only | |
| Outcomes | 1. systemic and gastro‐intestinal side effects. 2. peripheral blood WBC count. 3. 5‐year survival. | |
| Notes | Guanxi Traditional Chinese Medical University, China. Fuyuan oral decoction was prepared in the authors' hospital. The contents of the decoction include Huangqi (Radix Astragali), Taizishen (Radix Pseudostellariae), Huangjin (Rhizoma Polygonati), Shudi (Radix Rehmanniae Preparata), Zhiheche (Placenta Hominis), Jixueteng (Caulis Spatholobi), Fulin (Poria). | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bao 1992 | "Randomised selection control group" was mentioned, bu there was no evidence of random allocation. |
| Chen 1999 | Duplicate publication. Same as Wang 2000. |
| Guo 1999‐exclude | Duplicate publication. Same as Guo 1999. |
| Huang 2002 | Data unusable. 67 late stage patients, including 27 who had not been previously treated, were allocated to inject TianMa injection or FMC chemotherapy. We were unable to identify which patients in the TianMa injection group were treated with chemotherapy. |
| Jiang 2001 | Controlled study, no random allocation. |
| Li 1999 | Randomised design was mentioned. But the interventions were allocated by non‐random procedures. |
| Li 2002a | Data unusable. Just simply mentioned that there were no differences in Karnofsky score and blood cell count between two groups, but the patients in the Chinese herbs group had better appetite and less dirrhoea than the patients in the chemotherapy alone group. |
| Li 2002b | Controlled study, no random allocation. |
| Liu 2001 | Controlled study, no random allocation. |
| Wang 2000 | Randomised design was mentioned. But the interventions were allocated by non‐random procedures. |
| Wang 2001 | Duplicate publication. Same as Wang 2000. |
| Xu 1996 | Randomisation was mentioned, but the allocation procedure was unclear. Furthermore, the number of participants of each group did not balance(57:34), and the data unusable. |
| Zheng 2002 | Controlled study, no random allocation. |
Contributions of authors
Wu Taixiang: protocol development, searching for trials, quality assessment of trials, data extraction, data analysis, review development. Alastair J Munro: data analysis, review development Liu Guanjian: data analysis
Sources of support
Internal sources
Chinese Cochrane Centre, West China Hospital of Sichuan University, Chinese Medical Board of New York(CMB), China.
External sources
Danish acronym: ViFAB and the Nordic Cochrane Centre, Cochrane Colorectal Cancer Group, Denmark.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Guo 1999 {published data only}
- Guo ZX. FuZheng Anticancer Prescription in Postoperative Treatment for Colorectal Cancers. Chinese Journal of integrated Traditional Chinese and Western Medicine 1999;19(1):20‐2. [PubMed] [Google Scholar]
He 1995 {published data only}
- He YH, Wang YQ. Chinese medicinal herbs in the treatment of liukopenia result from chemotherapy for postoperative of bowel cancer. Liaoning Jounal of Traditional Chinese Medince 1995;22(3):125‐6. [Google Scholar]
Li 2000 {published data only}
- Li HS, Li HH, Tang ZJ, Zhang S, Gao F, Li GD, Wang CX. Effect of Chang'ai Kangfu Decoction on Immunity in Postoperational patients with Bowel Cancer [Effect of Chang'ai Kangfu Decoction on Immunity in Postoperational Patients with Large Intestine Cancer]. Chinese Journal of of Integrated Traditional Chinese and Western Medicine 2000;20(8):580‐2. [PubMed] [Google Scholar]
Xiao 1998 {published data only}
- Xiao ZQ, Luo CD. Observation for effect of FuYuan oral decoction in the treatment of 50 cases of postoperation and chemotherapy. Liaoning Journal of Traditional Chinese Medicine 1998;25(11):516‐7. [Google Scholar]
References to studies excluded from this review
Bao 1992 {published data only}
- Bao SZ, Sun ZD, Wang ZS, Wu LC. Chinese herbs "FuFang ShanGengTang" decoction combined with chemotherapy in the treatment of 120 intermediate and late stage bowel cancer cases. Journal of Liao Ning Traditional Chinese Medicine 1992;7:33‐4. [Google Scholar]
Chen 1999 {published data only}
