Abstract
Capsaicin, the irritant principle of hot peppers, is a vanilloid agonist known to activate the transient receptor potential channel vanilloid subfamily member 1 (VR1), recently reported to be involved in neurodegeneration. The present study investigated the role of VR1 in a model of global cerebral ischemia in gerbils.
Over the dose range tested, capsaicin (0.01, 0.025, 0.05, 0.2 and 0.6 mg kg−1), given 5 min after recirculation, dose-dependently antagonized the ischemia-induced electroencephalographic total spectral power decrease and restored relative frequency band distribution evaluated 7 days after ischemia.
Capsaicin, at all tested doses, fully prevented ischemia-induced hyperlocomotion evaluated 1 day after ischemia.
Capsaicin dose-dependently antagonized ischemia-induced memory impairment evaluated in a passive avoidance task, 3 days after ischemia.
Capsaicin showed a dose-dependent hypothermic effect evaluated for 2 h after recirculation.
At 7 days after ischemia, a progressive survival of pyramidal cells in the CA1 subfield in capsaicin-treated gerbils, with a maximum of 80%, at a dose of 0.2 mg kg−1, was obtained.
The selective VR1 antagonist, capsazepine (0.01 mg kg−1), reversed capsaicin-induced protective effects, in a competitive manner.
These results suggest that the neuroprotective effect of capsaicin may be attributable, at least in part, to VR1 desensitization and provide a valuable target for development of interventional pharmacological strategies.
Keywords: Capsaicin, vanilloid agonist, vanilloid antagonist, ischemia, EEG, memory, motor activity, rectal temperature, CA1, neuroprotection
Introduction
Capsaicin (N-(3-methoxy-4-hydroxy-benzyl)-8-methyl-6-trans-nonenamide or N-vanillyl-8-methyl-6-trans-nonenamide), the irritant principle of hot peppers, is a vanilloid agonist known to activate the transient receptor potential channel vanilloid subfamily member 1 (TRPV1), named vanilloid receptor 1 (VR1) (Gunthorpe et al., 2002; van der Stelt & Di Marzo, 2004) and to possess analgesic (Perkins & Campbell, 1992), anti-inflammatory (Kim et al., 2003) and hypolocomotor effects (Di Marzo et al., 2001).
TRPV1 is believed to function as a molecular integrator of noxious stimuli (Szallasi & Blumberg, 1999; Cortright & Szallasi, 2004). However, the recent discovery in both human and rat brains of TRPV1, where it is unlikely to be gated by noxious stimuli, has suggested additional functional roles for this channel (Sasamura & Kuraishi, 1999; Hayes et al., 2000; Mezey et al., 2000) in physiological processes such as satiety, cognition and motor control (Szallasi et al., 1995). There is also some evidence of involvement of TRPV1 in neurodegeneration: (i) Pathological changes in brain temperature or pH, for example after a severe stroke, may influence TRPV1 activity (Marinelli et al., 2002) suggesting an important role in events occurring after ischemia (like modulation of glutamate release and consequent activation of excitatory amino-acid receptors); (ii) TRPV1 is present in brain regions (hippocampus) that are highly susceptible to neurodegenerative insults, suggesting that this ion channel might contribute to the cellular processes involved in neuronal death (Mezey et al., 2000); and (iii) capsaicin and the vanilloid antagonist capsazepine (CPZ), peripherally administered, have been shown to exhibit neuroprotection against ouabain-induced excitotoxicity in rats through a mechanism not fully identified (Veldhuis et al., 2003).
In addition, TRPV1 can be activated by endogenously generated compounds such as anandamide and lipoxygenase products, which accumulate during brain injury (Marinelli et al., 2002). In this context, capsaicin has been shown to stimulate the biosynthesis of endocannabinoids (Di Marzo et al., 2001), which may afford neuroprotection.
In the current study, we investigated the possible neuroprotectant effect of capsaicin in vivo using a model of transient global cerebral ischemia in the gerbil. Since capsaicin can be tested only up to 1.0 mg kg−1 due to its toxic effects in vivo (Di Marzo et al., 2001), we employed a wide range of doses (from 0.01 to 0.6 mg kg−1) below 1 mg kg−1. To quantify the ischemic damage, we measured, from 1 h to 7 days after recirculation, different parameters known to be hardly influenced by global cerebral ischemia: electroencephalographic (EEG) spectral power, spontaneous motor activity, memory function and hippocampal CA1 neuronal count. We measured motor activity on day 1, since ischemia-induced hyperlocomotion is maximal 24 h after occlusion (Araki et al., 1986), memory function on day 3 according to Sala et al. (1997), and EEG spectral power and neuronal counts on day 7 since EEG decrease has been related to pronounced damage of neurons on day 7 (Suzuki et al., 1983; Hunter et al., 1995; Peruche et al., 1995). Rectal temperature was also monitored during the first 2 h after recirculation.
The role of TRPV1 was studied using the vanilloid antagonist CPZ peripherally administered.
Methods
Animals
Male Mongolian gerbils (Meriones unguiculatus) (Charles River, Calco, Como, Italy) weighing 60–80 g were housed singly in standard laboratory conditions: air-conditioned room (22±2°C), 12-h light/12-h dark illumination cycle, free access to food and water.
The gerbils were allowed to acclimatize themselves to the environment for a period of 1 week prior to the surgical implantation of cortical electrodes. All gerbils were submitted to EEG electrodes implantation and then divided in different groups on the basis of the treatment received. Each animal was submitted to all tests.
All procedures were approved under Italian Governmental decree No. 94/2000-A.
Surgical procedure
Gerbils were anesthetized with an intraperitoneal injection of chloral hydrate (450 mg kg−1, Sigma, St Louis, MO, U.S.A.) dissolved in saline and given at a volume of 9 ml kg−1. Four electrodes (Bilaney, Dusseldorf, Germany) for EEG recordings were implanted, as described elsewhere (Sala et al., 1997), on the right and left of the parieto-occipital cortex according to the coordinates (anterior +2, posterior −3, lateral 2, ventral 1.6 from bregma) of a brain atlas (Loskota et al., 1974). A further electrode was inserted into the nasal bone as ground. The five electrodes were connected to a pedestal (Bilaney, Dusseldorf, Germany) and fixed with an acrylic cement (Palavit, New Galetti & Rossi, Milan, Italy). After the chronic implantation of the electrodes, 1 week was allowed for recovery from surgery before experiments started.
EEG recording
Freely moving awake gerbils were acclimatized in a sound-attenuated Faraday chamber and then their EEG was recorded for 1 h a day, for 3 days, to determine the basal total and relative spectral power. Spectral powers between 0 and 25 Hz (0.2–4.0 Hz δ, 4.2–8.0 Hz θ, 8.2–13 Hz α, 13.2–25 Hz β) were evaluated using a resolution of 0.2 Hz. The signals were recorded on paper and processed for fast Fourier transform spectral analysis. EEG recordings were also made during ischemia and 7 days after. Each 1 h spectral power was calculated as the mean of six 1 min recordings taken at 10 min intervals.
