Abstract
Background
This is an update of the original review published in Issue 1, 2005. It is standard clinical practice to combine chemotherapy and chest radiotherapy in treating patients with limited‐stage small cell lung cancer. However, the best way to integrate both modalities is unclear.
Objectives
To establish the best timing of chest radiotherapy with chemotherapy for patients with limited‐stage small cell lung cancer in order to improve long‐term survival.
Search methods
We ran a new search in January 2009. We searched MEDLINE (through PubMed), EMBASE (through Ovid), CINAHL (through EBSCO), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2009, Issue 1) and reference lists, handsearched journals and conference proceedings, and contacted experts to identify potentially eligible trials, published and unpublished.
Selection criteria
Randomised controlled clinical trials comparing different timing of chest radiotherapy in patients with limited‐stage small cell lung cancer.
Data collection and analysis
Seven randomised trials were included. There were differences in the timing and overall treatment time of chest radiotherapy, and the type of chemotherapy used.
Main results
We found no significant differences in overall survival, whether chest radiotherapy was delivered within 30 days after the start of chemotherapy or later, even after exclusion of the only study that delivered chest radiotherapy during cycles of non‐platinum chemotherapy (HR 0.86 in favour of early radiation, P = 0.11). The same was observed for studies having early chest radiotherapy delivered in an overall treatment time of less than 30 days compared to a longer treatment time (HR 0.82, P = 0.13). These results should be interpreted with caution because the largest trial has follow‐up data up to three years only. The outcome of longer follow up for overall survival remains to be seen. Local tumour control was not significantly different between early and late chest radiotherapy, nor the incidence of severe pneumonitis or severe oesophagitis. However, we observed a trend towards a higher chance of developing oesophagitis and pneumonitis when early chest radiotherapy was delivered during chemotherapy, which remained for oesophagitis, but not pneumonitis, after exclusion of studies with non‐platinum based chemotherapy.
Authors' conclusions
At present, it is uncertain whether the timing of chest radiotherapy as such is important for survival. The optimal integration of chemotherapy and chest radiotherapy in patients with limited‐stage small cell lung cancer is unknown. Further research is needed to establish the best combination of radiotherapy and chemotherapy in this disease.
Plain language summary
Early (less than 30 days after the start of chemotherapy) or late (more than 30 days after the start of chemotherapy) chest radiotherapy for patients suffering from limited small cell lung cancer
Between 7% and 8% of lung cancers are of the type known as limited‐stage small cell tumours. People with this type of cancer have a limited chance of being cured with chemotherapy and radiotherapy. It is not known when the optimum time to give chest radiotherapy is in relation to administering chemotherapy treatment. This review indicates that it is unclear whether administering chest radiotherapy within 30 days of beginning chemotherapy or later improves survival. The effect on patients' overall survival is not statistically different, although there is a possibility that the effect is in favour of early chest radiotherapy. The interpretation of the current data is difficult and further research is needed.
Background
Small cell lung cancer (SCLC) accounts for 20% to 25% of all lung cancer cases, with only one‐third of patients presenting with limited disease (LD‐SCLC) (Bunn 1997; Ihde 1995). Without treatment, tumour progression in patients with SCLC is rapid, with a median survival of two to four months. Chemotherapy has improved the prognosis substantially, but long‐term survival remains below 10% (Kelly 2000). Two meta‐analyses (Pignon 1992; Warde 1992) have shown an improvement of 5.4% in absolute survival at two years and three years in patients who received chest irradiation in addition to chemotherapy compared to those receiving chemotherapy alone, but the five‐year survival rate remains disappointingly low at 10% to 15% (Pignon 1992). The trials included in the meta‐analyses used cyclophosphamide and/or doxorubicin‐based chemotherapy regimens as induction treatment, whereas in later trials cisplatin/etoposide chemotherapy alone or as a component was used. Although evidence for a significant survival benefit from chest radiotherapy was provided by the meta‐analyses, no conclusions could be drawn about the optimal timing and sequencing of chemotherapy and radiation. Using meta‐analysis techniques, it appeared that a short time between the first day of chemotherapy and the last day of chest radiotherapy was associated with improved survival in LD‐SCLC (De Ruysscher 2006). Furthermore, the results of a recently published meta‐analysis suggest that it is essential to ensure that the delivery of chemotherapy is optimal when administered with early chest radiotherapy (Spiro 2006).
Despite the recent findings described above, several issues about the administration of chest radiotherapy in LD‐SCLC are still unresolved, including its timing with chemotherapy, the optimal overall treatment time, whether or not to deliver it concurrent with chemotherapy, and whether or not the chemotherapy has been delivered to a reasonable total dose and with a sufficient dose‐intensity.
Even adding information from trials published after the two meta‐analyses, the data for the optimal timing of chest radiotherapy (i.e. early versus late) are still conflicting (De Ruysscher 2000; Kumar 1997; Takada 2002).
Giving prophylactic cranial irradiation (PCI) to reduce the incidence of brain metastases has been evaluated in a meta‐analysis of seven randomised trials that included 987 patients in complete remission after chemotherapy or chemotherapy plus chest radiotherapy. The relative risk of death in the group who received PCI, as compared with the control group, was 0.84 (95% CI 0.73 to 0.97, P = 0.01) (Auperin 1999). An investigation of the relative effectiveness of different ways of administering chest radiotherapy must therefore control for this co‐intervention.
In conclusion, there is good evidence that chest radiotherapy given in addition to chemotherapy improves survival in patients with LD‐SCLC compared with chemotherapy alone. However, the best timing of chest radiotherapy with chemotherapy has not so far been defined and is the focus of this review. It has become clear in recent years that the overall radiation treatment time plays an important role in the outcome, therefore this variable was also examined (Bentzen 1991; De Ruysscher 2006; Withers 1988). Since the type of chemotherapy delivered together with radiotherapy may affect outcomes, we also stratified for this factor (Harari 2003; Kumar 1997).
This is an updated version of the original review published in Issue 1, 2005 of The Cochrane Library (Pijls‐Johannesma 2005). The original review has also been published in a different version (Pijls‐Johannesma 2007).
Objectives
The two objectives of this review were:
1. To compare the overall survival of patients suffering from limited‐disease small cell lung cancer (LD‐SCLC) who received either early or late chest radiotherapy. We stratified the results for the overall treatment time of chest irradiation and for administration of chest radiotherapy either during chemotherapy cycles or after completion of chemotherapy.
Only one study provided kinetic data on tumour cell clonogen proliferation of SCLC during and after chemotherapy and radiotherapy (De Ruysscher 2006). In this study, as in many common solid tumours, accelerated tumour cell clonogen proliferation occurred approximately 30 days after the start of effective cytotoxic therapy (Bentzen 1991; Davis 2000; Withers 1988). We therefore defined early chest radiotherapy as starting chest irradiation before 30 days after the start of chemotherapy. We defined late radiotherapy as starting chest irradiation 30 days or more after the start of chemotherapy.
2. To compare the relative toxicities of early and late chest radiotherapy and different combination regimens (including hyper‐fractionation) on local normal tissues (oesophagus and lungs) and on the bone marrow.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled clinical trials, fully published in journals and those identified from other sources (abstracts and proceedings of relevant scientific meetings as well as contacts with investigators) for which full details were available from investigators.
Types of participants
Patients of any age with histologically and cytologically proven SCLC, limited disease and with performance status 0‐2. For this review we used the following definition of 'limited disease': cancer confined to one hemi‐thorax, including contralateral mediastinal and hilar lymph nodes as well as ipsilateral and/or bilateral supraclavicular involvement, but excluding malignant pleural effusion. We excluded studies in which it was not possible to separate data on patients receiving early and late radiation, even after contacting the authors.
Types of interventions
Any regimen of chest radiotherapy given concurrently (chemotherapy was continued while the patient was having radiotherapy), or not, with any chemotherapy regimen. We stratified analyses according to total treatment time of chest irradiation and by the administration, or not, of concurrent chemotherapy. Prophylactic cranial irradiation may or may not have been given and, if so, was analysed separately.
Types of outcome measures
We studied the following clinically relevant outcomes:
1. survival: overall survival; 2. local control: cumulative local tumour control at five years; 3. toxicities: incidence of haematological, lung and oesophageal grade 3‐4 toxicity, if available or extractable from the manuscript; 4. compliance: percentage of intended total dose completed of radiotherapy or chemotherapy.
Search methods for identification of studies
For this update, we identified eligible studies by searching the Cochrane Lung Cancer Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 1, 2009), MEDLINE (1966 to January 2009, accessed through PubMed), EMBASE (1974 to January 2009, accessed through Ovid) and CINAHL (1982 to January 2009, accessed through EBSCO). We also contacted experts to identify potentially eligible trials, published and unpublished.
We undertook searches without restricting the language of studies retrieved.
We include in Appendix 1 the search strategy used to search MEDLINE. We adapted the strategy to the requirements of the other databases.
