Abstract
Background
Coronary artery bypass graft surgery (CABG) replaces obstructed vessels with ones from other parts of the body. Alternatively, obstructions are remodelled using catheter‐based techniques such as percutaneous coronary angioplasty with the use of stents. Though less invasive, stenting techniques are limited by the re‐narrowing of treated vessels (restenosis). We examined evidence on cardiac‐related outcomes occurring after CABG or stenting, with implications for resource use, resource allocation and informing patient choice.
Objectives
To examine evidence from randomised controlled trials (RCTs) on benefit of stents or CABG in reducing cardiac events in people with stable angina or acute coronary syndrome (ACS).
Search methods
CENTRAL (Issue 2 2004), EMBASE (1990 to 2004), MEDLINE (1990 to 2004) and handsearching to July 2004.
Selection criteria
Only RCTs comparing stents used with PTCA with CABG were included. Participants were adults with stable angina or ACS and unstable angina and had either single or multiple vessel disease. Published and unpublished sources were considered.
Data collection and analysis
Outcomes included composite event rate (major adverse cardiac event, event free survival), death, acute myocardial infarction (AMI), repeat revascularisation and binary restenosis as well as information on design and baseline characteristics. Quality assessment was completed independently. Meta‐analyses are presented as odds ratios, 95% confidence intervals (CI) using a fixed‐effect model. Heterogeneity between trials was assessed.
Main results
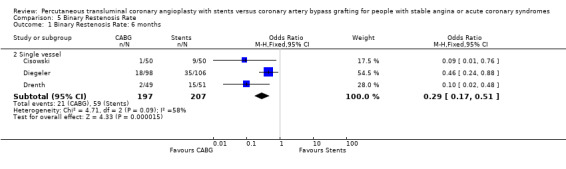
Nine studies (3519 patients) were included. Four RCTs included patients with multiple vessel disease, five focused on single vessel disease. Four studies reported beyond 1 year. No statistical differences were observed between CABG and stenting for meta‐analysis of mortality or AMI, but there was heterogeneity. Composite cardiac event and revascularisation rates were lower for CABG than for stents. Odds ratios resulting from meta‐analysis of event rate data at 1 year were, odds ratio 0.43 (95% CI 0.35 to 0.54) and at 3 years, odds ratio 0.37 (95% CI 0.29 to 0.48). Odds ratios for revascularisation at 1 year were, odds ratio 0.18 (95% CI 0.13 to 0.25) and at 3 years, odds ratio 0.09 (95% CI 0.02 to 0.34). Binary restenosis at 6 months (single vessel trials) favoured CABG, odds ratio 0.29 (95% CI 0.17 to 0.51).
Authors' conclusions
CABG is associated with reduced rates of major adverse cardiac events, mostly driven by reduced repeat revascularisation. However, the RCT data are limited by follow‐up, unrepresentative samples and rapid development of both surgical techniques and stenting. Research on real‐world patient population or patient level data meta‐analyses may identify risk factors and groupings who may benefit most from one strategy over the other.
Keywords: Humans; Coronary Artery Bypass; Stents; Angina Pectoris; Angina Pectoris/therapy; Angioplasty, Balloon, Coronary; Angioplasty, Balloon, Coronary/methods; Coronary Disease; Coronary Disease/therapy; Myocardial Infarction; Myocardial Infarction/therapy; Randomized Controlled Trials as Topic; Syndrome
Plain language summary
People who undergo bypass surgery for narrowed coronary vessels may be less likely to need re‐intervention than those treated using angioplasty with stents
Narrowing of coronary arteries can be alleviated by complete replacement using bypass surgery or, alternatively, unblocking and supporting the vessels open using angioplasty and stents. Analysis of RCTs to 2004 indicates re‐intervention (to alleviate subsequent narrowing) is needed less commonly after surgery than after stenting. Risk of death or heart attack following either treatment appeared the same, but this may be because too few trial participants were collected together in the review and variation between trials (heterogeneity) may be masking true differences. Further trials of new techniques in a greater variety of patients with subsequent systematic review are needed.
Background
Coronary artery bypass graft (CABG) is the surgical technique used to treat critical obstructions in coronary arteries caused by atherosclerotic plaque disease. New conduits are fashioned during the procedures to bypass the obstructions by the use of either reversed saphenous veins harvested from the legs or by using arterial conduits such as the internal mammary arteries which can be diverted to the coronary vessels or by using portions of the radial artery harvested from the arm. Surgery can provide relief from symptoms and prolong life, but being an invasive procedure, there is a risk of initial surgical mortality and morbidity with the need for a significant period of convalescence and surgical centres require specialised staff and facilitates.
These disadvantages of CABG prompted researchers to identify alternative treatments, such as Percutaneous Coronary Interventions (PCI), which include Percutaneous Transluminal Coronary Angioplasty (PTCA), PTCA with stenting, brachytherapy and atherectomy technologies. All involve methods for restoring blood flow in obstructed coronary vessels, but are performed generally under local anaesthetic through small vascular incisions in the groin or arm (percutaneously) without the need for major surgery except in the event of an emergency. These interventions can be completed in as little as 15 minutes and patients may return home the same day (Gunn 2003).
Although procedural success rates are high, complication rates low and most patients experience improvement in symptoms after intervention (Gunn 2003), rates of restenosis (re‐narrowing of the treated vessel), which may require re‐intervention has traditionally been a significant limitation of PCI. Restenosis following PTCA with the use of traditional (uncoated) stents, can result from the proliferation of smooth muscle cells into and around the stent. Rates of significant restenosis in PTCA with stents are reported (in systematic reviews by Meads 2000 and by Dundar 2004) as ranging between 20 and 50%, depending on the size, location and complexity of the lesion and the amount of routine angiographic monitoring performed in the study.
There remains therefore the debate on whether surgery or PCI is the most appropriate treatment for some people, particularly those with multivessel disease (Gunn 2003; Serruys 2001). In assessing the two technologies one must consider the relative merits of an invasive surgical procedure with lasting benefits against less invasive PCI with traditionally higher rates of reintervention.
This review compares PTCA with the use of stents to CABG for the treating people with coronary artery disease and will include investigation of outcomes in people with single or multivessel disease at follow‐up in the short and long term.
Objectives
To assess the clinical effects of the use of coronary artery stents (as part of Percutaneous Transluminal Coronary Angioplasty) compared to Coronary Artery Bypass Graft surgery for the treatment of people with coronary artery disease.
Methods
Criteria for considering studies for this review
Types of studies
Randomised Controlled Trials (RCTs), published or unpublished, where the use of coronary artery stents (in conjunction with Percutaneous Transluminal Coronary Angioplasty techniques) to relieve narrowing(s) of the coronary arteries is compared with the application of Coronary Artery Bypass Graft (CABG) techniques.
Types of participants
Adults with stable angina or Acute Coronary Syndrome (including AMI (ST segment elevation and depression, Q wave and non‐Q wave) and unstable angina). Adults with single or multivessel coronary artery disease.
Types of interventions
Percutaneous transluminal coronary angioplasty with stents versus coronary artery bypass grafting surgery.
Types of outcome measures
Clinical (1) Combined event rate or event free survival (e.g. Major Adverse Cardiac Events, Major Adverse Cardiac and Cerebrovascular Events, Target Vessel Failure or other composites of the events listed below); (2) Death (both cardiac and non‐cardiac death); (3) Acute Myocardial Infarction (AMI); (4) Target Vessel Revascularisation (TVR); (5) Target Lesion Revascularisation (TLR); (6) Repeat treatment (PTCA, stent or CABG).
Radiological Binary restenosis (greater than 50% luminal narrowing compared to diameter at completion of the procedure).
Quality of life Where quality of life (QoL) data were available the nature of the measures, timings of measurement and analytical tool used to assess QoL were recorded.
Search methods for identification of studies
The search incorporated a number of methods to identity completed or ongoing RCTs: (1) Searching of electronic databases; (2) Handsearching of recent journals and conferences in relevant fields; (3) Subscription to e‐mail‐based information newsletters and regular examination of webpages (including those supported by stent manufacturers) relevant to the review topic; (4) Searching of bibliographies of identified sources; (5) Use of submissions to National Institute for Clinical Excellence (NICE), London, UK.
Electronic searches included MEDLINE, EMBASE and CENTRAL (Issue 2 2004 of The Cochrane Library) for the period from 1990 (as it was in the early 1990s that coronary artery stents were introduced into practice) to July 2004. Electronic bibliographic databases were searched according to the strategy detailed in Table 1; Table 2; Table 3. The search was not limited to English. Handsearching of recent issues of cardiology journals and conference proceedings included American Heart Journal, American Journal of Cardiology, British Medical Journal, Catheterization and Cardiovascular Interventions, Circulation, European Heart Journal, Heart, International Journal of Cardiology, Journal of the American College of Cardiology, Journal of the American Medical Association, Journal of Thoracic and Cardiovascular Surgery, Lancet , New England Journal of Medicine, American College of Cardiology, American Heart Association, British Cardiac Society, European Society of Cardiology, Transcatheter Cardiovascular Therapeutics, Cardiovascular Revascularization Therapy.
1. MEDLINE (Ovid, 1966 to June Week 4 2004).
| 1 exp stents/ (18094) 2 stent$.tw. (21006) 3 or/1‐2 (24524) 4 exp coronary disease/ (138520) 5 exp myocardial infarction/ (95563) 6 exp angina pectoris/ (29604) 7 coronary.tw. (165294) 8 angina.tw. (29588) 9 myocardial infarction.tw. (71346) 10 or/4‐9 (289171) 11 3 and 10 (7736) 12 exp Coronary Artery Bypass/ (28297) 13 (coronary adj4 bypass$).tw. (21860) 14 cabg.tw. (5482) 15 or/12‐14 (34720) 16 11 and 15 (1491) 17 randomized controlled trial.pt. (190066) 18 controlled clinical trial.pt. (66483) 19 Randomized controlled trials/ (32727) 20 random allocation.sh. (50941) 21 double blind method.sh. (78441) 22 single‐blind method.sh. (8165) 23 or/17‐22 (321732) 24 exp animal/ not human/ (2813909) 25 23 not 24 (305055) 26 clinical trial.pt. (384488) 27 exp Clinical trials/ (155323) 28 (clin$ adj25 trial$).ti,ab. (100437) 29 ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).ti,ab. (75050) 30 placebos.sh. (23062) 31 placebo$.ti,ab. (84456) 32 random$.ti,ab. (286327) 33 research design.sh. (38205) 34 or/26‐33 (675439) 35 34 not 24 (627741) 36 35 not 25 (332926) 37 comparative study.sh. (1126924) 38 exp evaluation studies/ (489332) 39 follow up studies.sh. (284482) 40 prospective studies.sh. (174378) 41 (control$ or prospectiv$ or volunteer$).ti,ab. (1448168) 42 or/37‐41 (2896137) 43 42 not 24 (2216327) 44 43 not (25 or 36) (1777113) 45 25 or 36 or 44 (2415094) 46 16 and 45 (868) 47 limit 46 to yr=1990 ‐ 2004 (859) |