- Chen JX, Ma PZ, Wang HZ. Clinical study of MuDouHui glucoside tablets in the treatment of bowel cancer. Chinese Traditional and Herbal Drugs 1999;30(7):528‐9. [Google Scholar]
Guo 1999‐exclude {published data only}
- Guo ZX. FuZheng Anticancer Prescription in Postoperative Treatment for Colorectal Cancers. Chinese Journal of Integrated Traditional Chinese and Westen Surgery 1999;5(1):10‐3. [Google Scholar]
Huang 2002 {published data only}
- Huang GD, Huang CR, He YH. Clinical Observation of TianMa Injection in the treatment of late bowel cancer. Journal of Hunan College of TCM 2002;22(3):50‐1. [Google Scholar]
Jiang 2001 {published data only}
- Jiang YL, Pan B, Qiu XZ. JianPiXiaoAi with chemotherapy in the treatment of post‐operation of bowel cancer. Hunan Journal of Traditional Chinese Medicine 2001;17:5. [Google Scholar]
Li 1999 {published data only}
- Li YJ, Li QS. Enema with Traditional Chinese Herbs combined with intraperitoneal chemotherapy in the treatment of 60 progressive stage bowel cancer. Journal of Jiang Shu Traditional Chinese Medicine 1999;20(6):30. [Google Scholar]
Li 2002a {published data only}
- Li SW, Li Y. Clinical study of ChangAi I# in the treatment of bowel cancer. Chinese Journal of Clinical Oncology and Recovery 2002;9(1):75. [Google Scholar]
Li 2002b {published data only}
- Li ZY, Deng XJ. Observation of effects of turmeric rhiaone oil and other TCM preparations on late carcinoma of large intestines through regional perfusion with the implanted pump. GuangXi Journal of Traditional Chinese Medicine 2002;25(6):18‐9. [Google Scholar]
Liu 2001 {published data only}
- Liu H, Luo WH. Observation for the effect of anti‐tumour Shenbaipian with chemotherapy in the treatment of late stage bowel cancer. Hunan Jounal of Traditional Chinese Medicine 2001;17(6):13‐translete to 24. [Google Scholar]
Wang 2000 {published data only}
- Wang HZ, Wang SZ, Wang YH, Chen JX. Clinical Observation of "Mutouhui Glycoside Pill" in treating Carcinoma of Large Intestine". Journal of Shanghai Traditional Chinese Medicine 2000;12:16‐7. [Google Scholar]
Wang 2001 {published data only}
- Wang HZ, Wang YH, Chen JX, Wang SZ. A clinical study on the effect of radix patriniae beterophylla glucoside tablets on colorectal cancer. Chinese Jouranl of Clinical Oncology and Recovery 2001;8:37‐9. [Google Scholar]
Xu 1996 {published data only}
- Xu HY, Li CB, Xiong XD. Observation for effect of FuZhengJieDu herbs in the treatment of postoperation of bowel cancer. Journal of Shanghai Traditional Chinese Medicine 1996;2:12. [Google Scholar]
Zheng 2002 {published data only}
- Zheng YL, Wang XJ. A clinical study for ChangShunDa GuanChangYe in the treatment of bowel cancer. Henan Traditional Chinese Medicine 2002;22(1):12‐4. [Google Scholar]
References to studies awaiting assessment
Ran 1999 {published data only}
- Ran JH, Zhang JY, Wang X, Guo Q, Yang J. Effect of Kanglaite on Immunologic Function of Postoperative Patients with Large Intestine Cancer. Chinese Journal of Clinical Oncology and Recovery 1999;6(2):20‐1. [Google Scholar]
Wu 1999 {published data only}
- Wu HM, Song L, Zhu JC. FuZhengXiaoLiu decoction combine with interleukin II in the treatment of carcinoma of colon.. Chinese Journal of Integrated Traditional and Western medicine 1999;19(7):409. [Google Scholar]
Additional references
Beart 1992
- Beart RW, Steele GD Jr, Menck HR, Chmiel JS, Ocwieja KE, Winchester DP. Management and survival of patients with adenocarcinoma of the colon and rectum: a national survey of the Commission on Cancer.. J Am Coll Surg. 1995;181(3):225‐36. [PubMed] [Google Scholar]
Black 1997
- Black RJ, Bray F, Ferlay J, Parkin DM. Cancer incidence and mortality in the European Union. Cancer registry and estimates of national incidence for 1990.. Eur J Cancer 1997;33:1075‐1107. [DOI] [PubMed] [Google Scholar]
CCCG 2000
- Colorectal Cancer Collaborative Group. Surgery for colorectal cancer in elderly patients: a systematic review. The Lancet 2000;356(9234):968‐74. [PubMed] [Google Scholar]
CDC 2003
- Centers for Disease Control and Prevention. Colorectal Cancer Information. http://www.cdc.gov/cancer/screenforlife/info.htm 2003.