We scrutinised reference lists from identified studies for additional studies. We also undertook searches of the following oncology journals: International Journal of Radiation, Oncology, Biology and Physics (1985 to 2004); Radiotherapy and Oncology (1985 to 2004); Journal of Clinical Oncology (1985 to 2004); Clinical Oncology (1999 to 2004); Lung Cancer (1985 to 2004) and Thorax (1985 to 2004). We handsearched abstracts from the principal oncology conferences from 1985 onwards, with a minimum follow up of three years. We asked colleagues, collaborators and other experts in the field to identify missing and unreported trials.
Data collection and analysis
Selection of studies
We assessed randomised trials identified by the search to determine whether they met the inclusion criteria. Three independent review authors (DDR, MPJ, JV) assessed trials, both for the quality of the methods against pre‐determined criteria (see below) and for the results of key outcomes, which were identified and tabulated.
Data extraction and management
Two review authors (DDR, JV) extracted data independently to ensure validity, while a third review author (MPJ) was responsible for resolving discrepancies. We included all data extracted from randomised trials in these analyses.
We attempted to collect the following data from all trial reports: identifiers; gender; age; performance status at the time of randomisation; initial disease stage; definition of chemotherapy regimen; induction treatment that led to a complete response; start date of induction treatment; randomisation date; treatment allocated; overall treatment time of chest irradiation according to the protocol and updated information on survival; brain metastases; other metastases; and loco‐regional recurrence.
Two review authors (MPJ, RH) conducted the statistical analyses.
Assessment of risk of bias in included studies
We assessed the risk of bias using the tool described in the Cochrane Handbook for Systematic Reviews of interventions (Higgins 2008), addressing the following five domains: sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting of outcomes. Since blinding was not possible in chemoradiation studies we omitted this domain from the analysis. For each domain, we made a judgement (‘Yes’ for low risk of bias; ‘No’ for high risk of bias, or ‘Unclear’). In the 'Risk of bias' table, this judgement is followed by a text box providing a description of the design, conduct or observations that underlie the judgement. Consequences of the risk of bias assessment are discussed in the Results section.
Measures of treatment effect
We computed a weighted estimate of the typical treatment effect across studies for the study outcomes. For time‐to‐event outcomes such as survival, we used the log hazard ratio (lnHR) and the standard error (se(lnHR)) as the effect measure. We extracted these effect measures from the study publications using the methods described by Tierney 2007. For all studies except Work 1997, we calculated lnHR and se(lnHR) from the P value of the log‐rank test, total events and numbers randomised to each treatment arm. Since this information was not available for the study by Work 1997, we calculated lnHR and se(lnHR) from survival curve data in this case. For other outcomes than time‐to‐event, we used the risk ratio (RR) as the effect measure. We used the I2statistic to determine statistical heterogeneity among trials. I2 values above 50% are viewed as moderate; above 80% as high. As we anticipated that the trial results would be heterogeneous, we performed all analyses using a random‐effects model.
We carried out post‐hoc sensitivity analyses according to whether platinum‐based chemotherapy was used during chest radiotherapy or not and we investigated whether or not the chemotherapy was delivered with a reasonable total dose and with a sufficient dose‐intensity. We defined a 'reasonable' chemotherapy treatment as when the total dose and dose‐intensity was 80% or more.
We performed additional post‐hoc sensitivity analyses to determine the impact of the time difference between early and late chest radiotherapy on overall survival. For this, we took the difference in number of days between study arms as covariate in a mixed‐effects meta‐regression analysis. We carried out analysis in R version 2.10.0 using the metafor library. In order to perform a meta‐regression using overall survival data, we extracted lnHR and standard errors of lnHR from the selected studies. We used LnHR and its variance (i.e. the squared standard error) as input for this analysis.
Dealing with missing data
We did not impute missing outcome data. If only imputed outcome data were reported, we planned to contact trial authors to request data on the outcomes only among participants who were assessed.
Results
Description of studies
Eleven studies had been identified in the initial version of the review and another one was identified in the 2009 update. From these 12 studies, seven were suitable for survival analysis (Jeremic 1997; Murray 1993; Perry 1998; Skarlos 2001; Spiro 2006; Takada 2002; Work 1997). We excluded five trials (Blackstock 2005; Bonner 1999; Gregor 1997; Lebeau 1999; Turrisi 1999). We excluded three because chest radiotherapy started on the same day, thus making comparison between early and late radiation impossible (Blackstock 2005; Bonner 1999; Turrisi 1999). The study of the EORTC (European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group Study) (Gregor 1997) was not suitable for this analysis because in one arm chest radiotherapy started on day 49 and in the other on day 91; thus, according to our inclusion criteria, late radiotherapy was delivered in both arms. Likewise, we excluded Lebeau 1999 because chest radiotherapy started between day 30 and 64 in one arm, and day 36 and 47 or 64 and 75 in the other arm.
From the seven studies suitable for survival analysis, six gave enough information for local toxicity analysis, i.e. severe pneumonitis and severe oesophagitis (Jeremic 1997; Murray 1993; Perry 1998; Skarlos 2001; Spiro 2006; Takada 2002). Six studies provided sufficient information for haematological toxicity evaluation (Jeremic 1997; Perry 1998; Skarlos 2001; Spiro 2006; Takada 2002; Work 1997). Local tumour control data were available from six of these seven studies (Jeremic 1997; Murray 1993; Perry 1998; Spiro 2006; Takada 2002; Work 1997). However, in Takada 2002, only the site of first recurrence was available, whereas in the other trials the cumulative rate of local tumour failure at two and five years was available. The five‐year data were considered to be the most suitable for analysis as they are the least influenced by other confounding factors such as chemotherapy given for recurrence. Compliance, defined as the percentage of intended total dose completed, was registered in six of the seven studies (Jeremic 1997; Murray 1993; Skarlos 2001; Spiro 2006; Takada 2002; Work 1997).
Chemotherapy and radiotherapy were given sequentially in both arms in one of the seven studies (Work 1997). In another trial (Takada 2002), only one treatment group ‐ the early chest radiotherapy group‐ received concurrent radiation and chemotherapy, whereas the late radiation group received radiotherapy only after chemotherapy. As a consequence, there are not enough trials available to investigate the effect of the sequencing of chemotherapy and thoracic radiotherapy on survival.
Overall, all included studies delivered a reasonable chemotherapy dose to each treatment arm (Figure 1). In three studies the required delivered dose of 85% was not reached for some agents; that was for etoposide and cisplatin, early/late (E/L) 74%/81% and 70%/79% respectively (Spiro 2006), cisplatin E/L 84%/83% (Murray 1993) and cyclophosphamide E/L 84%/84% (Work 1997). The variance of the delivered doses between both arms was low, therefore it was not possible to stratify for this factor.
1.

Dose‐intensity chemotherapy
At least one group of patients in every trial received prophylactic cranial irradiation (PCI), so it was not possible to stratify for this variable.
One trial from Canada (Murray 1993) included 155 patients who were randomised to receive chest radiotherapy beginning at day 21, and another 153 patients who received chest radiotherapy on day 105. In both arms the radiotherapy dose was 40 Gy in 15 fractions in 19 days, which was given at the same time as a cycle of cisplatin and etoposide chemotherapy. Patients without progressive disease after chemotherapy and chest radiotherapy received PCI.
The Danish trial (Work 1997) randomised 199 patients to chest radiotherapy at a dose of 40 to 45 Gy in 20 to 25 fractions in 47 days, starting either on day 1 or on day 126. Patients who received chest radiotherapy on day 1 received half of their radiotherapy to the chest before starting chemotherapy. After two weeks of chemotherapy, patients continued split‐course chest radiotherapy. Chemotherapy was not given concurrently with radiation. Patients did not receive PCI in the first period of the study, but did in the second.
In the trial reported by Jeremic (Jeremic 1997), 52 patients received chest radiotherapy from day 1, and 51 patients from day 42. Both groups received a dose of 54 Gy in 36 fractions in 26 days. Platinum and etoposide were used during radiotherapy. After the end of chemotherapy, patients received PCI.
In a three‐arm trial, updated by Perry in 1998 (Perry 1998), 125 patients received chest radiotherapy concurrently with cyclophosphamide, vincristine and etoposide and, later in the study, cyclophosphamide, doxorubicin and etoposide (CDE) from day 1. Another 145 patients received radiotherapy together with CDE starting on day 64. This was the only study in which non‐platinum chemotherapy was administered concurrently with chest radiotherapy. In both arms, the radiotherapy dose was 50 Gy in 25 fractions in five weeks. A third arm, not relevant for the present analysis, did not include chest radiotherapy.
Skarlos 2001 reported on a randomised phase II trial in which 42 patients started chest radiotherapy on day 1 and 39 patients on day 56. In both arms, the radiation dose was 45 Gy in 30 fractions in 19 days, both concurrently with platinum and etoposide. PCI was given when a complete remission was achieved.
In a phase III trial from Japan (Takada 2002), patients were randomised to receive chest radiotherapy at a dose of 45 Gy in 30 fractions in 19 days, either starting on day 2 or on day 82. Both arms contained 114 patients. In the early radiotherapy arm, radiation was delivered concurrently with platinum and etoposide, whereas in the late radiotherapy arm, radiation was given after the end of chemotherapy. PCI was given after the end of chemotherapy in both arms.