2. EMBASE (Ovid, 1980 to 2004 Week 27).
| 1 coronary stent/ (4773) 2 stent$.tw. (19964) 3 or/1‐2 (21140) 4 exp Coronary Artery Disease/ (52238) 5 exp Coronary Artery Obstruction/ (10060) 6 exp coronary artery/ (17237) 7 exp heart infarction/ (76177) 8 exp angina pectoris/ (28196) 9 coronary.tw. (135313) 10 angina.tw. (24098) 11 myocardial infarction.tw. (58860) 12 or/4‐11 (209182) 13 3 and 12 (7595) 14 coronary artery bypass graft/ (17131) 15 (coronary adj4 bypass$).tw. (18718) 16 cabg.tw. (4807) 17 or/14‐16 (25814) 18 13 and 17 (1482) 19 random$.ti,ab. (252525) 20 factorial$.ti,ab. (5072) 21 (crossover$ or cross over$ or cross‐over$).ti,ab. (30031) 22 placebo$.ti,ab. (80297) 23 (double$ adj blind$).ti,ab. (65025) 24 (singl$ adj blind$).ti,ab. (5414) 25 assign$.ti,ab. (73603) 26 allocat$.ti,ab. (23112) 27 volunteer$.ti,ab. (75264) 28 Crossover Procedure/ (15069) 29 Double Blind Procedure/ (52125) 30 Randomized Controlled Trial/ (85815) 31 Single Blind Procedure/ (4797) 32 or/19‐31 (449620) 33 exp animal/ (79612) 34 nonhuman/ (2423534) 35 exp animal experiment/ (1040385) 36 or/33‐35 (2661811) 37 exp human/ (4657871) 38 36 not 37 (2340844) 39 32 not 38 (394258) 40 18 and 39 (239) |
3. CENTRAL (The Cochrane Library Issue 2 2004).
| #1 STENTS #2 stent* #3 (#1 or #2) #4 CORONARY DISEASE #5 CORONARY CIRCULATION #6 MYOCARDIAL REVASCULARIZATION #7 CORONARY VESSELS #8 coronary #9 angina #10 (myocardial next infarction) #11 (heart next infarction) #12 (#4 or #5 or #6 or #7 or #8 or #9 or #10 or #11) #13 (#3 and #12) #14 CORONARY ARTERY BYPASS #15 (coronary near bypass*) #16 cabg #17 (#14 or #15 or #16) #18 (#17 and #13) |
Internet‐based resources include: http://www.tctmd.com http://www.theheart.org http://www.crtonline.org http://www.atherothrombosis.org http://www.controlled‐trials.com/ http://www.update‐software.com/National/ Stent manufacturer webpages.
Details of CENTRAL, EMBASE MEDLINE and search strategies are provided in Table 1; Table 2; Table 3.
Selected triallists were contacted for further details of outcome data and trial progress. More information on contact with triallists will be provided by request to the corresponding author.
Date of the most recent search of the Group's specialised register: July 2004.
Data collection and analysis
Study selection and quality assessment Two reviewers independently selected the trials to be included in the review. Firstly, reviewers independently scanned the titles and abstracts of references identified by searching (electronic databases or handsearching). Full details of selected studies were obtained and assessed for inclusion in the review and if included, methodological quality. In particular, reviewers examined details of the randomisation method, concealment of allocation, whether the trial was masked (blinded), whether intention‐to‐treat analyses were possible from the available data and whether the number of patients lost to follow up or subsequently excluded from the study was recorded.
While not totally impossible, the reviewers anticipated that studies comparing surgery and stenting would have difficulty masking (blinding) outcome assessors to the treatment applied to participants in trials.
Quality was assessed using a scheme based onSchulz 1995, methods proposed by the Heart Collaborative Review Group (Heart CRG) and grading similar to that used inVillanueva 2004. Adequacy of the randomisation process A ‐ Adequate sequence generation is reported (such as computer generated random numbers and random number tables, whilst inadequate approaches will include the use of alternation, case record numbers, birth dates or days of the week); B ‐ Did not specify one of the adequate reported methods in (A) but mentioned randomisation method; C ‐ Other methods of allocation that appear to be unbiased. Adequacy of the allocation concealment process A ‐ Adequate measures to conceal allocations. Concealment will be deemed adequate where randomisation is centralised or pharmacy‐controlled, or where the following are used: serially numbered containers, on‐site computer‐based systems where assignment is unreadable until after allocation, other methods with robust methods to prevent foreknowledge of the allocation sequence to clinicians and patients; B ‐ Unclearly concealed trials, in which the authors either did not report an allocation concealment approach at all, or reported an approach that did not fall into one of the categories in (A); C ‐ Inadequately concealed trials, in which method of allocation is not concealed. Inadequate approaches will include: the use of alternation, case record numbers, days of the week, open random number lists and serially numbered envelopes even if opaque.
Potential for selection bias after allocation A ‐ Studies where an intention to treat analysis is possible and few exclusions (with adequate reporting of these exclusions); B ‐ Studies which reported exclusions as reported in (A), but exclusions were less than 10 percent; C ‐ No reporting of exclusions; exclusions of 10 percent or more or wide differences in exclusions between groups.
Adequacy of masking (see note on practicality of masking above) A ‐ Double (or triple) blind; B ‐ Single blind; C ‐ Non‐blind; D ‐ Unclear. If disagreement arose on the suitability of a trial for inclusion in the review or on its quality, the reviewers would attempt to reach a consensus by discussion and/or involve a third reviewer.
Data extraction Data were extracted by one reviewer using pre‐tested data extraction forms and checked by a second. Data extraction included the outcome measures (detailed above), as well as information on study design, participants (including baseline characteristics and co‐morbidity in terms of diabetes and previous heart disease
Data synthesis For binary outcome measures, data on the number of patients with each outcome event, by allocated treatment group, irrespective of compliance and whether or not the patient was later thought to be ineligible or otherwise excluded from treatment or follow‐up were sought to allow an intention‐to‐treat analysis.
No suitable continuous outcome data was available, but if these data were incorporated into the review, either mean change from baseline for each group or mean post‐treatment/intervention values and standard deviation for each group would be recorded. If clinically appropriate, a pooled estimate of treatment effect would be produced by calculating the weighted mean difference.
Time points for analysis of outcomes were 6 months, 1 year, 2 years, 2.5 to 3 years and 4 years. Given the restricted trial populations (see discussion of limitations, later) it was felt that CABG may have less favourable short term outcomes, but may have better long term outcomes. Therefore these multiple periods of follow‐up were used in order to attempt to record differences in short and longer term outcomes between CABG the use of stents. Heterogeneity between trial results was explored using a multi‐step process whereby: (1) Forest Plots were examined and the presence or absence of overlap in the confidence intervals noted. Lack of overlap of confidence intervals indicate heterogeneity; (2) The chi‐squared test for heterogeneity performed; (3) The I2 statistic obtained to describe the proportion of the variability due to heterogeneity; (4) Data analysed using a fixed effect model with odds ratio and 95% confidence intervals. If quantitative heterogeneity between trials was indicated, analysis using a random effect model are presented in addition to the fixed effect estimate.
Data were analysed using a fixed effect model with odds ratio and 95% confidence intervals. Where quantitative heterogeneity between trials was indicated, analysis using a random effects model is presented in addition to the fixed effect estimate.
Studies are grouped in the meta‐analysis plots according to whether the study recruited only patients with single vessel disease or a mix of patients with single or multiple vessel disease.
Where data were available, the following subgroups for analysis were proposed: (1) Vessels involved: single or multivessel cardiovascular disease; (2) Lesion characteristics: length, diameter of vessel, lesion category (American College of Cardiology/American Heart Association class: A, B1, B2 or C); (3) Surgical invention: 'standard' CABG or minimally invasive surgery; (4) Stent intervention: non drug‐eluting stents or drug‐eluting stents; (5) Co‐morbidity: diabetes.
Due to the lack of data specific to potential subgroups, only single and multivessel disease have been subject to separate analysis. Furthermore, even if data had been available subgroup analyses would only have been performed if judged to be clinically and methodologically appropriate.
Given the available trial reports we were also unable to separate out stable and unstable angina. However we would expect that as there was no stratification by presentation (with stable or unstable angina) at randomisation similar proportions of unstable patients are present in each arm of the trials. In practice most patients would have been semi‐elective in that they were eligible for both techniques and few patients were truly unstable in the setting of an acute myocardial infarction. Thus the distinction may be somewhat less relevant to this review than we anticipated when designing the review protocol.
A current version of the Review Manager/RevMan Analyses application was used for data analysis.
Results
Description of studies
Selection of included studies As a result of our electronic searches, 957 non‐duplicate records were identified, of which 48 records were selected for detailed examination for possible inclusion in the review. A total of 17 records were excluded after applying the inclusion/exclusion criteria, with the remaining 31 records attributed to a set of eight RCTs. The Grip study was identified by our examination of the bibliography of a systematic review (Biondi‐Zoccai 2003) identified in the electronic search, resulting in our total number of included studies raising to nine.
Two studies broadly fulfilled the inclusion criteria, but were not ultimately included in our review because we were unable to determine outcome data for patients receiving stents. In the AWESOME study surgery was compared to a number of interventional procedures ‐ of which 54% underwent procedures involving stents. During the development of our earlier health technology assessment (Hill 2004) we contacted the AWESOME triallists requesting information on those patients who received stents, but further information was not forthcoming. In AMIST all but one of the 48 people undergoing PTCA received a stent. As the study did not set out to compare elective (pre‐determined) use of stents with surgery, and we are (at this time) unable to determine specific data where use of stenting was planned before initiation of the PTCA procedure. Authors of the AMIST report kindly provided information on the circumstances around stenting of individuals allocated to the PTCA arm of the study, but declined to release specific outcome data on only those electively stented. It is possible that this information may be made available through individual patient data (IPD) analysis of AMIST and other trials, which we believe is to be conducted by the AMIST triallists.
Details of other records/studies excluded from the review are detailed in Characteristics of excluded studies.
Included studies Nine RCTs, involving a total of 3519 participants, are included in this review.
Our search identified peer‐reviewed publications for all but one of these trials. Information on Grip was only available as a single conference abstract. Three studies included patients with multivessel disease (ARTS; ERACI II; SOS), five included only people with single vessel disease (Cisowski; Diegeler; Drenth; Grip; SIMA) and one included a mix of vessel involvement (OCTOSTENT). The review made no restrictions on type of surgical technique employed in the included trials. Techniques varied between and within some trials, with four studies using mainly minimally invasive methods (Cisowski; Diegeler; Drenth; Grip), one internal mammary grafts (SIMA) and four employed an 'off‐pump' procedure, without the use of cardiopulmonary bypass (Diegeler; Drenth; Grip; OCTOSTENT). A variety of techniques were used within the remaining trials. All but two studies (Drenth; SIMA) appeared to exclude patients who had undergone previous revascularisation.