Cerebral ischemia
After basal EEG recordings, each gerbil was again lightly anesthetized with 2,2,2-tribromoethanol (200 mg kg−1, 10 ml kg−1; Sigma Aldrich, St Louis, MO, U.S.A.). Body temperature was kept at 37°C throughout surgery with a heating lamp during 10-min ischemia, which was induced by common carotid occlusion as previously described (Braida et al., 2000). The ischemia was verified qualitatively on paper by the complete flattening of the EEG. A group of animals (sham-operated) was submitted to the same surgical procedure except for carotid clamping.
Body temperature
The experiments were performed in the sound-attenuated Faraday chamber. Controls, sham-operated and ischemic gerbils were trained to the temperature measurements for 5 days before thermoregulator reactions were tested as described by Sala et al. (1997). Briefly, after a 1-h acclimatization period in the test room, maintained at 22°C, body temperature was monitored with a rectal thermistor probe (PRA-22002-A, Ellab, Roedovre, Denmark) inserted 3 cm into the colon. After a 30-s equilibration period, the temperature was recorded to the nearest 0.1°C on a CTD 85-M Thermometer (Ellab, Roedovre, Denmark). During temperature measurements, gerbils were unrestrained and were held gently by hand at the base of the tail. Temperature was measured three times (every 10 min) before induction of ischemia (basal) and again at 30, 60, 90 and 120 min after recirculation.
Locomotor activity
Spontaneous motor activity was evaluated as previously described (Braida et al., 2000) in an activity cage (43 cm long × 43 cm wide × 32 cm high; Ugo Basile, Varese, Italy) placed in a sound-attenuating room. The cage was fitted with two parallel horizontal infrared beams located 2 cm from the floor. Cumulative horizontal movement counts were recorded for 30 min, every 5 min, 1 day after ischemia.
Passive avoidance task
On the third day after ischemia, each gerbil was submitted to the passive avoidance task. The apparatus (Ugo Basile, Varese, Italy) consisted of a box divided by a guillotine door into two compartments of the same size (22 cm long × 22 cm wide × 21 cm high) in which the floor had a grid of stainless steel rod. One compartment was lit with a 10 W electric bulb, and the other compartment was dark. The step-through type passive avoidance task was used as described by Katoh et al. (1992), with some modifications. Adaptation was carried out 10 min before the training, and retention 1 day after the training. For the adaptation, a gerbil was placed in the light compartment, and allowed to explore both compartments by leaving the guillotine door open for 5 min. After 10 min, training started during which each gerbil was placed in the light compartment and allowed to enter the dark. When the gerbil entered the dark compartment, the door was automatically closed and an unavoidable scrambled foot shock (1.5 mA) was delivered for 5 s. Each gerbil was then allowed to stay there for 10 s. The procedure was immediately repeated twice. For the retention test, gerbils were placed in the light compartment and the latency to re-enter the dark compartment was recorded up to a maximum of 180 s.
Histology
At 7 days after the ischemic injury, all the gerbils were anesthetized with an overdose of chloral hydrate 5% and transcardially perfused with 4% paraformaldehyde (Sigma Aldrich, St Louis, MO, U.S.A.) for the histological determination as previously described (Braida et al., 2003). Then, brains were removed and placed in the same fixative overnight. Brains were embedded in paraffin wax. Five serial 5-μm coronal hippocampal sections at 1.5, 1.7 and 1.9 mm caudal to bregma were cut using a microtome (Leica, Mod. RM2125RT, Solms, Germany) and stained with cresyl violet (Sigma Aldrich, St Louis, MO, U.S.A.). Neurons with a normal appearance in the pyramidal cell layer of the CA1 sector were counted blind (from coded slides) in each section for each group.
Treatment
Gerbils submitted to ischemia were divided in 12 groups of five animals each, receiving s.c. vehicle+vehicle, vehicle+capsaicin (0.01, 0.025, 0.05, 0.2 and 0.6 mg kg−1) (Tocris Cookson Ltd, U.K.), CPZ (0.01 mg kg−1) (Tocris Cookson Ltd, U.K.)+vehicle and CPZ (0.01 mg kg−1)+capsaicin (0.01, 0.025, 0.05, 0.2 and 0.6 mg kg−1). Capsaicin was injected 5 min after recirculation, while CPZ was given 15 min before carotid occlusion. Vehicle was given 5 min after or 15 min before ischemia. Both drugs were dissolved in an appropriate vehicle (Tween 80, ethanol, saline, 1 : 1 : 8) and injected in a volume of 10 ml kg−1. A further group, sham-operated, received the same volume of vehicle.
Statistical analysis
Results were presented as mean±s.e.m. Total EEG spectral data were expressed as mean of the percent difference vs preischemic value while relative power was expressed as percent of total. For the temperature results, data were expressed as the means of °C deviation from the baseline. EEG, motor activity, latency time and neuronal count were analyzed by one-way analysis of variance (ANOVA) for multiple comparisons, followed by Tukey's test. Temperature data were analyzed by repeated measures analysis of variance (two-way ANOVA) with drug treatment as a between-subjects factor and time as a within-subjects factor. The post hoc individual comparisons were performed with Bonferroni's test or Student's t-test. The accepted level was P<0.05. All statistical analyses were performed using software Prism version 4 (GraphPad, U.S.A.).
Results
The physiological parameters, such as food and water consumption and body weight, were stable throughout the study in all groups (data not shown).
EEG
A quantitative EEG analysis of gerbils treated with increasing doses of capsaicin is given in Figure 1, in which significant between-group differences were observed in terms of percent vs preischemic value of mean total spectral power (F(11,48)=32.913, P<0.0001, ANOVA), evaluated on day 7. Post hoc analysis showed that, in comparison with sham-operated values, the vehicle group had a decrease of EEG power of about 80%. Increasing doses of capsaicin progressively antagonized the ischemia-induced EEG flattening. At higher doses (0.2 and 0.6 mg kg−1), a complete recovery was obtained. CPZ per se (0.01 mg kg−1) did not induce any change in comparison with the vehicle group (data not shown). Thus, this dose was chosen to study a possible antagonism. Pretreatment with CPZ only partially antagonized the protective effect observed with capsaicin alone at the doses of 0.2 and 0.6 mg kg−1.
Figure 1.