Finally, in a large phase III trial (Spiro 2006), 325 patients were randomised to receive chest radiotherapy beginning at day 21, with the second course of chemotherapy, or with the sixth cycle of chemotherapy. In both arms of the study, the radiotherapy dose was 40 Gy in 15 fractions in 19 days given with a cycle of cisplatin and etoposide chemotherapy. Cisplatin and etoposide were given as chemotherapeutic agents, together with chest radiotherapy. PCI was given to responding patients with a negative post‐treatment brain scan.
Risk of bias in included studies
The risk of bias of the included studies is shown in the table 'Characteristics of included studies' and is summarised in Figure 2 and Figure 3. All seven included studies reported on a randomisation process. In some studies (Jeremic 1997; Perry 1998; Work 1997) allocation concealment might be questionable. In four of seven studies, the analyses were performed according to intention‐to‐treat (Jeremic 1997; Perry 1998; Skarlos 2001; Work 1997). Although there was no blinding in any study, this is unlikely to affect outcome. All studies addressed incomplete outcome of data.
2.
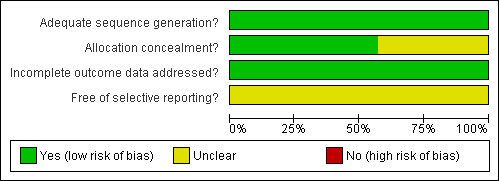
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
In all studies, the groups were similar at baseline for the most important prognostic indicators, the eligibility criteria were specified, and the co‐interventions that may have influenced the results were controlled for. However, there was no uniform definition of local tumour control or of the methods to assess it. The co‐interventions that may have influenced the local tumour control were not controlled for. We therefore chose the cumulative proportion of local tumour control at five years, because this would probably not be influenced by second‐line chemotherapy given for recurrence after primary treatment. However, as the proportion of the surviving patients at the end of study follow up was small, the numbers of patients with long‐term local control were obviously low as well, making these results more difficult to interpret. Also, no uniform criteria were used to assess toxicity. No other problems were detected in any of the seven studies that might have introduced a serious risk of bias.
Effects of interventions
Survival
All studies
Taking all seven studies into account, the overall survival was not significantly different between early or late chest radiotherapy.
Platinum during radiotherapy
When we excluded the one trial that delivered non‐platinum chemotherapy concurrently with chest radiotherapy (Perry 1998) in a sensitivity analysis, the HR of overall survival (HR 0.86 in favour of early chest radiotherapy, 95% CI 0.71 to 1.04, P = 0.11) remained non‐significant, although to a much lesser extent.
Chest radiotherapy less than 30 days
Considering studies with an overall treatment time of chest radiation of less than 30 days (Jeremic 1997; Murray 1993; Skarlos 2001; Spiro 2006; Takada 2002), the overall survival was again higher for early chest radiotherapy (HR 0.82, 95% CI 0.64 to 1.06, P = 0.13), however non‐significant.
Heterogeneity
Heterogeneity was moderate for all analyses with respect to survival. I2 values ranged from 58% to 75%.
Local tumour control
There was no significant effect on local tumour control from either early or late delivery of chest radiotherapy. This held true in a sensitivity analysis when we omitted the study not combining platinum‐based chemotherapy with thoracic radiotherapy (Perry 1998).
Toxicity
Severe pneumonitis
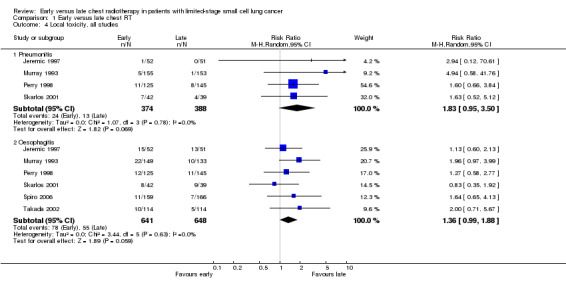
We only observed a trend towards a higher incidence of severe pneumonitis for early chest radiotherapy compared with late irradiation when all four studies (Jeremic 1997; Murray 1993; Perry 1998; Skarlos 2001) were considered (Figure 4) (RR 1.83, 95% CI 0.95 to 3.50, P = 0.07). When we excluded the one trial that delivered non‐platinum chemotherapy concurrently with chest radiation (Perry 1998) in a sensitivity analysis, the incidence of severe pneumonitis was not significantly different between early or late chest radiotherapy (Figure 5).
4.

Forest plot of comparison: 1 Early versus late chest RT, outcome: 1.8 Local toxicity, all studies.
5.

Forest plot of comparison: 1 Early versus late chest RT, outcome: 1.9 Local toxicity, excluding studies with non‐platinum CT.
Taking into account studies with an overall treatment time of chest radiation of less than 30 days (Jeremic 1997; Murray 1993; Perry 1998; Skarlos 2001), the incidence of severe pneumonitis was not significantly different between early or late chest radiotherapy.
Severe oesophagitis
For severe oesophagitis, we observed a trend between early and late chest radiotherapy when all six studies (Jeremic 1997; Murray 1993; Perry 1998; Skarlos 2001; Spiro 2006; Takada 2002) were taken into account (Figure 4) (RR 1.36, 95% CI 0.99 to 1.88, P = 0.06). In a sensitivity analysis, we excluded the one trial that delivered non‐platinum chemotherapy concurrently with chest radiation (Perry 1998), but the incidence of severe oesophagitis still showed this trend (Figure 5) (RR 1.39, 95% CI 0.97 to 1.98, P = 0.07). This was the same when taking into account studies with an overall chest radiation treatment time of less than 30 days (Figure 6) (Jeremic 1997; Murray 1993; Skarlos 2001; Spiro 2006).
6.

Forest plot of comparison: 1 Early versus late chest RT, outcome: 1.10 Local toxicity, only studies with overall treatment time of chest RT less than 30 days.
Severe leucopenia
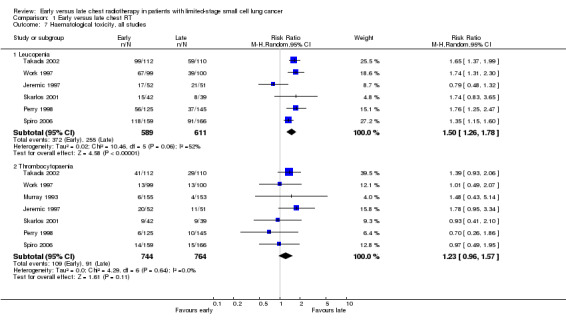
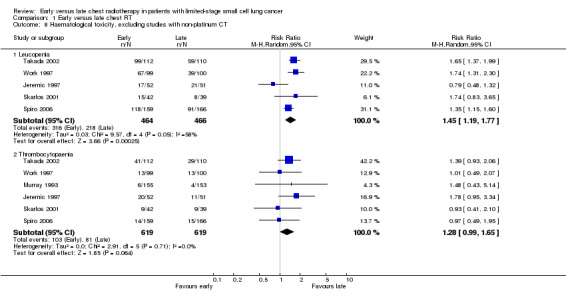
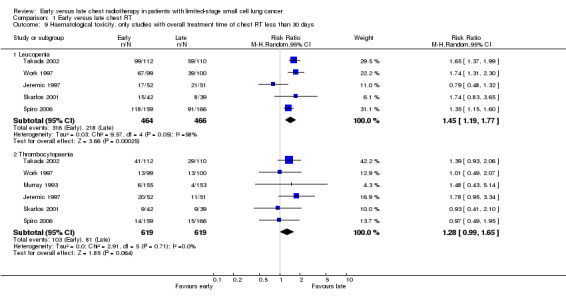
Taking all studies into account (Jeremic 1997; Perry 1998; Skarlos 2001; Spiro 2006; Takada 2002; Work 1997), severe leucopenia was significantly more frequent in patients receiving early chest radiotherapy (Figure 7) (RR 1.50, 95% CI 1.26 to 1.78, P < 0.00001). When we excluded the one trial that delivered non‐platinum based chemotherapy concurrently with chest radiation (Perry 1998) in a sensitivity analysis, the incidence of severe leucopenia was still significantly different between early and late chest radiotherapy, with early irradiation leading to more leucopenia (Figure 8) (RR 1.45, 95% CI 1.19 to 1.77, P = 0.0002). Taking into account studies with an overall chest radiotherapy treatment time of less than 30 days (Jeremic 1997; Skarlos 2001; Spiro 2006; Takada 2002; Work 1997), severe leucopenia, once again, occurred significantly more often in patients receiving early chest radiotherapy (Figure 9) (RR 1.45, 95% CI 1.19 to 1.77, P = 0.0002).
7.
