Abstract
The effects of the main component of the Tityus serrulatus scorpion venom, toxin TsTX-I, were studied on the contractility and release of neurotransmitters in the rat vas deferens. Since TsTX-I is known to act on sodium channels, we used veratridine, another sodium channel agent, for comparison.
Toxin TsTX-I induced concentration-dependent contractions with an EC50 value of 47.8±0.1 nM and a maximum effect of 84.4±10.4% of that for BaCl2.
Contractions by TsTX-I were abolished by denervation or tetrodotoxin (0.1 μM), showing that the toxin effects depend on the integrity of sympathetic nerve terminals.
To check for the presence of a noradrenergic component, experiments were conducted after removal of adrenergic stores in nerve terminals by reserpinization (10 mg kg−1, 24 h prior to experiments) or blockade of α1 adrenoceptors by prazosin (30 μM), showing that these procedures did not modify the response to TsTX-I, and therefore that adrenoceptors were not involved in contractions.
To check for the presence of a purinergic component, experiments were carried out after blockade of P2X receptors by suramin (0.1 mM) or desensitization by α,β-methylene-ATP (30 μM). These agents greatly abolished the contractile response to TsTX-I (about 83% by desensitization and 96% by suramin), showing the involvement of purinergic receptors.
The release of noradrenaline and purinergic agents (ATP, ADP, AMP and adenosine) was detected by HPLC. Together, the total release of purines in the presence of TsTX-I was about 42 times higher than in the control group. In contrast, TsTX-I did not modify the overflow of noradrenaline, showing that the release was selective for purines.
The release of purinergic agents was reduced by the N-type calcium channel blocker ω-conotoxin GVIA (1 μM) and by the P/Q-type blocker ω-conotoxin MVIIC (1 μM), showing that the effects of TsTX-I are calcium-dependent.
The results show that TsTX-I produced a selective release of purines from postganglionic sympathetic nerves in the rat vas deferens.
Keywords: VAS deferens, toxin TsTX-I, cotransmission, ATP, adrenoceptors
Introduction
In Brazil, Tityus serrulatus is responsible for most cases of scorpion envenomation (Dorce & Sandoval, 1994). Scorpion venoms are a rich source of neurotoxins, which are basic, low-molecular-weight polypeptides without enzymatic activity that modify the functioning of ionic channels (Couraud & Jover, 1984). Several toxins that act in a number of ionic channels have been purified. Voltage-gated sodium and potassium channels are the more frequent targets of scorpion toxins, but calcium channels from endoplasmic reticulum (Tripathy et al., 1998) and chloride channels of endothelial cells (DeBin et al., 1993) are also modulated by some neurotoxins. Sodium and potassium neurotoxins are the most abundant toxins in scorpion venoms and have been extensively used as neurobiological tools to study these channels (Ohizumi, 1997; Garcia et al., 1999). Scorpion toxins that act on sodium channels are divided into α toxins, which delay the channel inactivation, and β scorpion toxins, which include the most abundant component of T. serrulatus venom, TsTX-I. This toxin enhances the activation of sodium channels (Barhanin et al., 1982; 1983; 1984; Vijverberg et al., 1984; Jonas et al., 1986; Arispe et al., 1988; Yatani et al., 1988; Marcotte et al., 1997; Conceição et al., 1998; Catterall et al., 2003). Binding on ionic channels has turned scorpion toxins into very useful tools to study neurotransmission processes.
The vas deferens is supplied with a dense sympathetic innervation, and adenosine triphosphate (ATP) plays a role in neuroeffector mechanisms as a cotransmitter with noradrenaline (Burnstock, 2004). Stimulation of the nerves results in mechanical responses mediated both by P2X purinoceptor activation by ATP (Sneddon & Westfall, 1984) and by α1-adrenoceptor activation by noradrenaline (Pupo et al., 1997). The neurogenic response is biphasic and consists of an initial rapid twitch, followed by a maintained contraction. The first phase of the response is mediated mainly by ATP, whereas the second phase is mediated mainly by noradrenaline (Sneddon et al., 1984). The cotransmitters are released from the same type of nerves because pretreatment with 6-hydroxydopamine, an agent that specifically destroys adrenergic nerves, abolishes both phases of the neurogenic contraction (Fedan & Besse, 1980). However, in contrast to adrenal chromaffin cells, where it has been reported that ATP and noradrenaline are stored in the same synaptic vesicle (Todorov et al., 1996), in the vas deferens (as in other sympathetic nerves), ATP and noradrenaline may be stored in separate vesicles and can be released via independent mechanisms (Ellis & Burnstock, 1989; Todorov et al., 1996; Brock & Cunnane, 1999; Stjärne, 2001).