Reporting of outcomes extended beyond 1 year for ARTS; Drenth; ERACI II; SIMA; SOS (SOS reported median 2.0 years where as mean follow‐up was reported in ERACI II (18.5 months) and Drenth, at 'mid‐range' 3 and 4 years), but were restricted to 6 months for Cisowski; Diegeler; Grip. The Cisowski paper reported outcome data at 12 months, but for only half of those originally enrolled (Cisowski). As there was no clear explanation of why only a proportion of data where presented and with the possibility that the data where interim findings, we choose not to include these 12 month data in our analysis.
Further details are provided in Characteristics of included studies.
Risk of bias in included studies
Eight studies have been published as peer‐reviewed publications. As Grip was only available as a conference abstract, quality assessment was limited to the information provided that source.
The randomisation process was described and appeared adequate for five trials (ARTS; Diegeler; ERACI II; OCTOSTENT; SOS), these studies also appeared to use adequate allocation concealment. The four other included studies (Cisowski; Drenth; Grip; SIMA) did not provide details of the method of randomisation nor did they clearly describe steps taken to conceal allocation. There was little evidence of selection bias after allocation in the trials. An intention to treat analysis was presented for all studies and it was only Cisowski at 12 months that did not report on all but a few of those entering the trials. Potential for masking was limited in these studies due to the differing nature of the interventions involved. That being said, the OCTOSTENT trial implemented masking of treatment allocation from an independent committee of outcome assessors.
The results of the quality assessment are presented in Table 4.
4. Quality assessment.
| Study name | Adeq. Randomisation | Adeq. Concealment | Selection Bias | Adeq. Masking | Notes |
| ARTS | A | A | A | C | |
| Cisowski | B | B | A* | C* | |
| Diegeler | A | A | A | C | |
| Drenth | B | B | A | C | |
| ERACI II | A | A | A | C | |
| Grip | B | B | A | C | |
| OCTOSTENT | A | A | A | B/C ** | |
| SIMA | B | B | A | C | |
| SOS | A | A | A | C | |
| *Few exclusions up to 6 month (A); but 12 months data included only half of those allaocted to treatments ‐ suspect data are interium reporting, so not considered in quality assessment. | * Angiographic outcomes were assessed buy a committee 'not involved' in the study. This outcome may have been masked. ** An 'independant committee blinded to... allocation evaluated all events." Therefore it would appear that there may have been non‐blinded and blinded assessment of outcomes. |
Effects of interventions
Studies reported death, AMI, repeat revascularisation rate and a hierarchical composite of these outcomes in the form of major adverse cardiac events (MACE) or event free survival. Three studies, all focusing on patients with single vessel disease, reported binary restenosis rates ‐ the percentage of lesions with greater than 50% of luminal narrowing compared to diameter at completion of the procedure (Cisowski; Diegeler; Drenth).
Quality of life data were identified in five studies (ARTS; Grip; OCTOSTENT; SIMA; SOS) .
Data synthesis These syntheses report odds ratios using fixed effects comparisons. In the analyses, the studies are grouped according whether they focused on multiple or single vessel disease.
Composite cardiac event rate Event rates were significantly lower in the CABG arm at all time points analysed. For example, pooled effect estimates were odds ratio 0.43 (95% confidence interval 0.35 to 0.54) at 12 months and odds ratio 0.37 (95% confidence interval 0.29 to 0.48) at follow‐up within the 2.5 and 3.0 year category.
Death Although stents appeared to be favoured in terms of lower mortality, these differences were not statistically different.
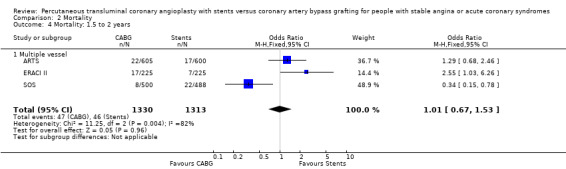
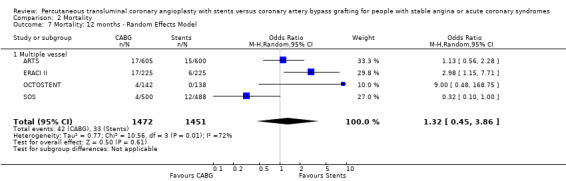
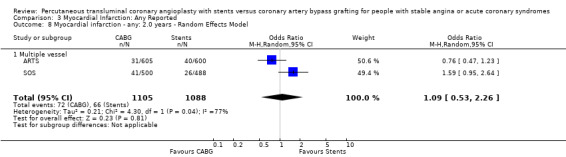
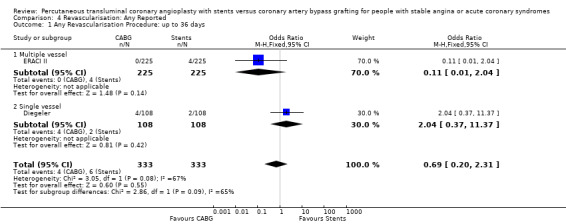
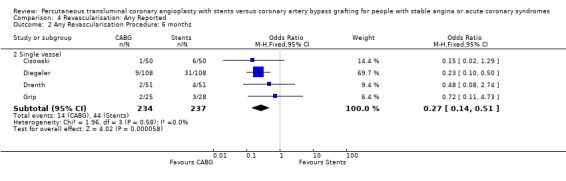
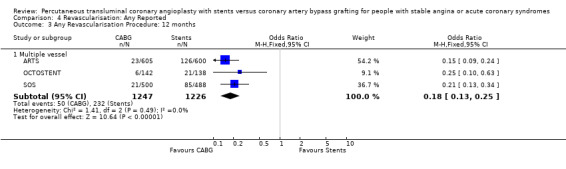
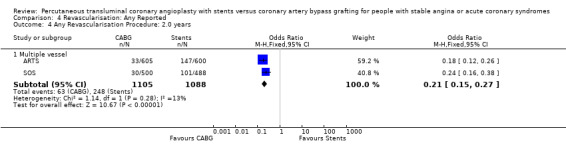
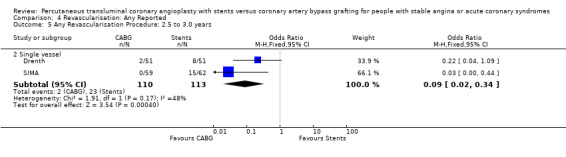
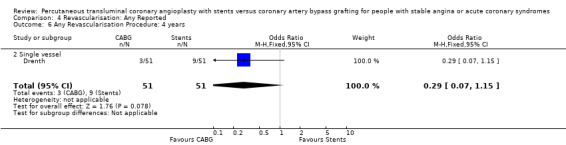
It is important to note that heterogeneity was apparent in analyses at 12 months and 1.5 to 2 years and that the SOS trial (which is included at these time points) experienced abnormally high rates of deaths in the stent arm. The SOS study reports eight cancer related deaths in the stent arm (as apposed to only one cancer death in the CABG group). The uneven distribution of non‐cardiac deaths in SOS would appear to contribute to it appearing to favour CABG and possibly to heterogeneity of these analyses. Within ERACI II a high number of deaths occurred in the CABG group early in the study (only 2 deaths in the stent group, but 13 in CABG). This high mortality in ERACI II could also be contributing to heterogeneity within the analyses of mortality. Application of random effects analysis, odds ratio 1.32 (95% confidence interval 0.45 to 3.86) at 12 months; odds ratio 1.04 (95% confidence interval 0.36 to 3.03) at 1.5 to 2 years, had little impact on effect size or statistical significance. AMI No significant difference observed, but there was moderate to high degree of heterogeneity at 36 days, 12 months and 2 years. Little difference was observed with application of random effects analysis. Trends within multiple and single vessel groups are discussed later. Revascularisation Repeat revascularisation procedures where less common in the CABG group within individual trials and the pooled analyses. This advantage was statistically significant in all but the early (up to 36 days) follow‐up period where only two trials are included and considerable heterogeneity is indicated. Multiple vessel disease trials are included in the analysis at 12 months and 2 years, resulting in odds ratio 0.18 (95% confidence interval 0.13 to 0.25) and odds ratio 0.21 (95% confidence interval 0.15 to 0.27), respectively. Analysis at 2.5 years included only single vessel disease studies, producing an odds ratio 0.09 (95% confidence interval 0.02 to 0.34).
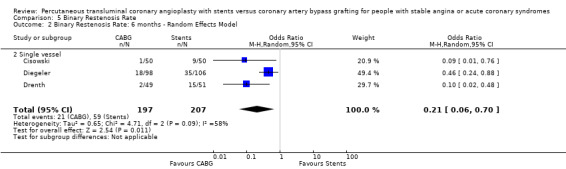
Binary restenosis Binary restenosis rate was reduced with CABG, odds ratio 0.29 (95% confidence interval 0.17 to 0.51) in the three single vessel trials reporting this outcome at 6 months; random effects odds ratio 0.21 (95% confidence interval 0.06 to 0.70).
Quality of life Generic quality of life (QoL) was reported in OCTOSTENT and SIMA , based on SF‐36 (Short Form 36), and ARTS and OCTOSTENT, based on EQ‐5D (EuroQol five Dimension). The SOS and SIMA trials report disease specific measures from the SAQ (Seattle Angina Questionnaire). The abstract for Grip referred to QoL data, but contained no clear information on the quality of life tool applied.
Improvements were recorded in studies comparing QoL immediately before and after treatment. Differences in generic QoL favouring of stents for SF‐36 (OCTOSTENT) and EQ‐5D (ARTS; OCTOSTENT) were reported in the immediate period, post intervention. However, few differences were discernible at follow‐up beyond 1 month (ARTS; OCTOSTENT; SIMA).
Overall, the available data do not permit conclusions to be drawn on between group differences in quality of life.
Discussion
Key findings The main findings of the meta‐analysis are that over the duration of follow‐up available from current RCTs, there is considerable benefit, in terms of reduction in repeat revascularisation rates, with CABG over stenting. These reductions were similar in single and multiple vessel disease studies and drove the differences seen in the composite cardiac event rates since revascularisation procedures account for approximately 75% of events and as a result also favour the CABG arms.
For the purposes of clinical decision making at the patient level, it is worth making further comment separately on observations for single and multivessel disease.
Multivessel disease The four studies (ARTS; ERACI II; OCTOSTENT; SOS) included in this meta‐analysis demonstrate some differences in mortality between CABG and stent groups, however these did not reach statistical significance but showed considerable heterogeneity. Similarly, the rates of AMI were also not significantly different and after combination were almost equal in the two pooled invention arms to 12 months. After 2 years the rates of AMI tend to favour surgery, but again this observation failed to reach statistical significance. The composite cardiac event endpoints favoured surgery at all time points compared to stenting, driven by the large differences in repeat revascularisation rates incurred by stenting. At 12 months the repeat revascularisation rates with CABG were approximately one fifth of the rates for stenting with an odds ratio 0.18; 95% confidence interval 0.13 to 0.25.