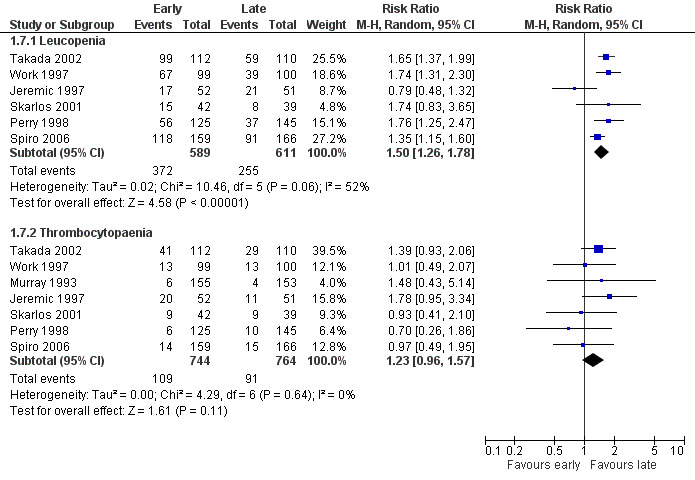
Forest plot of comparison: 1 Early versus late chest RT, outcome: 1.11 Haematological toxicity, all studies.
8.

Forest plot of comparison: 1 Early versus late chest RT, outcome: 1.12 Haematological toxicity, excluding studies delivering radiation concurrently with doxorubicin.
9.

Forest plot of comparison: 1 Early versus late chest RT, outcome: 1.13 Haematological toxicity, only studies with overall treatment time of chest RT less than 30 days.
Severe thrombocytopaenia
Taking all studies into account, severe thrombocytopaenia was not significantly different between early and late chest radiotherapy (Figure 7). When we excluded the one trial that delivered non‐platinum based chemotherapy concurrently with chest radiation therapy (Perry 1998), severe thrombocytopaenia was still not significantly different between early and late chest radiotherapy, although a trend was observed in this sensitivity analysis with more thrombocytopaenia for late irradiation (Figure 8) (RR 1.28, 95% CI 0.99 to 1.65, P = 0.06). Taking into account studies with an overall treatment time of chest radiation of less than 30 days (Jeremic 1997; Skarlos 2001; Spiro 2006; Takada 2002; Work 1997), we only observed a trend towards a higher incidence of severe thrombocytopaenia for late chest radiotherapy (Figure 9) (RR 1.28, 95% CI 0.99 to 1.65, P = 0.06).
Heterogeneity
Heterogeneity was absent for all toxicities (I2 = 0%), except for the analyses with respect to leucopenia. Here, heterogeneity was moderate, ranging from 52% to 58%.
Compliance
Compliance with chest radiotherapy
The percentage of intended total radiation dose completed was registered in six studies (Jeremic 1997; Murray 1993; Skarlos 2001; Spiro 2006; Takada 2002; Work 1997). The lowest percentage of compliance (74% to 78%) was observed in the Danish Trial (Work 1997), whereas the highest percentage (100%) was observed in Skarlos 2001. The results of compliance, both for radiotherapy and chemotherapy, are shown in the table 'Characteristics of included studies'.
Compliance with chemotherapy
Overall, there were no significant differences in the total dose of chemotherapy or the dose‐intensity between early and late thoracic irradiation. However, the percentage of cisplatin dose was lower in the early compared to the late radiotherapy group: 70% versus 79%, respectively (P = 0.01). The compliance rate in the early group was considerably lower compared to that of the late radiation group in two studies (Skarlos 2001; Spiro 2006), that is 71% versus 90% and 69% versus 80% (P = 0.03), respectively. A post‐hoc analysis showed that the overall survival was not statistically different between these low and high compliance groups (HR 1.11, 95% CI 0.89 to 1.37). No heterogeneity was observed for this analysis (I2 = 0%).
Meta‐regression
We explored sources of heterogeneity in the assessment of the primary outcome measure by mixed‐effects meta‐regression. We assessed the effect of the time difference in days between chest irradiation and the administration of chemotherapy on overall survival.
Since there was only one study that delivered non‐platinum based chemotherapy concurrently with chest radiation therapy (Perry 1998), no meta‐regression analyses could be performed to determine the association between delivering non‐platinum based or platinum‐based chemotherapy concurrently with chest radiation. However, we identified this factor as a potential source of the observed heterogeneity, and therefore we excluded the study from the mixed‐effects meta‐regression.
According to the mixed‐effects meta‐regression, the influence of the time difference between early and late chest radiotherapy on overall survival was in the expected direction but clearly non‐significant (regression coefficient 0.0037, SE 0.0040, P = 0.36, 95% CI ‐0.0042 to 0.0116). At the median time difference between early and late chest radiotherapy of 84 days for the included studies, the hazard ratio of early versus late chest radiotherapy would be estimated at 0.85 (95% CI 0.70 to 1.04).
Discussion
From two meta‐analyses, it has become clear that adding chest radiotherapy to chemotherapy in patients suffering from limited stage small cell lung cancer (SCLC) improves survival (Pignon 1992; Warde 1992). However, several issues about the administration of thoracic radiotherapy in LD‐SCLC are still unresolved, including timing with chemotherapy (De Ruysscher 2000; Warde 1992). We therefore focused this review on the best timing for administering chest radiotherapy in conjunction with chemotherapy. In recent years it has become clear that the overall treatment time of radiation plays an important role in the outcome of radiotherapy (Bentzen 1991; Withers 1988), in addition to the timing of chest radiotherapy (De Ruysscher 2006). Therefore, we decided to examine the overall treatment time of chest irradiation. Only scarce data are available on tumour cell clonogen proliferation of SCLC during and after chemotherapy and radiotherapy (De Ruysscher 2006). The results suggest that as in many common solid tumours, accelerated tumour cell clonogen proliferation occurs approximately 30 days after the start of effective cytotoxic therapy (Bentzen 1991; Davis 2000; De Ruysscher 2006; Withers 1988). We therefore defined early chest radiotherapy as starting chest irradiation before 30 days after the start of chemotherapy. We defined late radiotherapy as starting chest irradiation before 30 days after the start of chemotherapy. Since the type of chemotherapy delivered together with radiotherapy may affect outcomes, we also stratified for this (Harari 2003; Kumar 1997).
We identified seven studies enabling investigation of the best timing of chest radiotherapy for survival (Jeremic 1997; Murray 1993; Perry 1998; Skarlos 2001; Spiro 2006; Takada 2002; Work 1997). We excluded four well‐known trials from this analysis. In two of them, chest radiotherapy started on the same day in both arms of the trial, thus making comparison between early and late radiation impossible (Bonner 1999; Turrisi 2002). In another two studies, thoracic radiotherapy started in both arms after day 30 implying that, according to our inclusion criteria, late radiotherapy was delivered in both arms (Gregor 1997; Lebeau 1999).
From the seven studies suitable for survival analysis, four gave enough information for severe pneumonitis analysis (Jeremic 1997; Murray 1993; Perry 1998; Skarlos 2001); five for severe oesophagitis analysis (Jeremic 1997; Murray 1993; Perry 1998; Skarlos 2001; Spiro 2006); and six studies for haematological toxicity evaluation (Jeremic 1997; Perry 1998; Skarlos 2001; Spiro 2006; Takada 2002; Work 1997). Local tumour control data were available from five of these seven studies (Jeremic 1997; Murray 1993; Perry 1998; Takada 2002; Work 1997). However, in the study from Japan (Takada 2002), only the site of first recurrence was available, whereas in the other trials the cumulative rate of local tumour failure at both two and five years was available. We considered the five‐year data to be the most suitable for analysis, because they are the least influenced by other confounding factors such as chemotherapy given for recurrence.
In one of the seven studies, chest radiotherapy was only partly delivered during chemotherapy cycles in one arm, while in the second arm it was given after the end of chemotherapy (Work 1997). In another trial (Takada 2002), the early chest radiotherapy group received concurrent radiation and chemotherapy, while the late radiation group only received radiotherapy after chemotherapy. As a consequence, there are not enough trials available to investigate the effect of the sequencing of chemotherapy and thoracic radiotherapy on survival.
As in all studies PCI was given to at least one group of patients, it was not possible to stratify for this variable.
The effect of the timing of chest radiotherapy on survival is not clear. For all studies together, we observed no significant differences in overall survival rates. When we excluded the only trial in which non‐platinum chemotherapy was delivered concurrently with chest radiotherapy (Perry 1998), this did not change. However, these results should be interpreted with caution. First, the subgroups based on non‐platinum chemotherapy delivered during radiotherapy versus platinum‐based chemotherapy were a post‐hoc analysis by our group. Second, there are differences in the availability of survival data between the studies. For example, the NCI‐C trial (Murray 1993) contains survival data up to five years after treatment and does show a survival difference between the treatment regimens. A subsequent large study (Spiro 2006) with the same design and therapeutic regimen as the NCI‐C trial failed to show a survival difference between early and late chest radiotherapy with survival data available up to three years. Patients randomised to early chest radiation received in this trial significantly less chemotherapy than the late arm. It remains to be seen whether longer follow‐up data for this study will show anything significant. Third, the studies with the highest reported dose‐intensity of chemotherapy seem to have the highest survival rates and show the largest differences between early and late chest radiotherapy (Jeremic 1997; Murray 1993; Skarlos 2001; Takada 2002). The same studies also report the highest compliance rates. It seems that in order to have a benefit of early chest radiotherapy, an effective systemic treatment as well as a two‐year survival of at least 30% should be achieved. It is difficult to explain these findings, which may be interrelated. It is not unlikely that the beneficial effect of early chest radiation can only be observed in those patients in a good general condition, thoroughly staged and thus able to have a good compliance for an intensive, but effective chemotherapy regimen combined with chest radiotherapy. However, this hypothesis cannot be proved by the available data. At present, therefore, it is unclear whether early chest radiotherapy confers a survival benefit.