Chen 1978
- Chen W‐F, Patchefsky AS, Goldsmith HS. Colonic protection from dimethylhydrazine by a high‐fiber diet.. Surg Gynecol Obstet 1978;147(4):503‐6. [PubMed] [Google Scholar]
Chrystal 2002
- Chrystal K, Allan S, Forgeson G, Isaacs R. The use of complementary/alternative medicine by cancer patients in a New Zealand regional cancer treatment centre. N Z Med J 2002;116(1168):U296. [MEDLINE: ] [PubMed] [Google Scholar]
Clarke 2002
- Clarke M, Oxman AD, editors. Optimal search strategy for RCTs.. Reviewers' Handbook 4.1.5 [updated April 2002] 2002:appendix 5c..
Diaz‐Canton 1996
- Diaz‐Canton EA, Pazdur R. Medical treatment of colorectal cancer. Medicina (B Aires) 1996;56(4):414‐22. [PubMed] [Google Scholar]
Doll 1981
- Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today.. J Natl Cancer Inst 1981;66:1191‐1308. [PubMed] [Google Scholar]
Fleiszer 1978
- Fleiszer D, MacFarlane J, Murray D, Brown RA. Protective effect of dietary fibre against chemically induced bowel tumours in rats. The Lancet 1978;2(8089):552‐3. [DOI] [PubMed] [Google Scholar]
Fukutake 2000
- Fukutake M, Miura N, Yamamoto M, Fukuda K, Iijima O, Ishikawa H, Kubo M, Okada M, Komatsu Y, Sasaki H, Wakabayashi K, Ishige A, Amagaya S. Suppressive effect of the herbal medicine Oren‐gedoku‐to on cyclooxygenase‐2 activity and azoxymethane‐induced aberrant crypt foci development in rats. Cancer Lett 2000;157(1):9‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Giovannucci 1999
- E. Giovannucci, E. A. Platz. Colorectal cancer: the problems. In: W. Schmiegel, J. Scholmerich editor(s). Colorectal Cancer, Molecular Mechanisms, Premalignant State and its Prevention. Dordrecht, The Netherlands: KLUWER ACADEMIC PUBLISHERS, 1999:3‐15. [Google Scholar]
Haenszel 1968
- Haenszel W, Kurihara M. Studies of Japanese migrants. I. Mortality from cancer and other diseases among Japanese in the United States.. J Natl Cancer Inst 1968;40:43‐68. [PubMed] [Google Scholar]
Hilsden 1999
- Hilsden RJ, Meddings JB, Verhoef MJ. Complementary and alternative medicine use by patients with inflammatory bowel disease: An Internet survey. Can J Gastroenterol 1999;13(4):327‐32. [DOI] [PubMed] [Google Scholar]
Hua 1999
- Hua B, Wang A. Clinical study on treatment of mid‐late stage gastric carcinoma by composite xiansu capsule combined with chemotherapy. Zhongguo Zhong Xi Yi Jie He Za Zhi 1999;19(8):470‐2. [MEDLINE: ] [PubMed] [Google Scholar]
Jin 2001
- Jin H, Wu XW. Chinese herbal medicine combined with rediotherapy in the treatment of 30 cases of abdomen tumor. Journal of Traditional Chinese Medicine 2001;42(6):357‐9. [Google Scholar]
Juni 2001
- Juni P, Altman DG, Egger M. Assessing the quality of controlled clinical trials. British Medical Journal 2001;323:42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
King 1980
- King H, Locke FB. Cancer Mortality Among Chinese in the United States. J Natl Cancer Inst 1980;65:1141‐8. [PubMed] [Google Scholar]