The effects of toxin TsTX-I, initially named as toxin γ (Possani et al., 1977), have been studied in some tissues innervated by autonomic nervous system and are attributed to the neurotransmitter released by the toxin acting in presynaptic sites. In the rat heart, this toxin induced a negative chronotropic and a positive inotropic effect (Silveira et al., 1991; Couto et al., 1992; Drumond et al., 1995), due to the release of sympathetic and parasympathetic neurotransmitters. In guinea pig ileum, TsTX-I induced contractions, which were ascribed to the release of acetylcholine and neuropeptides (Matos et al., 1999). Also, release of nitric oxide from nitrergic nerves of rabbit corpus cavernosum was related to the relaxation caused by toxin TsTX-I (Fernandes de Oliveira et al., 2003). However, in these tissues, no direct neurotransmitter overflow experiments were performed to verify the participation of different neurotransmitters in the action of this toxin.
In the present work, we have studied the effects of toxin TsTX-I in the rat vas deferens to provide a further pharmacological characterization of the toxin and to verify the participation of noradrenaline and purines in its postjunctional effects. For comparison, we have used veratridine (VTD), a well-known sodium channel toxin that acts by delaying the opening time of sodium channel, leading to a persistent depolarization (Conceição et al., 1998).
Methods
Tissue preparation
Male Wistar rats weighing between 280 and 360 g (16–20 weeks old) from our own colony, BAW-2 (UNIFESP), were killed with an overdose of ether. Both vasa deferentia were removed and cleaned from surrounding tissues, and the lumens carefully washed with a nutrient solution of the following composition (mM): NaCl 138, KCl 5.7, CaCl2 1.8, NaH2PO4 0.36, NaHCO3 15 and glucose 5.5, pH 7.4. Each organ was mounted in a 10 ml chamber containing continuously aerated nutrient solution at 30°C (Jurkiewicz & Jurkiewicz, 1976). Isotonic contractions were recorded by means of isotonic transducers coupled to a recording system (Ugo Basile), under a load of 1.0 g. Surgically denervated vasa deferentia were also used. Denervation was performed as previously described (Kasuya et al., 1969; Pupo et al., 1999). Briefly, the rats were anesthetized by ether inhalation, and a 2 cm suprapubic incision was made to expose the deferential artery and vein close to the prostatic end of the vas, where the hypogastric plexus is located. In this area, the serous coat and both vessels were gently separated from the organ. After this procedure, the incision was sutured and the rat was killed 7–10 days later. The experimental procedures were approved by the Ethics Committee of UNIFESP.
Contraction induced by the toxins
After an initial equilibration period of 30 min, BaCl2 (10 mM) was added to the organ bath and washed out after achieving a maximal contraction. After a period of 30 min, toxin TsTX-I (1.5, 4.5, 15, 45, 150 or 450 nM) or VTD (3, 10, 30 or 100 μM) was added to the preparation and the effects recorded for 15 min. Due to the irreversibility of both drugs, only one concentration was applied in each organ. Concentration–response curves were obtained from the respective contractions for calculation of EC50 (effective concentration 50% of maximum response) values. Time–response curves were also made. Data were expressed as percent values of maximum contraction induced by BaCl2 (EmaxBa), which is able to contract the vas deferens to its maximum capacity (Jurkiewicz et al., 1969; 1976). These values were also used to calculate ρ (relative responsiveness) values, expressing the maximum effects of TsTX-I or VTD as a ratio in relation to the normalized value of EmaxBa, taken as 1.0 (Jurkiewicz et al., 1969; 1976).
Participation of purinergic and adrenergic components on TsTX-I- or VTD-induced contraction
Two experiments were conducted. In the first one, animals were divided in two groups. One of them was treated with an intraperitoneal (i.p.) injection of reserpine (RES, 10 mg kg−1, 24 h before experiments). The other group received an i.p. injection of the same volume of vehicle. In organs of RES-treated animals, 0.1 mM tyramine was applied to the bath to verify if catecholamine stores were depleted. Organs that responded to tyramine were discarded. In all experiments, contraction to BaCl2 was first determined as described above. After 15 min, 30 μM α,β-methylene-ATP (α,β-mATP) was added to some organs to desensitize purinergic receptors. After 15 min, toxin TsTX-I (45 nM) or VTD (10 μM) was applied. Consequently, the effects of toxins were evaluated in (1) normal organs, (2) organs of reserpinized rats, (3) organs with desensitized purinergic receptors or (4) organs of reserpinized rats with desensitized purinergic receptors. Maximum effects were expressed as percent of maximum initial BaCl2 contraction.