The five studies with an overall chest radiation treatment time of less than 30 days (Jeremic 1997; Murray 1993; Skarlos 2001; Spiro 2006; Takada 2002) did not show a significant benefit of early chest radiotherapy for overall survival. For the same reasons that are described above, the results should be interpreted with caution. No firm conclusion can be drawn as to whether the overall treatment time of chest radiotherapy affects survival or not.
Local tumour control was not significantly different between early and late chest radiotherapy. This is similar to the results of a potential survival benefit of early chest radiotherapy. The local control figures were much influenced by the data of Murray 1993, who observed a significantly better rate of survival for patients receiving early chest radiotherapy versus late radiation, but no significant difference in local tumour control. In the Danish trial (Work 1997), no differences between early and late radiotherapy were observed, but chest radiotherapy was not delivered concomitantly with chemotherapy, which might have influenced the results. Moreover, two of the four studies amenable to local tumour control evaluation had an overall treatment time of chest radiation of more than 30 days (Perry 1998; Work 1997), which also may have increased the heterogeneity among the studies. At present, no five‐year data on the local tumour control rate are available from the largest trial, that of Spiro et al (Spiro 2006).
Keeping all caveats for comparing local toxicity between studies in mind, severe pneumonitis and severe oesophagitis were not significantly different between early and late chest radiotherapy, whether non‐platinum chemotherapy was administered during chest radiotherapy or not (Jeremic 1997; Murray 1993; Perry 1998; Skarlos 2001; Spiro 2006; Takada 2002). Even when the overall treatment time of chest radiation was less than 30 days (Jeremic 1997; Murray 1993; Skarlos 2001; Spiro 2006), severe local toxicity was not significantly different between early and late chest radiotherapy. However, some trends were visible in the results. When taking all studies into account, we observed a trend towards a higher incidence of pneumonitis when delivering early chest radiotherapy. Furthermore, we observed a trend towards a higher incidence of oesophagitis in all analyses. Although this should be interpreted with caution, it may be that larger radiation fields were used when irradiation was carried out during the first or at the beginning of the second chemotherapy cycles, at a time when the primary tumour and the lymph node metastases were still larger than after later chemotherapy cycles.
Compliance rates in the studies, except for Skarlos 2001 and the early arms of Skarlos 2001 and Spiro 2006, were high (87% to 100%) (Jeremic 1997; Murray 1993; Takada 2002), which implies that these radiotherapy schedules are generally well‐tolerated. Overall, the delivered chemotherapy dose was similar in both treatment arms of each study, but the dose schedules and regimens differed between most studies (Figure 10). Since the studies Spiro 2006 and Murray 1993 used the same study design and treatment schedules, the different results in outcome between these studies could therefore not be explained by differences in the delivered dose‐intensity but may be explained by differences in compliance rates. To determine the effect of the dose‐intensity of chemotherapy on treatment outcome in the combined modality treatment with radiotherapy, investigation by future research is required.
10.
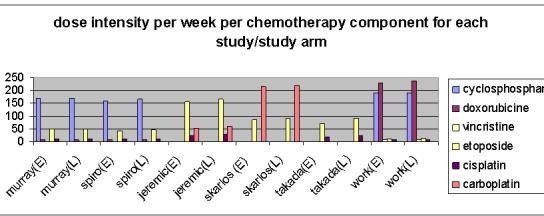
Distribution chemotherapy dose per study arm
Severe leucopenia was significantly more frequent in patients receiving early chest radiotherapy, regardless of whether all studies were taken into account; whether the study delivering non‐platinum chemotherapy during radiation (Perry 1998) was excluded; or when the overall chest radiotherapy treatment time was fewer than 30 days. This finding, however, may be attributed to the increased bone marrow damage of delivering concurrent chemotherapy and chest radiotherapy, which both cause leucopenia. Severe thrombocytopaenia was not significantly different between early and late chest irradiation when all studies were considered. When we excluded the one trial that delivered non‐platinum chemotherapy concurrently with chest radiation (Perry 1998), or when taking into account studies with an overall treatment time of chest radiation of less than 30 days (Jeremic 1997; Skarlos 2001; Spiro 2006; Takada 2002; Work 1997), a trend towards more severe thrombocytopaenia for late chest radiotherapy appeared. Once again, this may be due to bone marrow suppression caused by earlier chemotherapy treatments.
We identified no serious risks of bias. For some studies, more information was needed for a correct judgement on allocation concealment. For most of the studies, we judged sequence generation as correctly performed, but more information would be preferable to eliminate all doubts.
Authors' conclusions
Implications for practice.
At present, it is unclear whether the timing of chest radiotherapy (beginning either within 30 days after the start of chemotherapy or later) affects long‐term survival. The possible benefit of early chest radiotherapy for improved overall survival should be viewed with caution. Early chest radiotherapy could be more effective but since the compliance rate appeared to be very important, the influence of patient selection and of the systemic treatment should be taken into account. Early chest radiotherapy leads to a higher incidence of severe leucopenia, while in the late chest radiotherapy arm a trend towards more oesophagitis in all comparisons and for more thrombocytopaenia in some subgroups was observed.
Implications for research.
In view of the many uncertainties, more research is needed to investigate the optimal timing of chest radiotherapy, the best radiotherapy schedule in relation to the type of chemotherapy chosen, the optimal delivering of chemotherapy, the volumes to be treated and the patient groups for whom these treatments apply.
What's new
| Date | Event | Description |
|---|---|---|
| 14 December 2009 | New search has been performed | Updated with new methodology. Survival is now analysed as time‐to‐event data and we have added meta‐regression with time difference between early and late chest radiotherapy as a covariate. We also ran searches but no new studies were identified. |
History
Protocol first published: Issue 2, 2004 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 18 September 2008 | Amended | Converted to new review format. |
| 31 July 2004 | New citation required and conclusions have changed | Substantive amendment. |
Acknowledgements
We want to thank Dr A Kester of the Department of Methodology and Statistics of the University of Maastricht, for his support with the statistical analyses.
Appendices
Appendix 1. Search strategy designed for MEDLINE (via PubMed)
1. "lung neoplasms"[mh] 2. "carcinoma, small‐cell"[mh] 3. (lung*[tw] AND cancer*[tw]) 4. (lung*[tw] AND carcinoma*[tw]) 5. (lung*[tw] AND neoplasm*[tw]) 6. (pulmonary[tw] AND neoplasms*[tw]) 7. (lung*[tw] AND metast*[tw]) 8. "carcinoma,bronchogenic"[mh] 9. "bronchial neoplasms"[mh] 10. (bronch*[tiab] AND cancer*[tiab]) 11. (bronch*[tiab] AND carcinoma[tiab]) 12. "pleural neoplasms"[mh] 13. OR/1‐12 14. (lung*[tw] OR bronch*[tw] OR pulmonary[tw]) 15. "carcinoma, small cell"[mh] 16. ((small[tw] AND cell[tw]) AND (carcinoma*[tw] OR cancer*[tw])) 17. ((reserve[tw] AND cell[tw]) AND (carcinoma*[tw] OR cancer*[tw])) 18. ((oat[tw] AND cell[tw]) AND (carcinoma*[tw] OR cancer*[tw])) 19. OR/15‐18 20. 14 AND 19 21. 13 OR 20 22. "radiotherapy"[mh] 23. "radiotherapy, computer‐assisted"[mh] 24. "radiation dosage"[mh] 25. "radiotherapy dosage"[mh] 26. "radiotherapy,high‐energy"[mh] 27. "radiotherapy, adjuvant"[mh] 28. "dose fractionation"[mh] 29. "brachytherapy"[mh] 30. "radiation oncology"[mh] 31. radiotherap*[tw] 32. (thorac*[tiab] AND radiotherap*[tiab]) 33. (radiat*[tiab] AND therap*[tiab]) 34. (thorac*[tiab] AND radiat*[tiab]) 35. irradiation[tw] 36. OR/22‐35 37. 21 AND 36
Data and analyses
Comparison 1. Early versus late chest RT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 7 | Hazard Ratio (Random, 95% CI) | 0.91 [0.75, 1.10] | |
| 1.1 Platinum‐based CT | 6 | Hazard Ratio (Random, 95% CI) | 0.86 [0.71, 1.04] | |
| 1.2 Non‐platinum based CT | 1 | Hazard Ratio (Random, 95% CI) | 1.27 [0.97, 1.67] | |
| 2 Overall survival (only studies with OTT < 30 days) | 5 | Hazard Ratio (Random, 95% CI) | 0.82 [0.64, 1.06] | |
| 3 Local tumour control (5‐yrs) | 4 | 880 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.69, 1.07] |
| 3.1 Platinum‐based CT | 3 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.75, 1.15] |
| 3.2 Non‐platinum based CT | 1 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.57, 0.87] |
| 4 Local toxicity, all studies | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Pneumonitis | 4 | 762 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [0.95, 3.50] |
| 4.2 Oesophagitis | 6 | 1289 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.99, 1.88] |
| 5 Local toxicity, excluding studies with non‐platinum CT | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Pneumonitis | 3 | 492 | Risk Ratio (M‐H, Random, 95% CI) | 2.15 [0.82, 5.64] |
| 5.2 Oesophagitis | 5 | 1019 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.97, 1.98] |
| 6 Local toxicity, only studies with overall treatment time of chest RT less than 30 days | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Pneumonitis | 3 | 492 | Risk Ratio (M‐H, Random, 95% CI) | 2.15 [0.82, 5.64] |
| 6.2 Oesophagitis | 5 | 1019 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.97, 1.98] |
| 7 Haematological toxicity, all studies | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Leucopenia | 6 | 1200 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [1.26, 1.78] |
| 7.2 Thrombocytopaenia | 7 | 1508 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.96, 1.57] |
| 8 Haematological toxicity, excluding studies with non‐platinum CT | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Leucopenia | 5 | 930 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.19, 1.77] |
| 8.2 Thrombocytopaenia | 6 | 1238 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.99, 1.65] |
| 9 Haematological toxicity, only studies with overall treatment time of chest RT less than 30 days | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Leucopenia | 5 | 930 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.19, 1.77] |
| 9.2 Thrombocytopaenia | 6 | 1238 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.99, 1.65] |