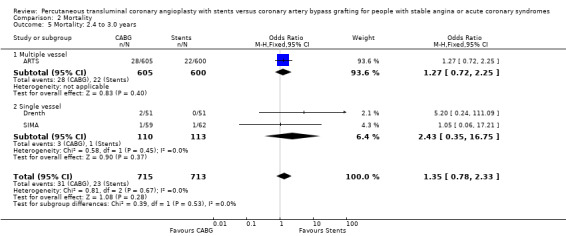
Single vessel disease In the four single vessel studies (Cisowski; Diegeler; Drenth; Grip; SIMA), given that mortality rates in the short term were generally low and the small number of total participants, the difference did not reach statistical significance, but would appear to favour stenting in contrast to the multivessel disease studies. The AMI and combined endpoint results closely mimic the respective results seen in the multivessel studies with CABG appearing to be better than stents in terms of composite event rate and repeat revascularisation at 6 months (odds ratio 0.27; confidence interval 0.14 to 0.51)
Clinical Interpretation The mortality rate trend seen in the single vessel studies favouring stenting was not surprising given that stenting is performed under local anaesthetic and does not entail the general anaesthesia required for surgery. Although larger studies are needed here to confirm this assumption, substantiation might have been achieved by meta‐analysis of single and multiple vessel studies, were it not for the mortality rates in the SOS trial. Among the multivessel trials at 12 months mortality differences vary, with the SOS trial having a higher mortality rate in the stent group (driven mainly by a large number of cancer deaths in the stent arm) contradictory to the results of ERACI II and OCTOSTENT with higher early mortality in the surgical groups, while ARTS had similar mortality rates for both arms.
Such results are difficult to translate into clinical practice, where registry data has shown higher peri‐procedural mortality for surgery compared to PTCA with or without stenting (Clark 2004; Srinivas 2002). The observations contained in this review are challenged further by the knowledge that large numbers of patients were screened compared to the number of patients finally enrolled and that some of these trials excluded patients with impaired left ventricular function often seen in clinical practice (ARTS; OCTOSTENT). Finally these trials are all relatively modest in size and are therefore not powered to look at mortality differences. To date, available trials report only medium term follow up (to 3 years), which may capture most of the repeat procedures needed with stent technology (i.e. restenosis in vessel treated by stenting) but do not have sufficient follow up to capture even 50% of venous graft failure which would require 10 years. Long‐term studies are however unlikely to be performed as the rate of technological advancement is likely to make the results of such trials obsolete (Bakhai 2000).
The mortality differences may yet have been different if mortality had been further split into cardiovascular and non‐cardiovascular causes, particularly since surgery is associated with higher rates of cerebrovascular events than percutaneous coronary intervention in the immediate post‐procedural phase (Pell 2001).
The results with respect to myocardial infarction are also worthy of discussion. While there would appear to be no significant difference in myocardial infarction rates at any time point, there is a trend in favour of CABG in those studies with longer follow up. This may be attributed to a number of factors: (1) the definitions for post‐procedural infarctions are likely to have had different enzyme rise thresholds for the two techniques, with a higher threshold set for CABG. This is because it has previously been considered 'not unreasonable' to allow for some cardiac enzyme leak from the heart due to cardiac manipulation, without defining this as an infarction. More sensitive markers such as troponins have not been universally used in these trials; (2) grafts which are invariably placed distally on native vessels may occlude with less myocardial impact than vessels opened proximally by stent procedures; (3) interventionists are more inclined to request cardiac enzymes on patients with post‐PCI chest pains then surgeons who are inclined to accept a degree of chest pain from patients due to the nature of the operative procedure; (4) finally, since the modest follow‐up duration is likely to capture stent failure more fully as opposed to graft failure, the limited follow‐up duration may introduce a bias against stenting.
Limitations This review has a number of limitations which are related to the limits of the trial data currently available: (1) patients entered into such studies had to be suitable for either intervention and were not typical of all patients seen by cardiologists or cardiothoracic surgeons; (2) practice changed over the periods of the trials and so results from different procedures or devices may not be the same; for example the frequent use of Glycoprotein IIB/IIIA has in more recent practice reduced early stent thrombosis and the amount of Ischaemic enzyme release peri procedurally which would favour the stent arm of trials in which such agents are used ‐ similarly, the increasing use of 'off pump' surgery may have reduced neurological defeats; (3) we could not consider subgroups of patients in the current meta‐analysis. There is potential for within and between study heterogeneity related to the patients entering the study. We understand that an IPD study is currently underway (SOS Investigators, Personal communication, January 2003); (4) analysis of 'other' adverse events (for example, neurological complications) were not completed as these were not commonly or consistently reported.
For the these reasons there are several factors which bias outcomes for and against each revascularisation technique compared to contemporary practice. There may therefore be limits to the generalisability of our review, but nonetheless it presents an up to date appraisal of available data. Future directions Stenting and surgical practice are developing and so, review of these evolving interventions should continue. Three RCTs comparing CABG to drug‐eluting stents (DES) are underway or pending analysis. (1) CARDia study included a DES subset and is expected to report within 2004; (2) FREEDOM which is recruiting patients with diabetes and multiple vessel disease, all receive DES; (3) SYNTAX trial (which is in the start‐up phase) will utilise DES and stratify patients by number of vessels disease, diabetes and left main vessel disease. We enquired as to the progress of release of CARDia data and contacted FREEDOM triallists, but did not receive replies. Surgical practice is also developing with the use of less invasive techniques and 'off‐pump' procedures.
As in practice, most patients with single vessel disease receive stents and those with more than two vessels involved are allocated to surgery, the studies of patients with multivessel disease (especially two vessel disease) are therefore of particular interest, as they focus on areas where there is the greatest margin for change in practice.
It is generally excepted that in the short term, traditional stenting may be less expensive, more patient friendly and incur lower peri‐procedural mortality and morbidity, but in the longer term, CABG may be better value with reduced need for repeat revascularisations. Since differences in major clinical outcomes such as mortality or quality of life are not apparent with the current evidence base, this review demonstrates the need for consideration of larger trials with long‐term data to fully populate clinical and economic data to allow formal lifetime cost‐effectiveness models to determine whether resources should be put preferentially into stenting or CABG. Since such a rigorous scientific approach is likely to require large‐scale nationally funded investment, we may never see appropriately powered and designed clinical trials answering the question ‐ for whom is surgery and for whom is stenting a preferable choice?
Such paucity of data may have important implications as physicians, patients, policy makers and device manufacturers, who driven by their own personal enthusiasm or biases, can make choices unrestricted by the evidence base.
Authors' conclusions
Implications for practice.
Considerably more data is needed to make firm long term conclusions on the implications for practice, but in the short to medium term, CABG has far less repeat revascularisation procedures than PTCA with stents currently in common clinical use.
Implications for research.
Re‐evaluation of these technologies will be required as the development of new surgical techniques and stent designs is ongoing. Although longer‐term follow‐up may be useful, interventions are changing to such an extent that long‐term data risks being inapplicable to what has become the subject of current research and/or practice. Future trials should recruit more realistic patient groupings, as the population selected for inclusion in the current review were prone to bias. In common with many clinical trials in this area, selection tended to focus on patients with generally less co‐morbidities and with better left ventricular function than the overall population presenting for revascularisation in the real world setting. Reviews could undertake IPD analyses to examine subgroups.
What's new
| Date | Event | Description |
|---|---|---|
| 12 May 2017 | Amended | This review is no longer maintained. A review with a similar title (Coronary artery bypass grafting surgery versus percutaneous coronary intervention for coronary artery disease) has been registered to provide up‐to‐date information. |
History
Protocol first published: Issue 1, 2004 Review first published: Issue 1, 2005
| Date | Event | Description |
|---|---|---|
| 13 December 2012 | Review declared as stable | Review team unable to update and Cochrane Heart Group was unable to find new review team. This review requires a new team to take this work forward. |
| 9 September 2008 | Amended | Converted to new review format. |
| 1 November 2004 | New citation required and conclusions have changed | Substantive amendment |
Notes
This review 'topic' has been included in work being carried out as part of a Health Technology Assessment commissioned by the National Institute for Clinical Excellence in England and Wales.
Details of this research is available via www.nice.org.uk and www.ncchta.org
This review is no longer maintained. A review with a similar title (Coronary artery bypass grafting surgery versus percutaneous coronary intervention for coronary artery disease) has been registered to provide up‐to‐date information.
Acknowledgements
We are grateful to the staff of the Heart CRG for co‐ordination (T Moore, KJ Wornell) and information specialist support (MA Burke) and to those triallists who provided us with information about their studies. We also wish to acknowledge the support and contribution of colleagues involved in the larger health technology assessment project (completed in 2003, Hill 2004): A Bagust, A Haycox, R Mujica Mota, DH Roberts, PR Williamson (for statistical advice) as well as experts and NICE appraisal consultees who commented on drafts of the NICE appraisal report.
Data and analyses
Comparison 1. Event Rate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2 Event Rate: 6 months | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.2 Single vessel | 3 | 418 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.18, 0.55] |
| 3 Event Rate: 12 months | 4 | 2575 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.35, 0.54] |
| 3.1 Multiple vessel | 3 | 2473 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.35, 0.55] |
| 3.2 Single vessel | 1 | 102 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.08, 0.93] |
| 4 Event Rate: 2 years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Multiple vessel | 1 | 1205 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.31, 0.54] |
| 5 Event Rate: 2.5 to 3.0 years | 3 | 1428 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.29, 0.48] |
| 5.1 Multiple vessel | 1 | 1205 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.30, 0.52] |
| 5.2 Single vessel | 2 | 223 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.11, 0.53] |
| 6 Event Rate: 4 years | 1 | 102 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.09, 0.87] |
| 6.2 Single vessel | 1 | 102 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.09, 0.87] |
1.2. Analysis.
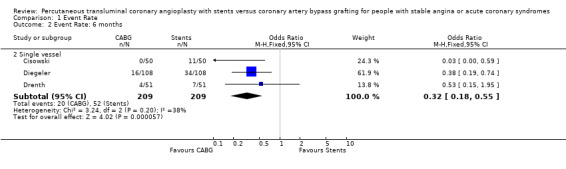
Comparison 1 Event Rate, Outcome 2 Event Rate: 6 months.
1.3. Analysis.
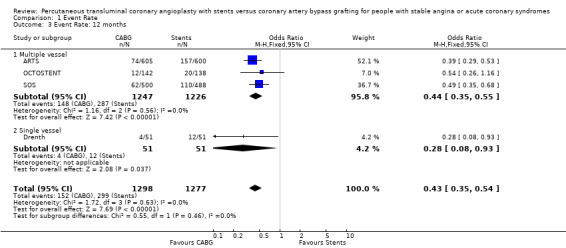
Comparison 1 Event Rate, Outcome 3 Event Rate: 12 months.