Cortically derived EEG total spectral power evaluated as the difference (Δ%) from the preischemic value in freely moving awake gerbils given increasing doses (mg kg−1) of capsaicin 5 min after recirculation either singly or in combination with CPZ at a dose of 0.01 mg kg−1 administered s.c. 15 min before bilateral carotid occlusion. Veh=vehicle. Each column represents the mean (±s.e.m.) of five animals. aP<0.001 compared with sham;bP<0.001 compared with capsaicin 0.2 and 0.6; cP<0.001 compared with vehicle; dP<0.05, eP<0.001 in comparison with CPZ+capsaicin (one-way ANOVA followed by Tukey's test).
There was a drug treatment effect in the relative distribution of power over frequency bands, except for β (F(12,52)=164.00, P<0.0001, ANOVA for δ) (F(12,52)=93.26, P<0.0001, ANOVA for θ) (F(12,52)=74.19, P<0.0001, ANOVA for α) (F(12,52)=0.96, NS, ANOVA for β) calculated 7 days after recirculation. Post hoc analyses revealed that ischemia significantly increased the relative EEG power density in the δ, θ and α frequency band in the vehicle-treated group (Table 1). Increasing doses of capsaicin progressively restored the power density distribution. No differences were found between vehicle- and CPZ-alone-treated gerbils, while the antagonist significantly reversed the protective effect of capsaicin starting from the dose 0.05 mg kg−1 shown for δ and θ bands.
Table 1.
Effect of capsaicin and CPZ on spectral power distribution evaluated 7 days after ischemia, in gerbils
| Drug (dose (mg kg−1 s.c.)) | δ (0.2–4.0 Hz) | θ (4.2–8.0 Hz) | α (8.2–13 Hz) | β (13.2–25 Hz) |
|---|---|---|---|---|
| Sham | 45±3.0 | 44±2.0 | 10±0.4 | 1.0±0.3 |
| Vehiclea | 62±2.0*** | 32±2.2** | 5±1.7** | 1.0±0.6 |
| Capsaicin (0.01)b | 65±1.9*** | 30±0.8** | 4±0.5** | 1.0±0.22 |
| Capsaicin (0.025)b | 58±1.2*** | 33±1.6** | 8±0.9 | 1.0±0.3 |
| Capsaicin (0.05)b | 50±1.6** | 39± 1.2* | 10±0.9$ | 1.0±0.3 |
| Capsaicin (0.2)b | 38±3.0$$$ | 49±3.0$$ | 13±0.8$ | 1.0±0.3 |
| Capsaicin (0.6)b | 40±2.0$$$ | 48±1.0$$ | 11±0.8$ | 1.5±0.4 |
| CPZ (0.01) | 63±1.0*** | 31±2.5** | 5± 1.5** | 1.0±0.5 |
| CPZ (0.01)c+capsaicin (0.01) | 62±1.0*** | 31±1.0** | 2±0.3** | 1.0±0.2 |
| CPZ (0.01)c+capsaicin (0.025) | 62±1.5*** | 30±2.0** | 7±0.9* | 1.0±0.4 |
| CPZ (0.01)c+capsaicin (0.05) | 60±2.0***### | 32±2.0**# | 7±1.0* | 1.0±0.2 |
| CPZ (0.01)c+capsaicin (0.2) | 58±1.5***### | 30±1.6**### | 11±0.6$ | 1.0±0.2 |
| CPZ (0.01)c+capsaicin (0.6) | 50±1.0**### | 37±.01*### | 12±1.0$ | 1.0±0.3 |
Vehicle was given 15 min before carotid occlusion or 5 min after recirculation.
Capsaicin was administered s.c. 5 min after recirculation.
CPZ was injected s.c. 15 min before carotid occlusion.
Each value represents mean (±s.e.m.) of five animals. CPZ=capsazepine. *P< 0.05, **P<0.01, ***P<0.001 compared with sham-operated group; $P<0.05, $$P<0.01, $$$P<0.001 compared with the vehicle group and CPZ alone; #P<0.05, ###P<0.001 compared with capsaicin alone, same dose (ANOVA followed by Tukey's test).
Spontaneous locomotor activity
Significant between-group modifications in spontaneous locomotor activity were found (F(11,48)=23.638, P<0.0001, ANOVA) (Figure 2). As expected, the vehicle group showed an increase of motility in comparison to the sham-operated group (Tukey's test). The post hoc Tukey's test showed that capsaicin completely antagonized ischemia-induced hyperlocomotion at all tested doses. CPZ (0.01 mg kg−1), which per se did not affect ischemia-induced hyperlocomotion (data not shown), reversed the protective effect of capsaicin at doses of 0.01 and 0.025 mg kg−1 (Tukey's test) but it failed to block the protective effect of capsaicin shown at higher doses.
Figure 2.
Effect of increasing doses (mg kg−1) of capsaicin given s.c. 5 min after recirculation either singly or in combination with CPZ at a dose of 0.01 mg kg−1 administered s.c. 15 min before bilateral carotid occlusion on spontaneous motor activity evaluated for 30 min, 1 day after ischemia in gerbils. Veh=vehicle. Each column represents the total number of horizontal counts (mean±s.e.m.) of five animals. aP<0.001 compared with sham; bP<0.001 compared with corresponding capsaicin alone; cP<0.01, dP<0.001 compared with vehicle (one-way ANOVA followed by Tukey's test).
Passive avoidance
The mean latency time revealed a significant difference between groups (F(11,48)=35.22, P<0.0001, ANOVA) (Figure 3). Post hoc analyses revealed that vehicle-treated gerbils exhibited a significant impairment as shown by the decrease of mean escape latency in comparison with the sham-operated group. In the range of doses between 0.01 and 0.2 mg kg−1, capsaicin produced a progressive increase of the latency. No effect was observed with the highest dose. When CPZ was given in combination with increasing doses of capsaicin, a complete antagonism was obtained (Tukey's test). CPZ alone did not modify the ischemia-induced mean escape latency (data not shown).
Figure 3.
Escape latency evaluated in the passive avoidance task 3 days after ischemia. Increasing doses (mg kg−1) of capsaicin were given s.c. 5 min after recirculation either singly or in combination with CPZ at a dose of 0.01 mg kg−1 administered s.c. 15 min before bilateral carotid occlusion. Veh=vehicle. Each column represents the mean (±s.e.m.) of five animals. aP<0.01, bP<0.001 compared with sham; cP<0.001 compared with corresponding capsaicin alone; dP<0.001 compared with vehicle (one-way ANOVA followed by Tukey's test).