1.1. Analysis.

Comparison 1 Early versus late chest RT, Outcome 1 Overall survival.
1.2. Analysis.

Comparison 1 Early versus late chest RT, Outcome 2 Overall survival (only studies with OTT < 30 days).
1.3. Analysis.
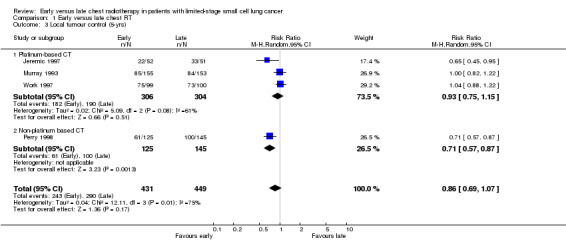
Comparison 1 Early versus late chest RT, Outcome 3 Local tumour control (5‐yrs).
1.4. Analysis.
Comparison 1 Early versus late chest RT, Outcome 4 Local toxicity, all studies.
1.5. Analysis.

Comparison 1 Early versus late chest RT, Outcome 5 Local toxicity, excluding studies with non‐platinum CT.
1.6. Analysis.

Comparison 1 Early versus late chest RT, Outcome 6 Local toxicity, only studies with overall treatment time of chest RT less than 30 days.
1.7. Analysis.
Comparison 1 Early versus late chest RT, Outcome 7 Haematological toxicity, all studies.
1.8. Analysis.
Comparison 1 Early versus late chest RT, Outcome 8 Haematological toxicity, excluding studies with non‐platinum CT.
1.9. Analysis.
Comparison 1 Early versus late chest RT, Outcome 9 Haematological toxicity, only studies with overall treatment time of chest RT less than 30 days.
Comparison 2. High (> 80%) versus low (< 80%) compliance chemotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 High (> 80%) versus low (< 80%) compliance CT | 2 | Hazard Ratio (Random, 95% CI) | 1.11 [0.89, 1.37] |
2.1. Analysis.

Comparison 2 High (> 80%) versus low (< 80%) compliance chemotherapy, Outcome 1 High (> 80%) versus low (< 80%) compliance CT.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jeremic 1997.
| Methods | Randomised, 2‐arm trial Allocation concealment: not reported ITT: yes | |
| Participants | 103 SCLC‐LD patients: 52 (E), 51(L) KPS 90‐100: 19%(E), 47%(L), KPS 50‐80: 48%(E), 53% (L) Age < 70 (Median E: 59, range 40 to 67; median L: 59, range 44 to 66) | |
| Interventions | Early RT (day 1) versus late RT (day 42) RT: 54 Gy/36 f/26d in both arms, twice daily CT: concurrent daily carboplatin/etoposide 30 mg each and 4 cycles of cisplatin/etoposide 30 mg/m2 and 120 mg/m2, respectively, on days 1 to 3 (no concurrent non‐platinum based CT in both arms). PCI if CR or PR: 98% (E), 84% (L) | |
| Outcomes | Survival (2‐ and 5‐ys), respectively 71% E/53% L and 30% E/15% L Local toxicity (grade 3‐4): oesophagitis 29% E/25% L and pneumonitis 2% E/0% L Haematological toxicity (grade 3‐4): leucopenia 33% E/41% L and thrombocytopaenia 38% E/22% L Local control (ys) | |
| Notes | Overall compliance*: 107 patients enrolled onto this study. Four (2 in each group) were excluded later: 3 patients because they were found to have extensive disease and one because simultaneous bladder cancer was detected during treatment. The actual dose‐intensity as a proportion of the planned dose‐intensity was greater than 96% in both arms. Overall conclusion: initial administration of thoracic RT with concurrent C/E seems to produce better local control and survival rates than delayed administration |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "patients were randomised..." |
| Allocation concealment? | Unclear risk | Insufficiently described |
| Incomplete outcome data addressed? All outcomes | Low risk | Quote: "No patient was lost to follow‐up evaluation" |
| Free of selective reporting? | Unclear risk | No protocol available. An expected outcome measure was reported. |
Murray 1993.
| Methods | Randomised, 2‐arm, multicentre trial Allocation concealment: not reported ITT: no | |
| Participants | 308 SCLC‐LD patients: 155 (E), 153 (L) ECOG 0‐1: 87.1%(E), 90.2%(L), ECOG 2‐3: 12.9%(E), 9.9% (L) Age < 80 (Median E: 62, median L: 62) | |
| Interventions | Early RT (day 21) versus late RT (day 105) RT: 40 Gy/15 f/19d, for both arms CT: cyclophosphamide, doxorubicin and vincristine alternating with etoposide and cisplatin every 3 weeks for 3 cycles of each chemotherapy regimen (no concurrent non‐platinum based CT in both arms) PCI if CR: 4% (E), 13% (L) | |
| Outcomes | Survival (2‐ and 5‐ys), respectively 40% E/34% L and 20% E/11% L Local toxicity: oesophagitis 29% E/26% L and pneumonitis 3% E/1% L Haematological toxicity (grade 4): thrombocytopaenia 4% E/3% L Local control (ys) | |
| Notes | Compliance RT: TI was administered to early: 96.1% and to late: 86.9%
Compliance CT: Cyclophosphamide: 87% (E)/87% (L). Doxorubicin: 86% (E)/87% (L). Vincristine: 89% (E)/86% (L) Etoposide: 86% (E)/86% (L). Cisplatin: 84% (E)/83% (L) Multicentre, stratified by centre Overall conclusion: the early administration of TI in the combined modality therapy of SCLC‐LD is superior to late or consolidation TI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "...patients were randomised..." |
| Allocation concealment? | Low risk | Quote: "After stratification by center...." |
| Incomplete outcome data addressed? All outcomes | Low risk | Reason why 24 patients were ineligible are described. It is assumed that no patients were lost to follow up (not reported) since the results of all total patients group are reported in the tables. |
| Free of selective reporting? | Unclear risk | No protocol available. An expected outcome measure was reported. |
Perry 1998.
| Methods | Randomised, 3‐arm trial Allocation concealment: not reported ITT: yes | |
| Participants | 270 SCLC‐LD patients: 125 (E), 145 (L) ECOG 0‐1: 86%(E), 87%(L), ECOG 2‐3: 13%(E), 9% (L) Any age (Median 60, range 32 to 79) | |
| Interventions | Early RT (day 1) versus late RT (day 64) and combined RT/CT versus CT alone RT: 50 Gy/25f/33 days in both arms CT: Cyclophosphamide, vincristine and etoposide (and later doxorubicin) (concurrent non‐platinum based CT in both arms) PCI | |
| Outcomes | Survival (2‐ and 5‐ys), respectively 15% E/25% L and 7% E/13% L Local toxicity: oesophagitis 10% E/8% L and pneumonitis 9% E/ 6% L Local toxicity: leucopenia 45% E/26% L and thrombocytopaenia (grade 2‐4) 5% E/7% L Local control (ys) | |
| Notes | 10‐year update: the third arm (n = 129) in which patients only received chemotherapy was not taken up in this review Overall compliance*: 7 patients who had no measurable disease after surgical resection and 2 for whom data on tumour response were missing were considered unavailable for response. Eleven patients died early or were lost to follow up before evaluation of response at week 9 and were considered not to have responded. Overall conclusion: no statistical difference in survival between early and late thoracic irradiation. Fatal toxicity by concurrent doxorubicin. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "Patients were stratified into four groups on basis of their initial CALGB performance score. After stratification, patients were randomly assigned to one of three treatment regimens. " |
| Allocation concealment? | Unclear risk | Insufficiently described |
| Incomplete outcome data addressed? All outcomes | Low risk | Quote: "...seven patients who had no measurable disease after surgical resection and two for whom data on tumour response were missing were considered unevaluable for response. Fifteen patients died early or were lost to follow‐up before the evaluation of response...." |
| Free of selective reporting? | Unclear risk | No protocol available. An expected outcome measure was reported. |
Skarlos 2001.