1.4. Analysis.
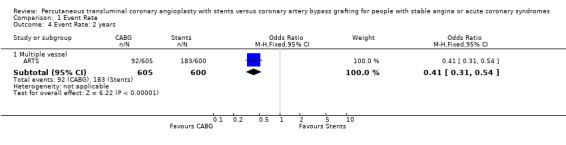
Comparison 1 Event Rate, Outcome 4 Event Rate: 2 years.
1.5. Analysis.
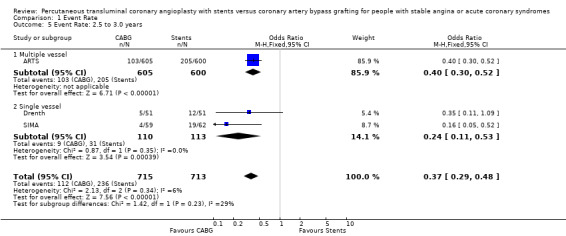
Comparison 1 Event Rate, Outcome 5 Event Rate: 2.5 to 3.0 years.
1.6. Analysis.
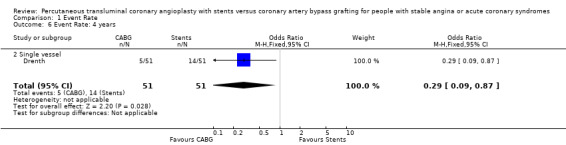
Comparison 1 Event Rate, Outcome 6 Event Rate: 4 years.
Comparison 2. Mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality: up to 36 days | 3 | 673 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.16 [1.38, 12.56] |
| 1.1 Multiple vessel | 1 | 450 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.84 [1.52, 30.66] |
| 1.2 Single vessel | 2 | 223 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.25, 9.41] |
| 2 Mortality: 6 months | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.2 Single vessel | 4 | 471 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.08 [0.51, 8.40] |
| 3 Mortality: 12 months | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Multiple vessel | 4 | 2923 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.80, 2.00] |
| 4 Mortality: 1.5 to 2 years | 3 | 2643 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.67, 1.53] |
| 4.1 Multiple vessel | 3 | 2643 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.67, 1.53] |
| 5 Mortality: 2.4 to 3.0 years | 3 | 1428 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.78, 2.33] |
| 5.1 Multiple vessel | 1 | 1205 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.72, 2.25] |
| 5.2 Single vessel | 2 | 223 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.43 [0.35, 16.75] |
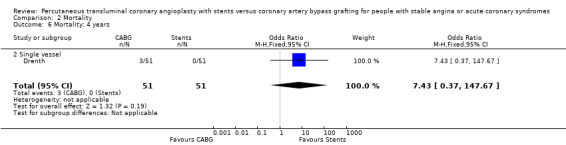
| 6 Mortality: 4 years | 1 | 102 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.43 [0.37, 147.67] |
| 6.2 Single vessel | 1 | 102 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.43 [0.37, 147.67] |
| 7 Mortality: 12 months ‐ Random Effects Model | 4 | 2923 | Odds Ratio (M‐H, Random, 95% CI) | 1.32 [0.45, 3.86] |
| 7.1 Multiple vessel | 4 | 2923 | Odds Ratio (M‐H, Random, 95% CI) | 1.32 [0.45, 3.86] |
| 8 Mortality: 1.5 to 2 years ‐ Random Effects Model | 3 | 2643 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.36, 3.03] |
| 8.1 Multiple vessel | 3 | 2643 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.36, 3.03] |
2.1. Analysis.
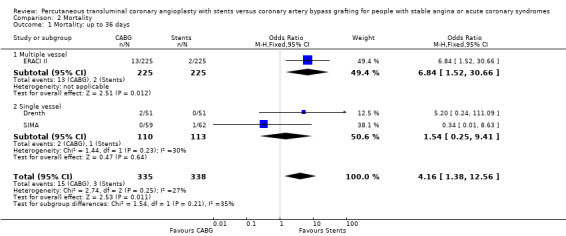
Comparison 2 Mortality, Outcome 1 Mortality: up to 36 days.
2.2. Analysis.
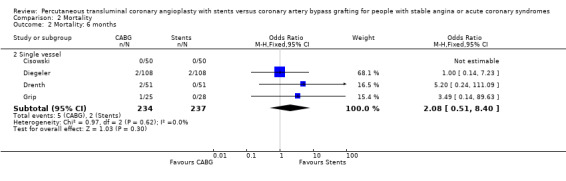
Comparison 2 Mortality, Outcome 2 Mortality: 6 months.
2.3. Analysis.
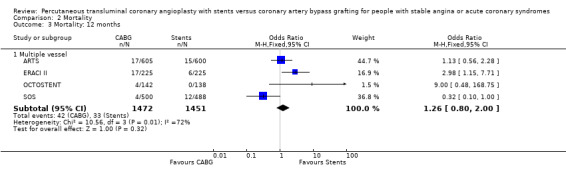
Comparison 2 Mortality, Outcome 3 Mortality: 12 months.
2.4. Analysis.
Comparison 2 Mortality, Outcome 4 Mortality: 1.5 to 2 years.
2.5. Analysis.
Comparison 2 Mortality, Outcome 5 Mortality: 2.4 to 3.0 years.
2.6. Analysis.
Comparison 2 Mortality, Outcome 6 Mortality: 4 years.
2.7. Analysis.
Comparison 2 Mortality, Outcome 7 Mortality: 12 months ‐ Random Effects Model.
2.8. Analysis.
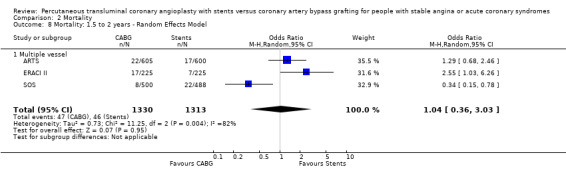
Comparison 2 Mortality, Outcome 8 Mortality: 1.5 to 2 years ‐ Random Effects Model.
Comparison 3. Myocardial Infarction: Any Reported.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Myocardial infarction ‐ any up to 36 days | 3 | 787 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.83 [1.17, 6.81] |
| 1.1 Multiple vessel | 1 | 450 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.84 [1.52, 30.66] |
| 1.2 Single vessel | 2 | 337 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.37, 4.14] |
| 2 Myocardial infarction ‐ any: 6 months | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.2 Single vessel | 4 | 471 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.29, 2.14] |
| 3 Myocardial infarction ‐ any: 12 months | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Multiple vessel | 3 | 2473 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.76, 1.53] |
| 4 Myocardial infarction ‐ any: 2.0 years | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Multiple vessel | 2 | 2193 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.76, 1.52] |
| 5 Myocardial infarction ‐ any: Typically 2.5 to 3.0 years | 3 | 1428 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.44, 1.07] |
| 5.1 Multiple vessel | 1 | 1205 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.47, 1.19] |
| 5.2 Single vessel | 2 | 223 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.10, 1.42] |
| 6 Myocardial infarction ‐ any: 4 years | 1 | 102 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.02, 1.63] |
| 6.2 Single vessel | 1 | 102 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.02, 1.63] |
| 7 Myocardial infarction ‐ any: 12 months ‐ Random Effects Model | 3 | 2473 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.65, 1.88] |
| 7.1 Multiple vessel | 3 | 2473 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.65, 1.88] |
| 8 Myocardial infarction ‐ any: 2.0 years ‐ Random Effects Model | 2 | 2193 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.53, 2.26] |
| 8.1 Multiple vessel | 2 | 2193 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.53, 2.26] |
3.1. Analysis.
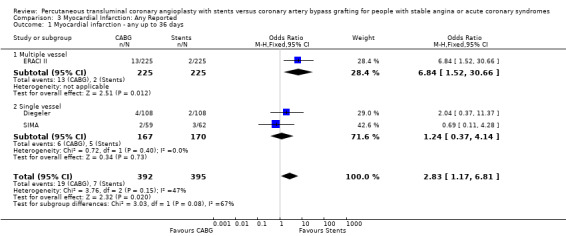
Comparison 3 Myocardial Infarction: Any Reported, Outcome 1 Myocardial infarction ‐ any up to 36 days.
3.2. Analysis.
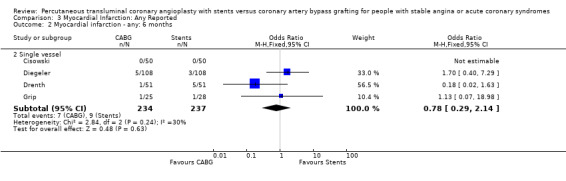
Comparison 3 Myocardial Infarction: Any Reported, Outcome 2 Myocardial infarction ‐ any: 6 months.
3.3. Analysis.
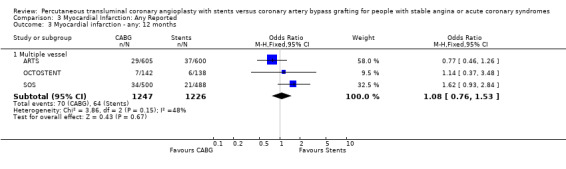
Comparison 3 Myocardial Infarction: Any Reported, Outcome 3 Myocardial infarction ‐ any: 12 months.
3.4. Analysis.
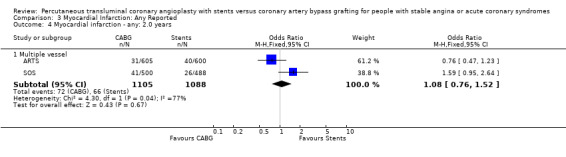
Comparison 3 Myocardial Infarction: Any Reported, Outcome 4 Myocardial infarction ‐ any: 2.0 years.
3.5. Analysis.
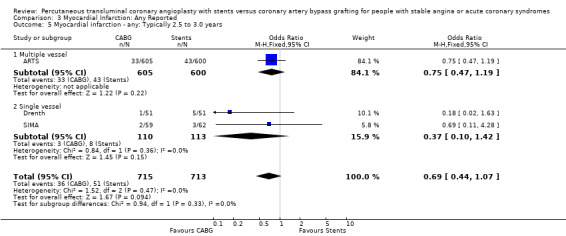
Comparison 3 Myocardial Infarction: Any Reported, Outcome 5 Myocardial infarction ‐ any: Typically 2.5 to 3.0 years.
3.6. Analysis.
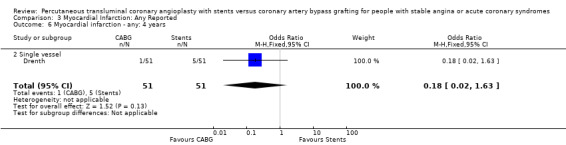
Comparison 3 Myocardial Infarction: Any Reported, Outcome 6 Myocardial infarction ‐ any: 4 years.