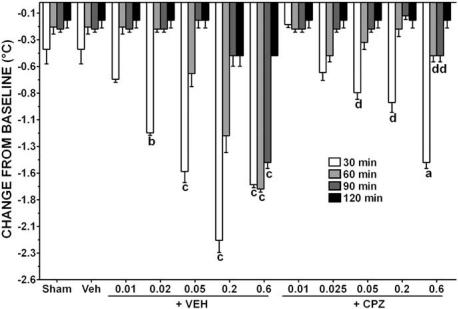
Body temperature
A repeated measures ANOVA revealed a significant dose (F(11,144)=55.62, P<0.0001, ANOVA), time (F(3,144)=622.60, P<0.0001, ANOVA) and interaction effect (F(33,144)=55.62, P<0.0001, ANOVA) of rectal temperature between groups evaluated at 30, 60, 90 and 120 min after recirculation (Figure 4). Post hoc test revealed a nonsignificant decrease of body temperature in both sham-operated and vehicle-treated groups at all tested intervals. Increasing doses of capsaicin progressively decreased the mean rectal temperature at 30 min. At 60 min, a hypothermic effect was still present for capsaicin 0.2 and 0.6, while at 90 min this effect was shown only for the highest dose. No significant hypothermic effect at 120 min was observed. Pretreatment with CPZ, which per se did not affect this parameter (data not shown), completely antagonized capsaicin-induced hypothermic effect in the range between 0.025 and 0.2 mg kg−1. The effect produced by the highest dose of capsaicin was reversed by CPZ starting from 60 min.
Figure 4.
Effect of increasing doses (mg kg−1) of capsaicin given s.c. 5 min after recirculation either singly or in combination with CPZ at a dose of 0.01 mg kg−1 administered s.c. 15 min before bilateral carotid occlusion on changes from baseline in rectal temperature. Veh=vehicle. Rectal temperatures were measured starting from 5 min after recirculation. Each column represents the mean (±s.e.m.) of five gerbils. aP<0.05, bP<0.01, cP<0.001 compared with sham; dP<0.001 compared with corresponding capsaicin alone (two-way ANOVA followed by Bonferroni's test).
Histology
Histological examination of the hippocampus at 7 days after recirculation following 10 min of occlusion showed severe alteration in the CA1 region (Figure 5b) in comparison to the sham-operated animals (Figure 5a). Most of the pyramidal cells were darkly stained and shrunken. Increasing doses of capsaicin led to a progressive survival of neurons (data not shown), except for the highest (0.6 mg kg−1). Maximal protection was obtained with capsaicin at 0.2 mg kg−1 (Figure 5c). Pretreatment with CPZ significantly antagonized capsaicin-induced neuroprotective effect (Figure 5d). ANOVA revealed a significant difference in the number of survived neurons in the CA1 region (F(7,32)=45.89, P<0.0001) (Figure 6). There was a mean loss of 80% of neuronal cells in vehicle-treated gerbils in comparison with the sham-operated group. A progressive survival in the animals given increasing doses of capsaicin compared to the vehicle group was obtained in the range of doses between 0.02 and 0.2 mg kg−1,with a peak of 75%.
Figure 5.
Photomicrographs of the hippocampal CA1 region of gerbils with or without 10-min ischemia, evaluated 7 days after ischemia. (a) Sham-operated animal. (b) Ischemic animal treated with vehicle 5 min after recirculation. (c) Ischemic animal treated with capsaicin (0.2 mg kg−1) 5 min after recirculation. (d) Ischemic animal treated with CPZ (0.01 mg kg−1) and capsaicin. Bar=50 μm.
Figure 6.
Effect of increasing doses of capsaicin (mg kg−1) on neuronal count evaluated 7 days after recirculation in the CA1 region of the hippocampus of sham-operated or ischemic gerbils. Capsaicin was given s.c. 5 min after recirculation. For the antagonism, capsaicin (0.2 mg kg−1) was given in combination with CPZ at a dose of 0.01 mg kg−1 given s.c. 15 min before bilateral carotid occlusion. Each column represents the mean (±s.e.m.) of five hippocampal sections from the same coronal plane for each animal. n=5 for each group. aP<0.05, bP<0.001 compared with sham; cP<0.05, dP<0.01, eP<0.001 compared with vehicle; fP<0.001 compared with capsaicin alone (one-way ANOVA followed by Tukey's test).
Discussion
We report here a full protection afforded by the VR1 agonist capsaicin on global cerebral ischemia in the gerbil. The neuroprotection was quantified in terms of complete recovery of total and relative spectral power, spontaneous motor activity, memory function and hippocampal CA1 neuronal density. These parameters are known to be affected by global cerebral ischemia as previously reported (Suzuki et al., 1983; Araki et al., 1986; Hunter et al., 1995; Peruche et al., 1995; Sala et al., 1997). In addition, both cognitive and sensorimotor deficits can be found in some human pathologies such as heart attack and coronary artery bypass surgery (Hunter et al., 1998). Furthermore, the histopathology seen in the gerbil is similar to that observed in the hippocampal CA1 region of human brain following cardiac arrest (Hunter et al., 1995). Capsaicin prevented all these mentioned neuropathological consequences of ischemia, suggesting its therapeutic potential.
For all behavioral parameters, a dose-dependent effect was obtained except for spontaneous motor activity, as capsaicin completely reduced ischemia-induced hyperlocomotion, at all tested doses. We can suggest that, even if tested on day 1 after treatment, capsaicin per se might exhibit hypolocomotor activity by increasing biosynthesis and release of anandamide, as previously found in rats by Di Marzo et al. (2001), using a similar range of doses.
Severe interference with cerebral blood flow has a pronounced effect on electrical activity, which is reflected in EEG flattening. The decrease in EEG spectral power observed 7 days after ischemia has been related to a severe neuronal damage (delayed death), since the cells are replaced by astrocytes in the hippocampal CA1 subfield (Peruche et al., 1995). The protection induced by capsaicin indicates that the compound may contrast the cascade of pathological events that lead to neuronal death. The hippocampus is a preferred area for global ischemia-induced damage and the CA1 sector is the most selectively vulnerable to the reduced cerebral blood flow (Schmidt-Kastner & Freund, 1991). The survival of CA1 neurons obtained 7 days after ischemia with capsaicin (0.2 mg kg−1) may account for the protection against EEG flattening in capsaicin-treated gerbils.
Results obtained from the EEG power spectral analysis indicate changes in the relative spectral composition of vehicle ischemic gerbils. There was a shift toward the lower frequency band, causing an increase in the relative power of the δ band. The increase in relative power of this frequency band is considered a sign of synchronization of neuronal activity (Frigeni et al., 2004). The increase was associated with a decrease in relative power of the θ and α frequency bands. Comparable EEG alterations have been reported for different kinds of focal brain ischemia and different animals species (Gloor et al., 1977; Hossmann & Schuier, 1980; Kataoka et al., 1987). Following treatment with capsaicin, as opposed to vehicle treatment, there was a significant recovery of spectral power distribution toward basal values, suggesting the presence of recovering neuronal activity.