| Methods | Randomised, 2‐arm trial Allocation concealment: centrally randomised at the HeCOG data office ITT: yes | |
| Participants | 81 SCLC‐LD patients: 42 (E), 39 (L) ECOG 0‐1: 76% (E), 85% (L), ECOG 2: 24% (E), 15% (L) Any age (Median E: 61, range 40 to 76; median L: 60, range 38 to 79) | |
| Interventions | Early RT (day 1) versus late RT (day 56) RT: 45 Gy/30f/19 days in both arms CT: 6 cycles carboplatin followed by etoposide 100 mg/m2 iv, every 3 weeks (no concurrent non‐platinum based CT in both arms) PCI if CR: 41% (E), 57% (L) | |
| Outcomes | Survival (2‐ and 5‐ys), respectively 36% E/29% L and 22% E/13% L Local toxicity (grade 3‐4): oesophagitis 19% E/23% L and pneumonitis 17% E/10% L Haematological toxicity (GRADE 3‐4): thrombocytopaenia 21% E/23% L and leucopenia 36% E/21% L | |
| Notes | Compliance RT: all patients received their planned dose of HFRT with a median delay of 1 week (mainly due to oesophagitis)
Compliance CT: 71% (E)/90% (L) Overall conclusion: 2 and 3‐year survival rates did not differ significantly between the 2 treatment groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "Randomized phase II study" |
| Allocation concealment? | Low risk | Quote: "...they were centrally randomized at the HeCOG..." |
| Incomplete outcome data addressed? All outcomes | Low risk | Reason why 5 patients were excluded from analysis are described. 1 patient was lost to follow up. |
| Free of selective reporting? | Unclear risk | No protocol available. An expected outcome measure was reported. |
Spiro 2006.
| Methods | Randomised, 2‐arm trial Allocation concealment: randomly assigned using minimisation with stratification per centre ITT: unclear | |
| Participants | 325 SCLC‐LD patients: 159 (E), 166 (L) ECOG 0‐1: 91% (E), 89% (L) Median age both arms: 62. Range: 34 to 74 (E) and 33 to 74 (L) | |
| Interventions | Early (day 21) versus late (day 105) RT RT: 40 GY/15f/21 days in both arms (no concurrent non‐platinum based CT in both arms) CT: 6 cycles every 21 days, cyclophosphamide 1000 mg/m2 iv, doxorubicin 50 mg/m2 iv, vincristine 2 mg iv day 1, alternating with cisplatin 25 mg/m2 iv and etoposide 100 mg/m2 iv days 1‐3 PCI if CR | |
| Outcomes | 3‐ys survival: 16% (E) and 20% (L) Local toxicity RT (grade 3‐4): oesophagitis 7% E/4% L Haematological tox. RT (grade 3‐4): leucopenia 74% E/55% L, thrombopenia 9% in each arm | |
| Notes | Compliance: RT: E/L: 92%/82% CT: E/L: 69%/80% (P = 0.03); percentage cisplatin dose: 70%/79% (P = 0.01) Overall conclusion: no difference in 3‐year survival between early and late chest radiotherapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "randomized clinical trial" |
| Allocation concealment? | Low risk | Quote: "Patients were randomly assigned using minimisation, with stratification by centre, ECOG performance status, sex, and whether or not they had undergone a CT brain scan" |
| Incomplete outcome data addressed? All outcomes | Low risk | Quote: "patients were withdrawn as a result of toxicity.... In addition table 3 reports reasons for not completing all CT courses". |
| Free of selective reporting? | Unclear risk | No protocol available. An expected outcome measure was reported. |
Takada 2002.
| Methods | Randomised, 2‐arm, multicentre trial Allocation concealment: centrally randomised at the JCOG data centre with the minimisation method ITT: no | |
| Participants | 228 SCLC‐LD patients: 114 (E), 114 (L) ECOG 0‐1: 95% (E), 95% (L), ECOG 2: 5% (E), 5% (L) Age < 75 (Median E: 65, range 39 to 74; median L: 64, range 30 to 74) | |
| Interventions | Early RT (day 2) concurrent with CT versus late RT (day 85) sequential with CT RT: 45 Gy/30f/19 days for both arms, twice daily CT: 4 cycles of cisplatin and etoposide every 3 weeks (sequential arm) or 4 weeks (concurrent arm) (no concurrent non‐platinum based CT in both arms) PCI if CR or near CR: 27% | |
| Outcomes | Survival (2‐ and 5‐ys), respectively 54% E/35% L and 24% E/18% L Local toxicity (grade 3‐4): oesophagitis 9% E/4% L Haematological toxicity (grade 3‐4): leucopenia 88% E/54% L and thrombocytopaenia 37% E/26% L | |
| Notes | Compliance RT: the actual dose‐intensity as a proportion of the planned dose‐intensity was greater than 90% in both arms
Compliance CT: Cisplatin: 94% (E)/91% (L). Etoposide: 93% (E)/90% (L) Overall conclusion: this study strongly suggests that concurrent (early) RT is more effective for the treatment of LD‐SCLC than sequential (late) RT |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "randomization was performed using the minimization method" |
| Allocation concealment? | Low risk | Quote: "randomization was performed using the minimization method of balancing institution and PS at the JCOG Data Center." |
| Incomplete outcome data addressed? All outcomes | Low risk | Quote: "Three patients in the sequential arm were ineligible..." In footnote of table 2 (toxicity) it is noted that data were not available for 7 patients in the sequential arm and 2 in the concurrent arm. |
| Free of selective reporting? | Unclear risk | No protocol available. An expected outcome measure was reported. |
Work 1997.
| Methods | Randomised, 2‐arm trial Allocation concealment: not reported ITT: yes | |
| Participants | 199 SCLC‐LD patients: 99 (E), 100 (L) KPS: 80‐100: 82% (E), 80% (L), KPS: 40‐70: 18% (E), 20% (L) Age < 70 (Median E: 61, range 36 to 70; median L: 59, range 36 to 69) | |
| Interventions | Early RT (day 1) versus late RT (day 126) RT: 40 to 45 Gy/15f/47 days in both arms (split‐course treatment, i.e. two treatment periods of 20 or 22.5 Gy in 11 fractions separated by an interval of 21 days in which cisplatin‐based chemotherapy was given). CT: 3 cycles of cisplatin 60 mg/m2 iv, etoposide 120 mg/m2 iv, and 6 cycles of cyclophosphamide 1000 mg/m2 iv, doxorubicin 45 mg/m2 iv, and vincristine 1.4 mg/m2 iv (no concurrent non‐platinum based CT in both arms) PCI if CR : 100% (E), 58% (L) | |
| Outcomes | Survival (2‐ and 5‐ys), respectively 20% E/19% L and 11% E/12% L Haematological toxicity: leucopenia 77% E/13% L and thrombocytopaenia 13% in both arms Local control (ys) | |
| Notes | Compliance RT:
40 Gy: 78% received 40 Gy, whereas 19% received less and 3% more than 40 Gy
45 Gy: 74% received 45 Gy, whereas 21% received less and 5% more than 45 Gy
Compliance CT:
Cyclophosphamide: 84% (E)/84% (L). Doxorubicin: 85% (E)/87% (L). Vincristine: 95% (E)/87% (L). Etoposide: 87% (E)/94% (L). Cisplatin: 97% (E)/99% (L) Overall conclusion: timing of CI did not significantly influence recurrences, or overall survival |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "randomization was on a 1:1 ratio based on a table of random numbers" |
| Allocation concealment? | Unclear risk | Insufficiently described |
| Incomplete outcome data addressed? All outcomes | Low risk | 199 patients were eligible and entered into the study. Same figure is reported in the tables assuming no loss to follow up. |
| Free of selective reporting? | Unclear risk | No protocol available. An expected outcome measure was reported. |
ABBREVIATIONS: C/E: carboplatin/etoposide; CI: chest irradiation; CR: complete response; CT: chemotherapy; d: day; E: early RT; ECOG: Eastern Co‐operative Oncology Group; f: fraction; Gy: Gray; HeCOG: Hellenic Co‐operative Oncology Group; ITT: intention‐to‐treat; iv: intravenous; JCOG: Japan Clinical Oncology Group; KPS: Karnofsky Performance Status; L: late RT; PCI: prophylactic cranial irradiation; RT: radiotherapy; SCLC‐LD: small cell lung cancer‐limited disease; TI: thoracic irradiation; ys: years
* Overall compliance: no data available to distinguish between compliance of radiotherapy and chemotherapy
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Blackstock 2005 | Chest radiotherapy was started on the same day in both arms, thus making comparison between early and late radiation impossible |
| Bonner 1999 | Chest radiotherapy was started on the same day in both arms, thus making comparison between early and late radiation impossible |
| Gregor 1997 | The study was not suitable for this analysis because in 1 arm chest radiotherapy started on day 49 and in the other arm on day 91, implying that according to our inclusion criteria late radiotherapy was delivered in both arms |
| Lebeau 1999 | This study was excluded because the start of chest radiotherapy varied between day 30 and 64 in one arm and day 36 and 47 or day 64 and 75 in the other arm |
| Turrisi 1999 | Chest radiotherapy was started on the same day in both arms, thus making comparison between early and late radiation impossible |
Contributions of authors
DDR, MPJ, JV assessed trials, both for the quality of the methods against pre‐determined criteria and for the results of key outcomes.