Labianca 1997
- Labianca R, Pessi MA, Zamparelli G. Treatment of colorectal cancer. Current guidelines and future prospects for drug therapy. Drugs 1997;53(4):593‐607. [DOI] [PubMed] [Google Scholar]
Lampert 2002
- Lampert N, Xu Y. Chinese herbal nephropathy. Lancet 2002;359(9308):796‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Li 2000
- Li H, Li H, Tang Z, Zhang S, Gang F, Li GD, Wang CX. Effect of Chang'ai Kangfu Decoction on Immunity in Postoperational Patients with Large Intestine Cancer. Chinese Journal of Integrated Traditional and Western Medicine 2000;20(8):580‐2. [MEDLINE: ] [PubMed] [Google Scholar]
Lin 1996
- Lin C, Lin X, Yang J. An observation on combined use of chemotherapy and traditional Chinese medicine to relieve cancer pain. J Tradit Chin Med 1996;16(4):267‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Liu 2000a
- Liu X, Wang B, Fu X. Clinical study on treatment of advanced non‐small cell lung cancer with Chinese herbal medicine combined with synchronous radio‐ and chemotherapy. Zhongguo Zhong Xi Yi Jie He Za Zhi 2000;20(6):427‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Liu 2000b
- Liu P, Cao Y, Qiao X. Clinical study on shenmai injection in promoting postoperative recovery in patients of breast cancer. Zhongguo Zhong Xi Yi Jie He Za Zhi 2000;20(5):328‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Locke 1980
- Locke FB, King H. Cancer mortality risk among Japanese in the United States. J Natl Cancer Inst 1980;65:1149‐56. [PubMed] [Google Scholar]
Lord 2001
- Lord GM, Cook T, Arlt VM, Schmeiser HH, Williams G, Pusey CD. Urothelial malignant disease and Chinese herbal nephropathy. Lancet 2001;358(9292):1515‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Martenson 1997
- Martenson JA Jr, Hyland G, Moertel CG, Mailliard JA, O'Fallon JR, Collins RT, Morton RF, Tewfik HH, Moore RL, Frank AR, Urias RE, Deming RL. Olsalazine is contraindicated during pelvic radiation therapy: results of a double‐blind, randomized clinical trial. Int J Radiat Oncol Biol Phys 1997;35(2):299‐303. [DOI] [PubMed] [Google Scholar]
Marvin 2002
- Marvin L. Corman, John A. Coller, Eric J. Daniels, Robert Gilliland, Anthony A. Goodman, Lester Gottesman, Victor W. Fazio, Jose Marcio Neves Jorge, Anthony M. Nyerges, Alberto Pena, John L. Petrini, Daniel Rosenthal, Jonathan M. Sackier, Ronald M. Stewart, Paula Erwin‐Toth, Steven D. Wexner. Translators: Jiang KW, Wang B. Colon cancer. In: Marvin L. Corman editor(s). Colon & rectal surgery. 4. Vol. Chinese edition, Beijing: People's Medical Publishing House, 2002:568‐662. [ISBN 7‐117‐04599‐X/R.4600] [Google Scholar]
Moertel 1993
- Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA. Hepatic toxicity associated with fluorouracil plus levamisole adjuvant therapy. J Clin Oncol 1993;11(12):2386‐90. [DOI] [PubMed] [Google Scholar]
NHS 2003
- NHS. UK Colorectal Cancer Screening Pilot. http://www.cancerscreening.nhs.uk/colorectal/ 2003.