3.7. Analysis.
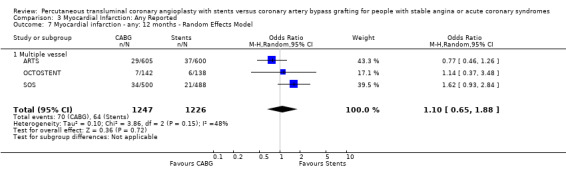
Comparison 3 Myocardial Infarction: Any Reported, Outcome 7 Myocardial infarction ‐ any: 12 months ‐ Random Effects Model.
3.8. Analysis.
Comparison 3 Myocardial Infarction: Any Reported, Outcome 8 Myocardial infarction ‐ any: 2.0 years ‐ Random Effects Model.
Comparison 4. Revascularisation: Any Reported.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any Revascularisation Procedure: up to 36 days | 2 | 666 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.20, 2.31] |
| 1.1 Multiple vessel | 1 | 450 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.04] |
| 1.2 Single vessel | 1 | 216 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.37, 11.37] |
| 2 Any Revascularisation Procedure: 6 months | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.2 Single vessel | 4 | 471 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.14, 0.51] |
| 3 Any Revascularisation Procedure: 12 months | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Multiple vessel | 3 | 2473 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.13, 0.25] |
| 4 Any Revascularisation Procedure: 2.0 years | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Multiple vessel | 2 | 2193 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.15, 0.27] |
| 5 Any Revascularisation Procedure: 2.5 to 3.0 years | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.2 Single vessel | 2 | 223 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.02, 0.34] |
| 6 Any Revascularisation Procedure: 4 years | 1 | 102 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.07, 1.15] |
| 6.2 Single vessel | 1 | 102 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.07, 1.15] |
4.1. Analysis.
Comparison 4 Revascularisation: Any Reported, Outcome 1 Any Revascularisation Procedure: up to 36 days.
4.2. Analysis.
Comparison 4 Revascularisation: Any Reported, Outcome 2 Any Revascularisation Procedure: 6 months.
4.3. Analysis.
Comparison 4 Revascularisation: Any Reported, Outcome 3 Any Revascularisation Procedure: 12 months.
4.4. Analysis.
Comparison 4 Revascularisation: Any Reported, Outcome 4 Any Revascularisation Procedure: 2.0 years.
4.5. Analysis.
Comparison 4 Revascularisation: Any Reported, Outcome 5 Any Revascularisation Procedure: 2.5 to 3.0 years.
4.6. Analysis.
Comparison 4 Revascularisation: Any Reported, Outcome 6 Any Revascularisation Procedure: 4 years.
Comparison 5. Binary Restenosis Rate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Binary Restenosis Rate: 6 months | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.2 Single vessel | 3 | 404 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.17, 0.51] |
| 2 Binary Restenosis Rate: 6 months ‐ Random Effects Model | 3 | 404 | Odds Ratio (M‐H, Random, 95% CI) | 0.21 [0.06, 0.70] |
| 2.2 Single vessel | 3 | 404 | Odds Ratio (M‐H, Random, 95% CI) | 0.21 [0.06, 0.70] |
5.1. Analysis.
Comparison 5 Binary Restenosis Rate, Outcome 1 Binary Restenosis Rate: 6 months.
5.2. Analysis.
Comparison 5 Binary Restenosis Rate, Outcome 2 Binary Restenosis Rate: 6 months ‐ Random Effects Model.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ARTS.
| Methods | cerebrovascular RCT Multicentre, International | |
| Participants | MVD 2 or more de novo lesion in different major arteries Total occlusion < 1month Lesion suitable for CABG or stenting | |
| Interventions | Palmaz‐Schatz Crown/Cross flex (Cordis) ‐ Conventional CABG Stents: 600 CABG: 605 | |
| Outcomes | Absence of major MACE for 1 year; angina status; medications; costs and cost‐effectiveness; QoL; combined end point of death, MI or stroke, death, MI, stroke, revascularisation procedures at 1 year | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Cisowski.
| Methods | RCT Single centre, Poland | |
| Participants | 1VD ACC/AHA A or B lesion in proximal LAD Angina CCS II or higher Lesion diameter 3 mm or greater/length 20mm or greater Lesion suitable for CABG or Stenting | |
| Interventions | Tristar, Tera, Penta (Guidant) ‐ MIDCAB (E‐ACAB) Stent: 50 CABG: 50 | |
| Outcomes | MACE (Death, MI, reoccurrence of angina pectoris); TVR; costs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Diegeler.
| Methods | RCT Multicentre, Germany | |
| Participants | 1VD Lesion =75% stenosis in proximal LAD or between origin of left circumflex and 1st septal branch | |
| Interventions | Various stents used ‐ MIDCAB Stent: 110 CABG: 110 | |
| Outcomes | Freedom from MACE within 6 months; cardiac death; MI; TVR; clinical status (CCS); need for antianginal drugs at 6 months; adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Drenth.
| Methods | RCT Single centre, Netherlands | |
| Participants | 1VD Angina II Lesion (Grade B2 or C) of proximal LAD Suitable for CABG or stenting | |
| Interventions | Stent type not described ‐ MICAB Stent: 51 CABG: 51 | |
| Outcomes | Freedom from MACCE at 3 years; angiographic outcome at 6 months; angina class (CCS); antianginal medication; clinical events MACCE without revascularisation 6 months; clinical outcome | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
ERACI II.
| Methods | RCT Multicentre, Argentina | |
| Participants | MVD Angina CSS III‐IV; no angina but large area of heart at risk; unstable =1 vessel to be treated Lesion>3.0mm | |
| Interventions | Gianturco Robin II (Cook) Primary device ‐ Conventional CABG Stents: 225 CABG: 225 | |
| Outcomes | MACE | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Grip.
| Methods | RCT Sweden | |
| Participants | 1VD engaging LAD Stable or unstable angina | |
| Interventions | Stent type not described ‐ MIDCAB Stent: 28 CABG: 25 | |
| Outcomes | QoL; Clinical events at 6 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
OCTOSTENT.
| Methods | RCT Multicentre, Europe | |
| Participants | M/1VD Moderate LV function CABG or stenting to be considered feasible | |
| Interventions | Stent type not described ‐ OPCAB Stent: 138 CABG: 142 | |
| Outcomes | Absence MACCE for 1 year (Death, stroke, TIA, reversible ischaemic neurological deficits, non fatal MI, repeat revascularisation by PCI or surgery) Angina status Medications Costs and cost‐effectiveness QOL Combined end point of death, MI or stroke, death, MI, stroke, revascularisation procedures at 1 year | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
SIMA.
| Methods | RCT Multicentre, Europe | |
| Participants | 1VD Symptomatic or silent ischaemia 1 LAD lesion Ejection fraction >45% Vessel >3.0mm | |
| Interventions | Any CE marked, but Palmaz‐Schatz recommended ‐ Conventional CABG/MIDCAB (10% of surgical procedures) Stent: 62 treated CABG: 59 treated | |
| Outcomes | Event free survival; angina functional class; exercise tolerance; antianginal medication; QoL; post procedural drug regimen | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
SOS.
| Methods | RCT Multicentre, International | |
| Participants | MVD Symptomatic 1 or more vessel suitable for stenting | |
| Interventions | No restriction on type of stent or surgical technique (3% of procedures OPCAB) Stent: 488 CABG: 500 | |
| Outcomes | Rate of repeat revascularisation; death Q‐wave MI; all‐cause mortality; angina (CCS); cardiac medication; LVF | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
MVD: multiple vessel disease; 1VD: single vessel disease; MIDCAB: minimally invasive direct coronary artery bypass (off‐pump proceedure); E‐ACAB: endoscopic atraumatic coronary artery bypass grafting; OPCAB: off‐pump coronary artery bypass; MACE: major adverse cardiac event; MACCE: major adverse cardiac or cerebrovascular event; MI: myocardial infarction; TVR: target vessel revascularisation; QoL: Quality of LIfe; CCS: Canadian Cardiovascular Society classification; LVF: Left ventricular ejection fraction
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| AMIST | PTCA versus CABG, unable to determine outcome data for those electively stented |
| AWESOME | PCI versus CABG, unable to determine outcome data for those stented. |
| Berger 2001 | PTCA versus CABG, no stent data (BARI) |
| Biondi‐Zoccai 2003 | Systematic review |
| Fischman 1994 | Stent versus PTCA study |
| Iakovou 2002 | Non RCT |
| Kapelak 1998 | Non RCT |
| Kim 2000 | Non RCT |
| Mathew 2000 | Non RCT |
| Murtaza 2001 | Correspondence about ARTS (not ARTS authors, no data) |
| Pohl 2002 | Intervention involved application of both stenting and surgery procedures to individuals |
| Ryan 2001 | Editorial about ERACI II |
| Serruys 1994 | Stent versus PTCA study |
| Shirai 2004 | Non RCT |
| Taggart 2001 | Correspondence about ARTS (not ARTS authors, no data) |
| Weintraub 1997 | Non RCT |
| Yock 2000 | Health Economics study (using PTCA versus CABG trial, BARI) |
Characteristics of ongoing studies [ordered by study ID]
CARDia.
| Trial name or title | CARDia |
| Methods | |
| Participants | 600 patients with diabetes, suitbale for revascularistaion by stenting or CABG, UK and Ireland |
| Interventions | PCI (with randomisation between non DES and Sirolimus‐elutng stents) CABG (few protocol restrictions on operative techniques) |
| Outcomes | Primary end point composite of death, MI, and cerebrovascular accident at 1 year. Data on cost effectiveness, QoL, neurocognitive function also collected. 30 days, 6 months, 1, 2, and 5 years |
| Starting date | Start date: 01/05/2002 End date: 31/01/2004 |
| Contact information | Dr. Akhil Kapur a.kapur@ic.ac.uk Department of Cardiology National Heart and Lung Institute at Hammersmith Hospital Imperial College School of Medicine Du Cane Road London W12 0HS, UK. Tel: +44 (0) 20 8383 1000 bleep 9136 Fax: +44 (0) 20 3383 2120 |
| Notes |
FREEDOM.
| Trial name or title | FREEDOM |
| Methods | |
| Participants | 2400 patients with diabetes and multi‐vessel coronary disease |
| Interventions | DES CABG |
| Outcomes | Not known |
| Starting date | 2004, 5 year project |
| Contact information | Dr. Valentin Fuster valentin.fuster@msnyuhealth.org The Zena and Michael A. Wiener Cardiovascular Institute The Mount Sinai Medical Center One Gustave L. Levy Place, Box 1030 New York , NY, USA 10029‐6574 Tel: 212‐241‐7911 |
| Notes |
SYNTAX.
| Trial name or title | SYNTAX |
| Methods | |
| Participants | Patients stratified by vessels disease, diabetes and left main vessel disease) |
| Interventions | DES CABG |
| Outcomes | MACCE at 1 year (all cuase mortality, MI, CVA, Repeat revascularistion) |
| Starting date | 2004, in start‐up phase |
| Contact information | To follow. Basic information presented 2004 at: http://www.europcr.com/ |
| Notes |
SYNTAX ‐ presented by Serruys, PCR 2004?