DDR, JV extracted data independently to ensure validity, while MPJ was responsible for resolving discrepancies.
MPJ, RH conducted the statistical analyses.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Jeremic 1997 {published data only}
- Jeremic B, Shibamoto Y, Acimovic L, Milisavljevic S. Initial versus delayed accelerated hyperfractionated radiation therapy and concurrent chemotherapy in limited small‐cell lung cancer: a randomized study. Journal of Clinical Oncology 1997;15(3):893‐900. [DOI] [PubMed] [Google Scholar]
Murray 1993 {published data only}
- Murray N, Coy P, Pater JL, Hodson I, Arnold A, Zee BC, et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited‐stage small‐cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group. Journal of Clinical Oncology 1993;11(2):336‐44. [DOI] [PubMed] [Google Scholar]
Perry 1998 {published data only}
- Perry MC, Herndon JE, Eaton WL, Green MR. Thoracic radiation therapy added to chemotherapy for small‐cell lung cancer: an update of Cancer and Leukemia Group B Study 8083. Journal of Clinical Oncology 1998;16(7):2466‐7. [DOI] [PubMed] [Google Scholar]
Skarlos 2001 {published data only}
- Skarlos DV, Samantas E, Briassoulis E, Panoussaki E, Pavlidis N, Kalofonos HP, et al. Randomized comparison of early versus late hyperfractionated thoracic irradiation concurrently with chemotherapy in limited disease small‐cell lung cancer: a randomized phase II study of the Hellenic Cooperative Oncology Group (HeCOG). Annals of Oncology 2001;12(9):1231‐8. [DOI] [PubMed] [Google Scholar]
Spiro 2006 {published data only}
- Spiro S, James LE, Rudd RM, Trask C, Tobias JS, Snee M, et al. Early compared with late radiotherapy in combined modality treatment for limited disease small‐cell lung cancer: a London Lung Cancer Group multicenter randomized trial and meta‐analysis. Journal of Clinical Oncology 2006;24(24):3823‐30. [DOI] [PubMed] [Google Scholar]
Takada 2002 {published data only}
- Takada M, Fukuoka M, Kawahara M, Sugiura T, Yokoyama A, Yokota S, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited‐stage small‐cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. Journal of Clinical Oncology 2002;20(14):3054‐60. [DOI] [PubMed] [Google Scholar]
Work 1997 {published data only}
- Work E, Nielsen OS, Bentzen SM, Fode K, Palshof T. Randomized study of initial versus late chest irradiation combined with chemotherapy in limited‐stage small‐cell lung cancer. Aarhus Lung Cancer Group. Journal of Clinical Oncology 1997;15(9):3030‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Blackstock 2005 {published data only}
- Blackstock AW, Bogart JA, Matthews C, Lovato JF, McCoy T, Livengood K, et al. Split‐course versus continuous thoracic radiation therapy for limited‐stage small‐cell lung cancer: final report of a randomized phase III trial. Clinical Lung Cancer 2005;6(5):287‐92. [DOI] [PubMed] [Google Scholar]
Bonner 1999 {published data only}
- Bonner JA, Sloan JA, Shanahan TG, Brooks BJ, Marks RS, Krook JE, et al. Phase III comparison of twice‐daily split‐course irradiation versus once‐daily irradiation for patients with limited stage small‐cell lung carcinoma. Journal of Clinical Oncology 1999;17(9):2681‐91. [DOI] [PubMed] [Google Scholar]
Gregor 1997 {published data only}
- Gregor A, Drings P, Burghouts J, Postmus PE, Morgan D, Sahmoud T, et al. Randomized trial of alternating versus sequential radiotherapy/chemotherapy in limited‐disease patients with small‐cell lung cancer: a European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group Study. Journal of Clinical Oncology 1997;15(8):2840‐9. [DOI] [PubMed] [Google Scholar]
Lebeau 1999 {published data only}
- Lebeau B, Urban T, Brechot JM, Paillotin D, Vincent J, Leclerc P, et al. A randomized clinical trial comparing concurrent and alternating thoracic irradiation for patients with limited small cell lung carcinoma. Cancer 1999;86(8):1480‐7. [PubMed] [Google Scholar]
Turrisi 1999 {published data only}
- Turrisi AT, Kim K, Blum R, Sause WT, Livingston RB, Komaki R, et al. Twice‐daily compared with once‐daily thoracic radiotherapy in limited small‐cell lung cancer treated concurrently with cisplatin and etoposide. New England Journal of Medicine 1999;340(4):265‐71. [DOI] [PubMed] [Google Scholar]
Additional references
Auperin 1999
- Auperin A, Arriagada R, Pignon JP, Pechoux C, Gregor A, Stephens RJ, et al. Prophylactic cranial irradiation for patients with small‐cell lung cancer in complete remission. New England Journal of Medicine 1999;341(7):476‐84. [DOI] [PubMed] [Google Scholar]
Bentzen 1991
- Bentzen SM, Thomas HD. Clinical evidence for tumor clonogen regeneration: interpretations of the data. Radiotherapy and Oncology 1991;22:161‐6. [DOI] [PubMed] [Google Scholar]
Bunn 1997
- Bunn PA Jr, Carney DN. Overview of chemotherapy for small cell lung cancer. Seminars in Oncology 1997;24(2 Suppl 7):69S‐74S. [PubMed] [Google Scholar]
Davis 2000
- Davis AJ, Tannock IF. Repopulation of tumor cells between cycles of chemotherapy: a neglected factor. The Lancet Oncology 2000;1:86‐93. [DOI] [PubMed] [Google Scholar]
De Ruysscher 2000
- Ruysscher D, Vansteenkiste J. Chest radiotherapy in limited‐stage small cell lung cancer: facts, questions, prospects. Radiotherapy and Oncology 2000;55(1):1‐9. [DOI] [PubMed] [Google Scholar]
De Ruysscher 2006
- Ruysscher D, Pijls‐Johannesma M, Bentzen SM, Minken A, Wanders R, Lutgens L, et al. Time between the first day of chemotherapy and the last day of chest radiation is the most important predictor of survival in limited‐disease small‐cell lung cancer. Journal of Clinical Oncology 2006;24(7):1057‐63. [DOI] [PubMed] [Google Scholar]
Harari 2003
- Harari PM, Mehta MP, Ritter MA, Petereit DG. Clinical promise tempered by reality in the delivery of combined chemoradiation for common solid tumors. Seminars in Radiation Oncology 2003;13(1):3‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Ihde 1995
- Ihde DC. Small cell lung cancer. State‐of‐the‐art therapy 1994. Chest 1995;107(6 Suppl):243S‐248S. [DOI] [PubMed] [Google Scholar]
Kelly 2000
- Kelly K. New chemotherapy agents for small cell lung cancer. Chest 2000;117(4 Suppl 1):156S‐162S. [DOI] [PubMed] [Google Scholar]
Kumar 1997
- Kumar P. The role of thoracic radiotherapy in the management of limited‐stage small cell lung cancer: past, present, and future. Chest 1997;112(4 Suppl):259S‐65S. [DOI] [PubMed] [Google Scholar]
Pignon 1992
- Pignon JP, Arriagada R, Ihde DC, Johnson DH, Perry MC, Souhami RL, et al. A meta‐analysis of thoracic radiotherapy for small‐cell lung cancer. New England Journal of Medicine 1992;327(23):1618‐24. [DOI] [PubMed] [Google Scholar]
Pijls‐Johannesma 2007
- Pijls‐Johannesma M, Ruysscher DK M, Lambin P, Rutten I, Vansteenkiste JF. Timing of chest radiotherapy in patients with limited stage small cell lung cancer: a systematic review and meta‐analysis of randomised controlled trials. Cancer Treatment Reviews 2007;33(5):461‐73. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8(16):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turrisi 2002
- Turrisi AT, Sherman CA. The treatment of limited small cell lung cancer: a report of the progress made and future prospects. European Journal of Cancer 2002;38(2):279‐91. [DOI] [PubMed] [Google Scholar]
Warde 1992
- Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited‐stage small‐cell carcinoma of the lung? A meta‐analysis. Journal of Clinical Oncology 1992;10(6):890‐5. [DOI] [PubMed] [Google Scholar]
Withers 1988
- Withers HR, Taylor JM, Maciejewski B. The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncologica (Stockholm, Sweden) 1988;27:131‐46. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Pijls‐Johannesma 2005
- Pijls‐Johannesma M, Ruysscher DKM, Lambin P, Rutten I, Vansteenkiste JF. Early versus late chest radiotherapy in patients with limited stage small cell lung cancer. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD004700.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