Capsaicin promoted an important protection against motor hyperactivity. This paradoxical behavior may indicate a reduction of the ischemic animal's ability to form spatial maps (O'Neill & Clemens, 2000) because of the loss of CA1 neurons. These findings are further sustained by memory results. Capsaicin prevented ischemia-induced impairment of memory retention as shown by the longer mean escape latency, in comparison to vehicle-treated ischemic gerbils. It is widely reported that a brief period of cerebral ischemia induces neurological dysfunction in laboratory animals, which is reflected in the deterioration of memory function due to a more vulnerability of CA1 pyramidal cells (LaPoncin-Lafitte et al., 1981; Kiyota et al., 1985).
The protective effect of capsaicin was accompanied by a clear dose-dependent hypothermic effect. Presently, postischemic hypothermia provides the best long-term functional and histological protection after global ischemia (Freund et al., 1990; Colbourne & Corbett, 1995; Colbourne et al., 1999b; Hickey et al., 2000). For instance, prolonged mild hypothermia persistently reduces CA1 zone delayed neuronal death even when cooling begins up to 12 h postischemia (Colbourne et al., 1999a). Hypothermia-induced improvement in postischemic functional recovery has been recently demonstrated, which correlated with decreased production of ascorbyl radicals, considered as a marker of oxidative stress either in vitro or in vivo (Sharma et al., 1994; Vergely et al., 1998) and in isolated rat heart model (Gambert et al., 2004). Capsaicin given s.c. from 0.1 to 5 mg kg−1 has been reported to cause a fall of body temperature in several animal species (Sasamura & Kuraishi, 1999), acting directly on thermoregulatory neurons of the hypothalamus (Kobayashi et al., 1998; Osaka et al., 2000). Our data suggest that VR1 is required for the hypothermic effect in agreement with Caterina et al. (2000) who found no decrease in body temperature in VR1−/− mice when compared with wild-type mice. However, no antagonism on rectal temperature with CPZ (30 mg kg−1) pretreatment in mice given capsaicin (0.3 mg kg−1) i.v. (Di Marzo et al., 2000) was observed. The different animal species, route of administration, doses and experimental protocol used in the present study probably account for this discrepancy.
The protective effect of capsaicin is supported by previous findings in which the VR1 agonist, given i.p. at a dose of 1 mg kg−1, led to neuroprotection against ouabain-induced in vivo excitotoxicity in Wistar rats (Veldhuis et al., 2003). The proposed mechanism suggested by the authors was a VR1 desensitization or endocannabinoids biosynthesis stimulation.
In our ischemic model, pretreatment with CPZ, at a dose that per se was ineffective, reversed, in a competitive manner, total spectral power, behavioral correlates and body temperature, suggesting that the protective effect of capsaicin was VR1 mediated.
The mechanism by which capsaicin exhibits its protective effect is still unclear. However, it has been reported that during brain injury, N-acyl-ethanolamines (including anandamide) and other membrane breakdown products accumulate in the brain (Hansen et al., 2002). These compounds activate VR1 with subsequent Ca2+ influx (Szallasi & Blumberg, 1999), glutamate release (Marinelli et al., 2002) and substantial contribution to neuronal excitotoxicity (apoptosis) (Maccarrone et al., 2000; Hail, 2003). Conversely, exogenous compounds capable of quickly desensitizing VR1, such as capsaicin, might exert neuroprotective action by preventing VR1 activation by endogenous stimuli. In this respect, pretreatment with CPZ may prevent VR1 desensitization. A block of capsaicin-induced desensitization has been reported to occur also centrally (Hajos et al., 1987). It can be argued that a simple dose of capsaicin (0.2 mg kg−1) may not be enough to produce vanilloid receptor desensitization. It is well documented that anandamide binds with great affinity to cannabinoid receptor type 1 (CB1) leading to a neuroprotection, most likely via a dominant presynaptic inhibition of glutamate release (Shen & Thayer, 1998; Nagayama et al., 1999; Sinor et al., 2000). In addition, a great amount of the endovanilloid ligand anandamide accumulates considerably in response to ischemia (Hansen et al., 2002), which causes a homologous desensitization of the capsaicin-induced response and vice versa in human VR1-transfected cells (Smart et al., 2000). Thus, an additive effect of capsaicin and anadamide in producing a massive increase of Ca2+, leading to desensitization, can be hypothesized.
The antagonism obtained with CPZ does not agree with the findings of Ray et al. (2003), who found that CPZ per se protected against cell death in an oxygen glucose deprivation model of organotypic hippocampus slices culture derived from both VR1 knockout and wild-type mice, via a non-VR1-mediated mechanism. In addition, a high dose of CPZ (10 mg kg−1) led to neuroprotection as previously reported in an in vivo rat model of excitotoxicity (Veldhuis et al., 2003). The low dose and the different model of injury used in the present study probably account for this discrepancy. Experiments are in progress to verify if higher doses of CPZ lead to neuroprotection in our model. A possible explanation for the lack of a protective effect of CPZ may be the presence in the central nervous system of an as yet undefined CBn/VRn receptor, as already suggested by different authors (Brooks et al., 2002; Hajos & Freund, 2002; Veldhuis et al., 2003), sensitive to capsaicin and CPZ at a very low dose, which might play a role in neuroprotection.
It is widely reported that large increases in Ca2+ influx during and following brain ischemia contribute to both acute and delayed neuronal death. On the other hand, moderate increase in Ca2+ influx may activate cell survival programs and promote resistance to hypoxia or ischemia, increasing the antiapoptotic protein, protein kinase B, and the MAP kinase ERK levels. It has been recently reported that calcimycin or ionomycin Ca2+ preconditioning greatly reduced cell death in CA1–CA3 and dentate neurons following 7 days after simulated ischemia induced by oxygen and glucose deprivation (Bickler & Fahlman, 2004). It can be argued that our single dose of capsaicin moderately increased Ca2+ influx that served as potent preconditioning stimulus via VR1 activation, inducing a tolerance of subsequent larger elevation in Ca2+ influx. However, since capsaicin was given after recirculation, it is unlikely that it worked as preconditioning stimulus.
Since cytotoxic and vasogenic edema appears during and after bilateral carotid occlusion in gerbils (Hakamata et al., 1997), we cannot exclude the possibility that other mechanisms may have contributed to the protection afforded by capsaicin. For example, capsaicin has potent anti-inflammatory properties by inhibiting the activity of cyclooxygenase-2 and the expression of inducible nitric oxide synthase protein in lipopolysaccharide-stimulated murine peritoneal macrophages (Kim et al., 2003) and in vivo via inhibition of nuclear factor-κB, a transcription factor essentially involved in reactive oxygen species-induced apoptosis (Sancho et al., 2002), through a mechanism not VR1 mediated. Furthermore, the possibility that capsaicin's neuroprotection could involve a release of neuropeptides from sensory neurons cannot be ruled out. A number of studies have shown that capsaicin-induced desensitization results in a transient depletion of some neuropeptides such as calcitonin gene-related peptide (CGRP), substance P and somatostatin storage (Shin & Hong, 2004). An inhibition of neurogenic inflammation, motor and cognitive deficits development following a traumatic brain injury in rats after depletion of the above neuropeptides has been shown (Nimmo et al., 2004).