Contributions of authors
All reviewers listed below contributed to the development of the protocol, data selection and analysis. All reviewers took part in the editing and production of the completed review.
Specific contributions of reviewers: AB lead reviewer, clinical advisor RH co‐ordination, data input, analysis and display YD search strategy development, information management RD methodological advisor, review management TW review management, data interpretation
Sources of support
Internal sources
No sources of support supplied
External sources
NCCHTA Project Ref: 02/16/01 (NICE appraisal of Coronary Artery Stents), UK.
Declarations of interest
Ameet Bakhai has worked on projects involving Abbott Vascular Devices, Bristol Myers Squibb and Merck & Co. USA. Through his appointment with the Harvard Clinical Research Institute (Boston, USA), he was a member of an academic department which is involved in collaborations with Boston Scientific and Guidant Corporation.
No further relevant competing interests exist.
Edited (no change to conclusions)
References
References to studies included in this review
ARTS {published data only}
- Abizaid A, Costa MA, Centemero M, Abizaid AS, Legrand VM, Limet RV et al (Group ARTS). Clinical and economic impact of diabetes mellitus on percutaneous and surgical treatment of multivessel coronary disease patients: insights from the Arterial Revascularization Therapy Study (ARTS) trial. Circulation 2001;104(5):533‐8. [DOI] [PubMed] [Google Scholar]
- Anonymous. Arterial Revascularization Therapies Study (ARTS). Indian Heart Journal 2001;53(2):239. [PubMed] [Google Scholar]
- Ekstein S, Elami A, Merin G, Gotsman MS, Lotan C. Balloon angioplasty versus bypass grafting in the era of coronary stenting. Israel Medical Association Journal 2002;4(8):583‐9. [PubMed] [Google Scholar]
- Farquhar D. Bypass surgery or stenting for multivessel coronary artery disease?. Canadian Medical Association Journal 2001;164(12):1742. [PMC free article] [PubMed] [Google Scholar]
- Gruberg L, Milo S, Ben TM, Lotan C, Merin G, Braun S, et al. Comparison of bypass surgery and stenting for the treatment of multivessel disease: results from the ARTS trial in Israel. Israel Medical Association Journal 2003;5(8):539‐42. [PubMed] [Google Scholar]
- Legrand VM, Serruys PW, Unger F, Hout BA, Vrolix MC, Fransen GM et al (Investigators ARTS). Three‐year outcome after coronary stenting versus bypass surgery for the treatment of multivessel disease. Circulation 2004;109(9):1114‐20. [DOI] [PubMed] [Google Scholar]
- Serruys PW, Unger F, Sousa JE, Jatene A, Bonnier HJ, Schonberger JP et al (Group ARTS). Comparison of coronary‐artery bypass surgery and stenting for the treatment of multivessel disease. New England Journal of Medicine 2001;344(15):1117‐24. [DOI] [PubMed] [Google Scholar]
- Serruys PW, Unger F, Hout BA, Brand MJB, Herwerden LA, Es GA, Morel MA, Bonnier JJRM, Colombo A, Morice MC, Simon R, Wijns W, Kremer D, Mohr F, Petterson G, Santoli C, Breeman A, Vandormael M, Firth BG, Madonna O, Marshall PR, Hugenholtz PG. The ARTS (Arterial Revascularization Therapies Study): Background, goals and methods. International Journal of Cardiovascular Interventions 1999;2(1):41‐50. [DOI] [PubMed] [Google Scholar]
- Serruys PW, Unger F, Hout BA, Brand MJ, Herwerden LA, Es GA, et al. The ARTS study (Arterial Revascularization Therapies Study). Seminars in Interventional Cardiology 1999;4(4):209‐19. [DOI] [PubMed] [Google Scholar]
- Unger F, Serruys PW, Yacoub MH, Ilsley C, Paulsen PK, Nielsen TT, et al. Revascularization in multivessel disease: comparison between two‐year outcomes of coronary bypass surgery and stenting. Journal of Thoracic and Cardiovascular Surgery 2003;125(4):809‐20. [DOI] [PubMed] [Google Scholar]
- Feyter PJ, Serruys PW, Unger F, Beyar R, V V, Milo S, Simon R, et al. Bypass surgery versus stenting for the treatment of multivessel disease in patients with unstable angina compared with stable angina. Circulation 2002;105(20):2367‐72. [DOI] [PubMed] [Google Scholar]
- Brand MJ, Rensing BJ, Morel MA, Foley DP, V V, Breeman A, et al. The effect of completeness of revascularization on event‐free survival at one year in the ARTS trial. Journal of the American College of Cardiology 2002;39:559‐64. [DOI] [PubMed] [Google Scholar]
Cisowski {published data only}
- Cisowski M, Drzewiecki J, Drzewiecka‐Gerber A, Jaklik A, Kruczak W, Szczeklik M, et al. Primary stenting versus MIDCAB: preliminary report‐comparision of two methods of revascularization in single left anterior descending coronary artery stenosis. Annals of Thoracic Surgery 2002;74(4):1334‐9. [DOI] [PubMed] [Google Scholar]
Diegeler {published data only}
- Diegeler A, Spyrantis N, Matin M, Falk V, Hambrecht R, Autschbach R, et al. The revival of surgical treatment for isolated proximal high grade LAD lesions by minimally invasive coronary artery bypass grafting. European Journal of Cardiothoracic Surgery 2000;17(5):501‐4. [DOI] [PubMed] [Google Scholar]
- Diegeler A, Thiele H, Falk V, Hambrecht R, Spyrantis N, Sick P, et al. Comparison of stenting with minimally invasive bypass surgery for stenosis of the left anterior descending coronary artery. New England Journal of Medicine 2002;347(8):561‐6. [DOI] [PubMed] [Google Scholar]
Drenth {published data only}
- Drenth DJ, Veeger NJ, Grandjean JG, Mariani MA, Boven AJ, Boonstra PW. Isolated high‐grade lesion of the proximal LAD: a stent or off‐pump LIMA?. European Journal of Cardio‐Thoracic Surgery 2004;25(4):567‐71. [DOI] [PubMed] [Google Scholar]
- Drenth DJ, Veeger NJ, Winter JB, Grandjean JG, Mariani MA, Boven van AJ, et al. A prospective randomized trial comparing stenting with off‐pump coronary surgery for high‐grade stenosis in the proximal left anterior descending coronary artery: three‐year follow‐up. Journal of the American College of Cardiology 2002;40(11):1955‐60. [DOI] [PubMed] [Google Scholar]
- Drenth DJ, Winter JB, Veeger NJ, Monnink SH, Boven AJ, Grandjean JG, et al. Minimally invasive coronary artery bypass grafting versus percutaneous transluminal coronary angioplasty with stenting in isolated high‐grade stenosis of the proximal left anterior descending coronary artery: six months' angiographic and clinical follow‐up of a prospective randomized study. Journal of Thoracic and Cardiovascular Surgery 2002;124(1):130‐5. [DOI] [PubMed] [Google Scholar]
ERACI II {published data only}
- Brennan FJ, Palacios IF, Rodriguez A. A randomized trial of multivessel stent versus coronary bypass. Journal of the American College of Cardiology 2001;38(1):286‐7. [DOI] [PubMed] [Google Scholar]
- Rodriguez A. Argentine randomized study: coronary angioplasty with stenting vs coronary artery bypass surgery in patients with multiple vessel disease (ERACI II): 30‐day and long‐term follow‐up results. American Heart Journal 2000;139(2 (Pt 1)):362‐3. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Bernardi V, Navia J. Erratum: Argentine randomized study: Coronary angioplasty with stenting versus coronary bypass surgery in patients with multiple‐vessel disease (ERACII): 30‐Day and one‐year follow‐up results. Journal of the American College of Cardiology 2001;37(3):973. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Bernardi V, Navia J, Baldi J, Grinfeld L, Martinez J, et al. Argentine randomized study: Coronary angioplasty with stenting versus coronary bypass surgery in patients with multiple‐vessel disease (ERACI II): 30‐day and one‐year follow‐up results. Journal of the American College of Cardiology 2001;37(1):51‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Rodriguez AM, Baldi J, Navia J, Delacasa A, Vogel D, et al. Coronary stenting versus coronary bypass surgery in patients with multiple vessel disease and significant proximal LAD stenosis: results from the ERACI II study. Heart 2003;89(2):184‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grip {published data only}
- Grip L, Wahrborg P, Odell A, Albertsson P, Berglin E, Brandrup‐Wognesen G, et al. Coronary artery bypass beating heart surgery with LIMA graft, versus coronary angioplasty with stent for patients with single left anterior descending artery ‐ a pilot study. European Heart Journal 2001;22 (Suppl):597. [Google Scholar]
OCTOSTENT {published data only}
- Eefting F, Nathoe H, van DD, Jansen E, Lahpor J, Stella P, et al. Randomized comparison between stenting and off‐pump bypass surgery in patients referred for angioplasty. Circulation 2003;108(23):2870‐6. [DOI] [PubMed] [Google Scholar]
- Brand, Nierich AP, Eefting FD, Buskens E, Nathoe HM, Jansen EW. The Octopus Study: rationale and design of two randomized trials on medical effectiveness, safety, and cost‐effectiveness of bypass surgery on the beating heart. Controlled Clinical Trials 2000;21(6):595‐609. [DOI] [PubMed] [Google Scholar]
SIMA {published data only}
- Goy J‐J, Kaufmann U, Goy‐Eggenberger D, Garachemani A, Hurni M, Carrel T, et al. A prospective randomized trial comparing stenting to internal mammary artery grafting for proximal, isolated de novo left anterior coronary artery stenosis: The SIMA trial. Mayo Clinic Proceedings 2000;75(11):1116‐23. [DOI] [PubMed] [Google Scholar]
SOS {published data only}
- SoS Investigators. Coronary artery bypass surgery versus percutaneous coronary intervention with stent implantation in patients with multivessel coronary artery disease (the Stent or Surgery trial): a randomised controlled trial.[comment]. Lancet 2002;360(9338):965‐70. [DOI] [PubMed] [Google Scholar]
- Stables RH. Design of the 'Stent or Surgery' trial (SoS): a randomized controlled trial to compare coronary artery bypass grafting with percutaneous transluminal coronary angioplasty and primary stent implantation in patients with multi‐vessel coronary artery disease. Seminars in Interventional Cardiology 1999;4(4):201‐7. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Mahoney EM, Stables RH, Booth J, Nugara F, Spertus JA, et al. Disease‐specific health status after stent‐assisted percutaneous coronary intervention and coronary artery bypass surgery: one‐year results from the Stent or Surgery trial. Circulation 2003;108(14):1694‐700. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Weintraub WS, Mahoney EM, Spertus JA, Booth J, Nugara F, et al. Relative benefit of coronary artery bypass grafting versus stent‐assisted percutaneous coronary intervention for angina pectoris and multivessel coronary disease in women versus men (one‐year results from the Stent or Surgery trial). American Journal of Cardiology 2004;93(4):404‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
AMIST {published data only}
- Angelini G. AMIST A multicenter trial of minimally invasive bypass grafting versus angioplasty +/‐ stenting for single vessel disease of the left anterior descending coronary vessel. http://www.cardiosource.com/ 1999.