Nortier 2000
- Nortier JL, Martinez MC, Schmeiser HH, Arlt VM, Bieler CA, Petein M, Depierreux MF, Pauw L, Abramowicz D, Vereerstraeten P, Vanherweghem JL. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi) Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi). N Engl J Med. 2000;342(23):1686‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ohnishi 1998
- Ohnishi Y, Fujii H, Hayakawa Y, Sakukawa R, Yamaura T, Sakamoto T, Tsukada K, Fujimaki M, Nunome S, Komatsu Y, Saiki I. Oral administration of a Kampo (Japanese herbal) medicine Juzen‐taiho‐to inhibits liver metastasis of colon 26‐L5 carcinoma cells.. Jpn J Cancer Res 1998;89(2):206‐13. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Quasar 2000
- Quasar Collaborative Group. Comparison of fluorouracil with additional levamisole, higher folinic acid, or both, as adjuvant chemotherapy for colorectal cancer. Lancet 2000;355(9215):1588‐1596. [PubMed] [Google Scholar]
Shike 1999
- Shike M. Diet and lifestyle in the prevention of colorectal cancer. An overview. Am J Med 1999;106:11S‐15S. [DOI] [PubMed] [Google Scholar]
Shumay 2001
- Shumay DM, Maskarinec G, Kakai H, Gotay CC. Why some cancer patients choose complementary and alternative medicine instead of conventional treatment. J Fam Pract 2001;50(12):1067. [MEDLINE: ] [PubMed] [Google Scholar]
SIGN 2003
- SIGN Scottish Intercollegiate Guideline Network. Management of Colorectal Cancer. SIGN Edinburgh http://www.sign.ac.uk/guidelines/fulltext/67/index.html 2003; Vol. 67.
Tough 2002
- Tough SC, Johnston DW, Verhoef MJ, Arthur K, Bryant H. Complementary and alternative medicine use among colorectal cancer patients in Alberta, Canada. Altern Ther Health Med 2002;8(2):54‐6, 58‐60, 62‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Veronese 2004
- Veronese, M.L, O'Dwyer, P.J. Monoclonal antibodies in the treatment of colorectal cancer. Eur J Cancer 2004;40:1292‐1301. [DOI] [PubMed] [Google Scholar]
Wang 1999
- Wang C, Deng Z, Lou X. Clinical study on treatment of rectal carcinoma with Chinese herbal medicine and high dose fluorouracil emulsion via rectal infusion. Zhongguo Zhong Xi Yi Jie He Za Zhi 1999;19(7):389‐91. [MEDLINE: ] [PubMed] [Google Scholar]
Wang 2002
- Wang R, Gao C, Liu D. Effects of shenmai injection on expression of TNF‐alpha mRNA in peritoneal macrophages of scald mice.. Chin Med J (Engl) 2002;115(2):293‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Winawer 1997
- Winawer SJ, Fletcher RH, Miller L, Godlee F, Stolar MH, Mulrow CD, Woolf SH, Glick SN, Ganiats TG, Bond JH, Rosen L, Zapka JG, Olsen SJ, Giardiello FM, Sisk JE, Antwerp RV, Brown‐Davis C, Marciniak DA, Mayer RJ. Colorectal Cancer Screening: Clinical Guidelines and Rationale. Gastroenterology 1997;112(2):594‐642. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Winawer 2003
- Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, Ganiats T, Levin T, Woolf S, Johnson D, Kirk L, Litin S, Simmang C, Gastrointestinal Consortium Panel. Colorectal cancer screening and surveillance: clinical guidelines and rationale‐Update based on new evidence.. Gastroenterology 2003;124(2):544‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Xin 2001
- Xin JH, Chen YQ, Ji MX, Zhu SG, Gong XQ. Clinical Study on Effect of Ginsenoside in Inducing Rectal Cancer Cell Apoptosis. Chinese Journal of Integrated Traditional and Western Medicine 2001;21(4):260‐1. [PubMed] [Google Scholar]
Ye 2002
- Ye F, Xui L, Yi J, Zhang W, Zhang DY. Anticancer activity of Scutellaria baicalensis and its potential mechanism. J Altern Complement Med 2002;8(5):567‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zhou 1999
- Zhou K, Wang J, Liu B. Clinical study on effect of shenqi fuzheng injection combined with chemotherapy in treating gastric cancer. Zhongguo Zhong Xi Yi Jie He Za Zhi 1999;19(1):11‐3. [MEDLINE: ] [PubMed] [Google Scholar]