In summary, we have demonstrated that capsaicin protected gerbil's brain from global ischemia. This effect was blocked by CPZ, suggesting a VR1 mediation. These findings contribute to develop new exogenous VR1 agonists as valuable targets for therapy against brain injury.
Acknowledgments
This research was supported by a grant (FIRST 2002) from the Ministry of Scientific Research and Technology.
Abbreviations
- ANOVA
analysis of variance
- CPZ
capsazepine
- EEG
electroencephalographam
- TRPV1
transient receptor potential channel vanilloid subfamily member 1
- VR1
vanilloid receptor 1
References
- ARAKI H., NOJIRI M., KAWASHIMA K., KIMURA M., AIHARA H. Behavioral, electroencephalographic and histopathological studies on mongolian gerbils with occluded common carotid arteries. Physiol. Behav. 1986;38:89–94. doi: 10.1016/0031-9384(86)90136-8. [DOI] [PubMed] [Google Scholar]
- BICKLER P.E., FAHLMAN C.S. Moderate increases in intracellular calcium activate neuroprotective signals in hippocampal neurons. Neuroscience. 2004;127:673–683. doi: 10.1016/j.neuroscience.2004.05.035. [DOI] [PubMed] [Google Scholar]
- BRAIDA D., PEGORINI S., ARCIDIACONO M.V., CONSALEZ G.G., CROCI L., SALA M. Post-ischemic treatment with cannabidiol prevents electroencephalographic flattening, hyperlocomotion and neuronal injury in gerbils. Neurosci. Lett. 2003;346:61–64. doi: 10.1016/s0304-3940(03)00569-x. [DOI] [PubMed] [Google Scholar]
- BRAIDA D., POZZI M., SALA M. CP 55,940 protects against ischemia-induced electroencephalographic flattening and hyperlocomotion in Mongolian gerbils. Neurosci. Lett. 2000;296:183–191. doi: 10.1016/s0304-3940(00)01634-7. [DOI] [PubMed] [Google Scholar]
- BROOKS J.W., PRYCE G., BISOGNO T., JAGGAR S.I., HANKEY D.J., BROWN P., BRIDGES D., LEDENT C., BIFULCO M., RICE A.S., DI MARZO V., BAKER D. Arvanil-induced inhibition of spasticity and persistent pain: evidence for therapeutic sites of action different from the vanilloid VR1 receptor and cannabinoid CB(1)/CB(2) receptors. Eur. J. Pharmacol. 2002;439:83–92. doi: 10.1016/s0014-2999(02)01369-9. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., LEFFLER A., MALMBERG A.B., MARTIN W.J., TRAFTON J., PETERSEN-ZEITZ K., KOLTZENBURG M., BASBAUM A.I., JULIUS D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- COLBOURNE F., CORBETT D. Delayed postischemic hypothermia: a six month survival study using behavioral and histological assessments of neuroprotection. J. Neurosci. 1995;15:7250–7260. doi: 10.1523/JNEUROSCI.15-11-07250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLBOURNE F., LI H., BUCHAN A.M. Indefatigable CA1 sector neuroprotection with mild hypothermia induced 6 h after severe forebrain ischemia in rat. J. Cereb. Blood Flow Metab. 1999a;19:742–749. doi: 10.1097/00004647-199907000-00003. [DOI] [PubMed] [Google Scholar]
- COLBOURNE F., SUTHERLAND G.R., AUER R.N. Electron microscopic evidence against apoptosis as the mechanism of neuronal death in global ischemia. J. Neurosci. 1999b;19:4200–4210. doi: 10.1523/JNEUROSCI.19-11-04200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORTRIGHT D.N., SZALLASI A. Biochemical pharmacology of the vanilloid receptor TRPV1. An update. Eur. J. Biochem. 2004;271:1814–1819. doi: 10.1111/j.1432-1033.2004.04082.x. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., BREIVOGEL C., BISOGNO T., MELCK D., PATRICK G., TAO Q., SZALLASI A., RAZDAN R.K., MARTIN B.R. Neurobehavioral activity in mice of N-vanillyl-arachidonyl-amide. Eur. J. Pharmacol. 2000;406:363–374. doi: 10.1016/s0014-2999(00)00687-7. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., LASTRES-BECKER I., BISOGNO T., DE PETROCELLIS L., MILONE A., DAVIS J.B., FERNANDEZ-RUIZ J.J. Hypolocomotor effects in rats of capsaicin and two long chain capsaicin homologues. Eur. J. Pharmacol. 2001;420:123–131. doi: 10.1016/s0014-2999(01)01012-3. [DOI] [PubMed] [Google Scholar]
- FREUND T.F., BUZSAK I.G., LEON A., SOMOGYI P. Hippocampal cell death following ischemia: effects of brain temperature and anesthesia. Exp. Neurol. 1990;108:251–260. doi: 10.1016/0014-4886(90)90131-b. [DOI] [PubMed] [Google Scholar]
- FRIGENI V., MIRAGOLI L., GROTTI A., LORUSSO V. Neurotolerability of contrast agents in rats with brain ischemia induced by transient middle cerebral artery occlusion: EEG evaluation. Invest. Radiol. 2004;36:1–8. doi: 10.1097/00004424-200101000-00001. [DOI] [PubMed] [Google Scholar]
- GAMBERT S., BES-HOUTMANN S., VANDROUX D., TISSIER C., VERGELY-VANDRIESSE C., ROCHETTE L., ATHIAS P. Deep hypothermia during ischemia improves functional recovery and reduces free-radical generation in isolated reperfused rat heart. J. Heart Lung Transplant. 2004;23:487–491. doi: 10.1016/S1053-2498(03)00211-0. [DOI] [PubMed] [Google Scholar]
- GLOOR P., BALL G., SCHAUL N. Brain lesions that produce delta waves in the EEG. Neurology. 1977;27:326–333. doi: 10.1212/wnl.27.4.326. [DOI] [PubMed] [Google Scholar]
- GUNTHORPE M.J., BENHAM C.D., RANDALL A., DAVIS J.B. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol. Sci. 2002;23:183–191. doi: 10.1016/s0165-6147(02)01999-5. [DOI] [PubMed] [Google Scholar]
- HAIL N., JR Mechanisms of vanilloid-induced apoptosis. Apoptosis. 2003;8:251–262. doi: 10.1023/a:1023620821878. [DOI] [PubMed] [Google Scholar]