- Reeves BC, Angelini GD, Bryan AJ, Taylor FC, Cripps T, Spyt TJ, et al. A multi‐centre randomised controlled trial of minimally invasive direct coronary bypass grafting versus percutaneous transluminal coronary angioplasty with stenting for proximal stenosis of the left anterior descending coronary artery. Health Technology Assessment 2004;8(16):1‐43. [DOI] [PubMed] [Google Scholar]
AWESOME {published data only}
- Morrison DA, Sethi G, Sacks J, Henderson W, Grover F, Sedlis S, et al. Percutaneous coronary intervention versus coronary artery bypass graft surgery for patients with medically refractory myocardial ischemia and risk factors for adverse outcomes with bypass: A multicenter, randomized trial. Journal of the American College of Cardiology 2001;38(1):143‐9. [DOI] [PubMed] [Google Scholar]
Berger 2001 {published data only}
- Berger PB, Velianou JL, Aslanidou VH, Feit F, Jacobs AK, Faxon DP, et al. the BARI, I. Survival following coronary angioplasty versus coronary artery bypass surgery in anatomic subsets in which coronary artery bypass surgery improves survival compared with medical therapy. Results from the Bypass Angioplasty Revascularization Investigation (BARI). Journal of the American College of Cardiology 2001;38(5):1440‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Biondi‐Zoccai 2003 {published data only}
- Biondi‐Zoccai GG, Abbate A, Agostoni P, Parisi Q, Turri M, Anselmi M, et al. Stenting versus surgical bypass grafting for coronary artery disease: systematic overview and meta‐analysis of randomized trials. Italian Heart Journal: Official Journal of the Italian Federation of Cardiology 2003;4(4):271‐80. [PubMed] [Google Scholar]
Fischman 1994 {published data only}
- Fischman DL, Leon MB, Baim DS, Schatz RA, Savage MP, Penn I, et al. A randomized comparison of coronary‐stent placement and balloon angioplasty in the treatment of coronary artery disease. New England Journal of Medicine 1994;331(8):496‐501. [DOI] [PubMed] [Google Scholar]
Iakovou 2002 {published data only}
- Iakovou I, Dangas G, Mehran R, Lansky AJ, Stamou SC, Pfister AJ, et al. Minimally invasive direct coronary artery bypass (MIDCAB) versus coronary artery stenting for elective revascularization of the left anterior descending artery. American Journal of Cardiology 2002;90(8):885‐7. [DOI] [PubMed] [Google Scholar]
Kapelak 1998 {published data only}
- Kapelak B, Sadowski J, Pfitzner R, Garlicki M, Zmudka K, Pietrzak I, et al. Coronary bypass grafting after failed percutaneous angioplasty (PTCA). Przeglad Lekarski 1998;55(11):591‐5. [PubMed] [Google Scholar]
Kim 2000 {published data only}
- Kim SW, Hong MK, Lee CW, Kim JJ, Park SW, Park SJ. Multivessel coronary stenting versus bypass surgery in patients with multivessel coronary artery disease and normal left ventricular function: immediate and 2‐year long‐term follow‐up. American Heart Journal 2000;139(4):638‐42. [DOI] [PubMed] [Google Scholar]
Mathew 2000 {published data only}
- Mathew V, Clavell AL, Lennon RJ, Grill DE, Holmes DR, Jr. Percutaneous coronary interventions in patients with prior coronary artery bypass surgery: changes in patient characteristics and outcome during two decades. American Journal of Medicine 2000;108(2):127‐35. [DOI] [PubMed] [Google Scholar]
Murtaza 2001 {published data only}
- Murtaza M, Singh M, Dharmarajan L. Coronary‐artery bypass surgery versus stenting for multivessel disease. New England Journal of Medicine 2001;345(22):1642‐3. [PubMed] [Google Scholar]
Pohl 2002 {published data only}
- Pohl T, Kupatt C, Giehrl W, Raake S, Paul H, Reichenspurner P, et al. Retroinfusion‐supported stenting in high‐risk patients for interventional treatment and bypass surgery: results of the prospective randomized myoprotect study. American Journal of Cardiology 2002;90((Suppl 6A)):94H. [DOI] [PubMed] [Google Scholar]
Ryan 2001 {published data only}
- Ryan TJ. Present‐day PTCR versus CABG: a randomized comparison with a different focus and a new result. Journal of the American College of Cardiology 2001;37(1):59‐62. [DOI] [PubMed] [Google Scholar]
Serruys 1994 {published data only}
- Serruys PW, de JP, Kiemeneij F, Macaya C, Rutsch W, Heyndrickx G, et al. A comparison of balloon‐expandable‐stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. New England Journal of Medicine 1994;331(8):489‐95. [DOI] [PubMed] [Google Scholar]
Shirai 2004 {published data only}
- Shirai K, Lansky AJ, Mehran R, Dangas GD, Costantini CO, Fahy M, et al. Minimally invasive coronary artery bypass grafting versus stenting for patients with proximal left anterior descending coronary artery disease. American Journal of Cardiology 2004;93(8):959‐62. [DOI] [PubMed] [Google Scholar]
Taggart 2001 {published data only}
- Taggart DP, Banning A, Channon K. Coronary‐artery bypass surgery versus stenting for multivessel disease. New England Journal of Medicine 2001;345(22):1641‐2. [DOI] [PubMed] [Google Scholar]
Weintraub 1997 {published data only}
- Weintraub WS, Jones EL, Morris DC, King SB, III, Guyton RA, Craver JM. Outcome of reoperative coronary bypass surgery versus coronary angioplasty after previous bypass surgery. Circulation 1997;95(4):868‐77. [DOI] [PubMed] [Google Scholar]
Yock 2000 {published data only}
- Yock CA, Boothroyd DB, Owens DK, Winston C, Hlatky MA. Projected long‐term costs of coronary stenting in multivessel coronary disease based on the experience of the Bypass Angioplasty Revascularization Investigation (BARI). American Heart Journal 2000;140(4):556‐64. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
CARDia {published and unpublished data}
- CARDia Trial [webpage]. http://www.cardia.org.uk.
- Kapur A, Malik I, Bagger J, Anderson J, Beatt K, Hall R, Investigators. ftC. eHEART: www.heartjnl.com [The Coronary Artery Revascularisation in Diabetes (CARDia) trial: a prospective, randomised comparison of optimal coronary angioplasty with use of stenting and abciximab recommended versus up to date coronary artery bypass grafting in patients with diabetes mellitus suitable for either intervention]. Heart 2003;89(5):550. [Google Scholar]
FREEDOM {unpublished data only}
- MSSM. Valentin Fuster MD, PhD to Lead New $25 Million NIH Study. http://www.mssm.edu/cvi/clinical_services.shtml (accessed 22/04/2004). The Zena and Michael A. Wiener Cardiovascular Institute and The Marie‐Josse and Henry R. Kravis Center for Cardiovascular Health 2004.
SYNTAX {unpublished data only}
- SYNTAX trial. EuroPCR 2004, Paris Course on Revascularization, Paris, 24‐27 May 2004. http://www.europcr.com/ 2004.
Additional references
Bakhai 2000
- Bakhai A, Stable RH, Prasad S, Sigwart U. Trials comparing coronary artery bypass greafting with percutaneous transluminal coronary angioplasty and primary stent implantation in patients with multivessel coronary artery disease. Current Opinion in Cardiology 2000;15(6):388‐94. [DOI] [PubMed] [Google Scholar]
Clark 2004
- Clark MA, Bakhai A, Lacey M, Pelletier E, Cohen DJ. Clinical and economic outcomes of percutaneous coromary interventions in the elderly: an analysis of medicare claims data. Circulation 2004;110(3):259‐64. [DOI] [PubMed] [Google Scholar]
Dundar 2004
- Dundar Y, Hill RA, Bakhai A, Dickson R, Walley T. Angioplasty and stents in coronary artery disease: a systematic review and meta‐analysis. Scandinavian Cardiovascular Journal 2004;38(4):200‐10. [DOI] [PubMed] [Google Scholar]
Gunn 2003
- Gunn J, Crossman D, Grech ED, Cumberland D. New developments in percutaneous coronary intervention. BMJ 2003;327(7407):150‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heart CRG
- Brunner E, Burke M, Cleland J, Conti A, Dans A, Ebrahim S, et al. Heart Group. The Cochrane Library 2004, Issue 1. [Google Scholar]
Hill 2004
- Hill R, Bagust A, Bakhai A, Dickson R, Dundar Y, Haycox A, et al. Coronary artery stents and appraisal of drug‐eluting sents: a rapid and systematic review. Health Technology Assessment 2004;8(35):1‐256. [DOI] [PubMed] [Google Scholar]
Meads 2000
- Meads C, Cummins C, Jolly K, Stevens A, Burls A, Hyde C. Coronary artery stents in the treatment of ischaemic heart disease: a rapid and systematic review. Health Technology Assessment (Winchester, England) 2000;4(23):1‐153. [PubMed] [Google Scholar]
Pell 2001
- Pell JP, Walsh D, Norrie J, Berg G, Colquhoun AD, Davidson K, et al. Outcomes following coronary artery bypass grafting and percutaneous transluminal coronary angioplasty in the stent era: a prospective study of all 9890 consecutive patients operated on in Scotland over a two year period. Heart 2001;85(6):662‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Journal of the American Medical Association 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Serruys 2001
- Serruys PW, Unger F, Sousa JE, Jatene A, Bonnier H, Schonberger J, et al. Comparison of coronary‐artery bypass surgery and stenting for the treatment of multivessel disease. The Arterial Revascularization Therapies Study Group. New England Journal of Medicine 2001;344(15):1117‐24. [DOI] [PubMed] [Google Scholar]
Srinivas 2002
- Srinivas VS, Brooks MM, Detre KM, King SB, III, Jacobs AK, Johnston J, et al. Contemporary Percutaneous Coronary Intervention Versus Balloon Angioplasty for Multivessel Coronary Artery Disease: A Comparison of the National Heart, Lung and Blood Institute Dynamic Registry and the Bypass Angioplasty Revascularization Investigation (BARI) Study. Circulation 2002;106(13):1627‐33. [DOI] [PubMed] [Google Scholar]
Villanueva 2004
- Villanueva EV, Wasiak J, Petherick ES. Percutaneous transluminal rotational atherectomy for coronary artery disease (Cochrane Review). The Cochrane Library 2004, Issue 4. [DOI] [PubMed] [Google Scholar]