- HAJOS M., JANCSO G., ENGBERG G. Capsaicin-induced excitation of locus coeruleus neurons. Acta Physiol. Scand. 1987;129:415–420. doi: 10.1111/j.1748-1716.1987.tb08086.x. [DOI] [PubMed] [Google Scholar]
- HAJOS N., FREUND T.F. Distinct cannabinoid sensitive receptors regulate hippocampal excitation and inhibition. Chem. Phys. Lipids. 2002;121:73–82. doi: 10.1016/s0009-3084(02)00149-4. [DOI] [PubMed] [Google Scholar]
- HAKAMATA Y., HANYU S., KUROIWA T., ITO U. Brain edema associated with progressive selective neuronal death or impending infarction in the cerebral cortex. Acta Neurochir. Suppl. (Wien) 1997;70:20–22. doi: 10.1007/978-3-7091-6837-0_6. [DOI] [PubMed] [Google Scholar]
- HANSEN H.S., MOESGAARD B., PETERSEN G., HANSEN H.H. Putative neuroprotective actions of N-acyl-ethanolamines. Pharmacol. Ther. 2002;95:119–126. doi: 10.1016/s0163-7258(02)00251-6. [DOI] [PubMed] [Google Scholar]
- HAYES P., MEADOWS H.J., GUNTHORPE M.J., HARRIES M.H., DUCKWOR D.M., CAIRNS W., HARRISON D.C., CLARKE C.E., ELLINGTON K., PRINJHA R.K., BARTON A.J., MEDHURST A.D., SMITH G.D., TOPP S., MURDOCK P., SANGER G.J., TERRETT J., JENKINS O., BENHAM C.D., RANDALL A.D., GLOGER I.S., DAVIS J.B. Cloning and functional expression of a human orthologue of rat vanilloid receptor-1. Pain. 2000;88:205–215. doi: 10.1016/S0304-3959(00)00353-5. [DOI] [PubMed] [Google Scholar]
- HICKEY R.W., FERIMER H., ALEXANDER H.L., GARMAN R.H., CALLAWAY C.W., HICKS S., SAFAR P., GRAHAM S.H., KOCHANEK P.M. Delayed, spontaneous hypothermia reduces neuronal damage after asphyxial cardiac arrest in rats. Crit. Care Med. 2000;28:3511–3516. doi: 10.1097/00003246-200010000-00027. [DOI] [PubMed] [Google Scholar]
- HOSSMANN K.A., SCHUIER F.J. Experimental brain infarcts in cats. Stroke. 1980;11:583–592. doi: 10.1161/01.str.11.6.583. [DOI] [PubMed] [Google Scholar]
- HUNTER A.J., GREEN A.R., CROSS A.J. Animal models of acute ischaemic stroke: can they predict clinically successful neuroprotective drugs. Trends Pharmacol. Sci. 1995;16:123–128. doi: 10.1016/s0165-6147(00)88999-3. [DOI] [PubMed] [Google Scholar]
- HUNTER A.J., MACKAY K.B., ROGERS D.C. To what extent have functional studies of ischaemia in animals been useful in the assessment of potential neuroprotective agents. Trends Pharmacol. Sci. 1998;19:59–66. doi: 10.1016/s0165-6147(97)01157-7. [DOI] [PubMed] [Google Scholar]
- KATAOKA K., GRAF R., ROSNER G. Differentiation between cortical and subcortical lesions following focal ischemia in cats by multimodality evoked potentials. J. Neurol. Sci. 1987;79:117–127. doi: 10.1016/0022-510x(87)90266-8. [DOI] [PubMed] [Google Scholar]
- KATOH A., ISHIBASHI C., SHIOMI T., TAKAHARA Y., EIGYO M. Ischemia-induced irreversible deficit of memory function in gerbils. Brain Res. 1992;577:57–63. doi: 10.1016/0006-8993(92)90537-j. [DOI] [PubMed] [Google Scholar]
- KIM C.S., KAWADA T., KIM B.S., HAN I.S., CHOE S.Y., KURATA T., YU R. Capsaicin exhibits anti-inflammatory property by inhibiting IkB-a degradation in LPS-stimulated peritoneal macrophages. Cell. Signal. 2003;15:299–306. doi: 10.1016/s0898-6568(02)00086-4. [DOI] [PubMed] [Google Scholar]
- KIYOTA Y., HAMAJO K., MIYAMOTO M., NAGAOKA M. Effect of idebenone (CV-2619) on memory impairment observed in passive avoidance task in rats with cerebral embolization. Jpn. J. Pharmacol. 1985;37:300–302. doi: 10.1254/jjp.37.300. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI A., OSAKA T., NAMBA Y., INOUE S., LEE T.H., KIMURA S. Capsaicin activates heat loss and heat production simultaneously and independently in rats. Am. J. Physiol. 1998;275:R92–R98. doi: 10.1152/ajpregu.1998.275.1.R92. [DOI] [PubMed] [Google Scholar]
- LAPONCIN-LAFITTE M., GROSDEMOGUE C., ROYBILLON C., POTRAT P., LESPINASSE P., RAPIN J.R. Short-term memory and cerebral ischemia: pharmacological application. Eur. Neurol. 1981;20:265–269. doi: 10.1159/000115245. [DOI] [PubMed] [Google Scholar]
- LOSKOTA W.J., LOMAX P.L., VERITY M.A. A Stereotaxic Atlas of the Mongolian Gerbil (Meriones unguiculatus) Ann Arbor: Ann Arbor Science Publishers; 1974. pp. 1–157. [Google Scholar]
- MACCARRONE M., LORENZON T., BARI M., MELINO G., FINAZZI-AGRO' A. Anandamide induces apoptosis in human cells via vanilloid receptors. Evidence for a protective role of cannabinoid receptors. J. Biol. Chem. 2000;275:31938–31945. doi: 10.1074/jbc.M005722200. [DOI] [PubMed] [Google Scholar]
- MARINELLI S., VAUGHAN C.W., CHRISTIE M.J., CONNOR M. Capsaicin activation of glutamatergic synaptic transmission in the rat locus coeruleus in vitro. J. Physiol. 2002;543:531–540. doi: 10.1113/jphysiol.2002.022863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEZEY E., TOTH Z.E., CORTRIGHT D.N., ARZUBI M.K., KRAUSE J.E., ELDE R., GUO A., BLUMBERG P.M., SZALLASI A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc. Natl. Acad. Sci. U.S.A. 2000;97:3655–3660. doi: 10.1073/pnas.060496197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGAYAMA T., SINOR A.D., SIMON R.P., CHEN J., GRAHAM S.H., JIN K., GREENBERG D.A. Cannabinoids and neuroprotection in global and focal cerebral ischemia and in neuronal cultures. J. Neurosci. 1999;15:2987–2995. doi: 10.1523/JNEUROSCI.19-08-02987.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIMMO A.J., CERNAK I., HEATH D.L., HU X., BENNETT C.J., VINK R. Neurogenic inflammation is associated with development of edema and functional deficits following traumatic brain injury in rats. Neuropeptides. 2004;38:40–47. doi: 10.1016/j.npep.2003.12.003. [DOI] [PubMed] [Google Scholar]
- O'NEILL M.J., CLEMENS J.A.Preclinical models of neurologic and psychiatric disorders Current Protocols in Neuroscience 2000New York: Wiley; 9.5.1–9.5.25.ed. Crawley, J.N., Gerfen, C.R., Mckay, R., Rogawski, M.A., Sibley, D.R. & Skolnick, P. pp [Google Scholar]
- OSAKA T., KOBAYASHI A., NAMBA Y., INOUE S., KIMURA S. Lack of integrative control of body temperature after capsaicin administration. Korean J. Intern. Med. 2000;15:103–108. doi: 10.3904/kjim.2000.15.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERKINS M.N., CAMPBELL E.A. Capsazepine reversal of the antinociceptive action of capsaicin in vivo. Br. J. Pharmacol. 1992;107:329–333. doi: 10.1111/j.1476-5381.1992.tb12746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERUCHE B., KLAASSENS H., KRIEGLSTEIN J. Quantitative analysis of the electrocoricogram after forebrain ischemia in the rat. Pharmacology. 1995;50:229–237. doi: 10.1159/000139287. [DOI] [PubMed] [Google Scholar]
- RAY A.M., BENHAM C.D., ROBERTS J.C., GILL C.H., LANNEAU C., GITTERMAN D.P., HARRIES M., DAVIS J.B., DAVIES C.H. Capsazepine protects against neuronal injury caused by oxygen glucose deprivation by inhibiting I(h) J. Neurosci. 2003;23:10146–10153. doi: 10.1523/JNEUROSCI.23-31-10146.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALA M., LEONE M.P., LAMPUGNANI P., MATTURRI L., GORI E. Polydeoxyribonuclotide (defibrotide) protects against post-ischemic behavioural, electroencephalographic and neuronal damage in the gerbil. Eur. J. Pharmacol. 1997;328:143–152. doi: 10.1016/s0014-2999(97)83040-3. [DOI] [PubMed] [Google Scholar]
- SANCHO R., LUCENA C., MACHO A., CALZADO M.A., BLANCO-MOLINA M., MINASSI A., APPENDINO G., MUNOZ E. Immunosuppressive activity of capsaicinoids: capsiate derived from sweet peppers inhibits NF-kappaB activation and is a potent antiinflammatory compound in vivo. Eur. J. Immunol. 2002;32:1753–1763. doi: 10.1002/1521-4141(200206)32:6<1753::AID-IMMU1753>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- SASAMURA T., KURAISHI Y. Peripheral and central actions of capsaicin and VR1 receptor. Jpn. J. Pharmacol. 1999;80:275–280. doi: 10.1254/jjp.80.275. [DOI] [PubMed] [Google Scholar]
- SCHMIDT-KASTNER R., FREUND T.F. Selective vulnerability of the hippocampus in brain ischemia. Neuroscience. 1991;40:599–636. doi: 10.1016/0306-4522(91)90001-5. [DOI] [PubMed] [Google Scholar]
- SHARMA M.K., BUETTNER G.R., SPENCER K.T., KERBER R.E. Ascorbyl free radical as a real-time marker of free radical generation in briefly ischemic and reperfused hearts – an electron paramagnetic resonance study. Circ. Res. 1994;74:650–658. doi: 10.1161/01.res.74.4.650. [DOI] [PubMed] [Google Scholar]
- SHEN M., THAYER S.A. Cannabinoid receptor agonists protect cultured rat hippocampal neurons from excitotoxicity. Mol. Pharmacol. 1998;54:459–462. doi: 10.1124/mol.54.3.459. [DOI] [PubMed] [Google Scholar]
- SHIN H.K., HONG K.W. Importance of calcitonin gene-related peptide, adenosine and reactive oxygen species in cerebral autoregulation under normal and diseased conditions. Clin. Exp. Pharmacol. Physiol. 2004;31:1–7. doi: 10.1111/j.1440-1681.2004.03943.x. [DOI] [PubMed] [Google Scholar]
- SINOR A.D., IRVIN S.M., GREENBERG D.A. Endocannabinoids protect cerebral cortical neurons from in vitro ischemia in rats. Neurosci. Lett. 2000;278:157–160. doi: 10.1016/s0304-3940(99)00922-2. [DOI] [PubMed] [Google Scholar]
- SMART D., GUNTHORPE M.J., JERMAN J.C., NASIR S., GRAY J., MUIR A.I., CHAMBERS J.K., RANDALL A.D., DAVIS J.B. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Br. J. Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUZUKI R., YAMAGUCHI T., KIRINO T., ORZI F., KLATZO I. The effects of 5-min ischemia in Mongolian gerbils: I. Blood–brain barrier, cerebral blood flow, and local cerebral glucose utilization changes. Acta Neuropathol. 1983;60:207–216. doi: 10.1007/BF00691868. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- SZALLASI A., NILSSON S., FARKAS-SZALLASI T., BLUMBERG P.M., HOKFELT T., LUNDBERG J.M. Vanilloid (capsaicin) receptors in the rat: distribution in the brain, regional differences in the spinal cord, axonal transport to the periphery, and depletion by systemic vanilloid treatment. Brain Res. 1995;703:175–183. doi: 10.1016/0006-8993(95)01094-7. [DOI] [PubMed] [Google Scholar]
- VAN DER STELT M., DI MARZO V. Endovanilloids. Putative endogenous ligands of transient receptor potential vanilloid 1 channels. Eur. J. Biochem. 2004;271:1827–1834. doi: 10.1111/j.1432-1033.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- VELDHUIS W.B., VAN DER STELT M., WADMAN M.W., VAN ZADELHOFF G., MACCARRONE M., FEZZA F., VELDINK G.A., VLIEGENTHART J.F., BAR P.R., NICOLAY K., DI MARZO V. Neuroprotection by the endogenous cannabinoid anandamide and arvanil against in vivo excitotoxicity in the rat: role of vanilloid receptors and lipoxygenases. J. Neurosci. 2003;15:4127–4133. doi: 10.1523/JNEUROSCI.23-10-04127.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERGELY C., MAUPOIL V., BENDERITTER M., ROCHETTE L. Influence of the severity of myocardial ischemia on the intensity of ascorbyl free radical release and on postischemic recovery during reperfusion. Free Radic. Biol. Med. 1998;24:470–479. doi: 10.1016/s0891-5849(97)00282-7. [DOI] [PubMed] [Google Scholar]