In the second experiment, after an initial equilibration period plus barium contraction and washout, the drugs, prazosin (PZS, 30 μM), suramin (SUR, 0.1 mM) or both, were incubated for 30 min to block α-adrenoceptors and purinergic receptors, respectively. This was followed by adding toxin TsTX-I (45 nM) or VTD (10 μM) to record the corresponding maximum effects.
Overflow experiments for noradrenaline and purinergic agents
After removal and cleaning as described before, the vas deferens was opened longitudinally and placed in a chamber of total volume of 500 μl. The chamber was filled with 300 μl of nutritive solution and organs were maintained with aeration during 60 min with solution changes at 10-min intervals. Following this period, the chamber was emptied and replenished with 300 μl of nutritive solution or nutritive solution plus TsTX-I (45 or 150 nM) or VTD (30 μM). After 90 s (to measure noradrenaline release) or 30 s (to measure purines), the incubation medium was collected in ice-cold tubes. To check whether or not the effect of TsTX-I is calcium-dependent, experiments were conducted with the blocker of N-type calcium channel (Hirata et al., 1997) ω-conotoxin GVIA (1 μM), or of P/Q-type channel (Randall & Tsien, 1995) ω-conotoxin MVIIC (1 μM). In this case, the procedure was exactly the same, except that the blockers were added 60 min before TsTX-I (45 nM), and maintained in the solution thereafter.
Since the vas deferens releases metabolic enzymes that rapidly degrade the initially released ATP into adenosine diphosphate (ADP), adenosine monophosphate (AMP), and adenosine (Todorov et al., 1996; 1997), we have quantified these purines separately, as a more dependable way to measure initially released ATP, as proposed by Todorov et al. (1999).
High-performance liquid chromatography assays
For noradrenaline measurements, the incubation medium (300 μl) was acidified with 33 μl of 1 M perchloric acid and assayed on an High-performance liquid chromatography (HPLC) system (Shimadzu Co., Japan, model LC-10VP) coupled to an electrochemical detector (Hewlett Packard, Germany, model 1049A) with glass-carbon electrode set at +0.65 V versus a solid state Ag/AgCl reference electrode. Noradrenaline separation was achieved using a shim-pack μC18 column of 250 × 4.5–mm (length × diameter) and 5 μm particle size (shim-pack CLC-ODS, Shimadzu Co.), using an isocratic gradient. Mobile phase was subjected to continuous degassing by helium bubbling and consisted of 50 mM Na2HPO4, 1.2 mM heptane-1-sulfonic acid, 0.2 mM EDTA and methanol 5% v v−1 in deionized water, with pH adjusted to 2.6 with phosphoric acid.
For measurement of purines, the HPLC system used was the same as that for noradrenaline quantification, except that a fluorescence detector (Shimadzu Co., model RF-10Axl) was used instead of an electrochemical one. Purines were quantified by the method previously described (Levitt et al., 1984) with minor modification. To the incubation medium (300 μl), 135 μl of citrate phosphate buffer, pH 6.0, and 15 μl of 2-chloracetaldehyde were added. This solution was heated at 80°C for 40 min for the formation of etheno (ɛ) derivatives. The formed derivatives (ɛ)-ATP, (ɛ)-ADP, (ɛ)-AMP and (ɛ)-adenosine were separated by a Chromolith RP-18e C18 column (Merck, Germany) of 100 × 4.6 mm (length × diameter) size, also using isocratic gradient. Mobile phase consisted of 0.2 M Na2HPO4, 0.2 M NaH2PO4 and methanol 5% v v−1 in deionized water, pH 6.0. Detection of (ɛ)-purines was performed at an excitation wavelength of 233 nm and emission wavelength of 415 nm.
Identification of released neurotransmitter peaks was performed by comparison with retention time of injected standards. Amounts of noradrenaline or individual purines in samples were estimated by the measurement of the peak area per picomole relative to those obtained with known amounts of standards.
Statistical analysis
ANOVAs followed by Newman–Keuls test were performed to determine significance of differences. The significance level was considered as P<0.05.
Drugs
Toxin TsTX-I was isolated from crude lyophilized T. serrulatus venom (supplied by the Arthropods Section of Butantan Institute) as previously described (Carvalho et al., 1998; Conceição et al., 1998). Noradrenaline (L-arterenol HCl), PZS HCl, SUR, RES, VTD, α,β-mATP and tyramine HCl were from Sigma (U.S.A.), and tetrodotoxin was purchased from Alamone Labs (Israel). RES was diluted in distilled water with 4% propylene glycol and 4% ethanol v v−1, and VTD was dissolved in dimethyl sulfoxide. All other drugs were diluted in distilled water.
Results
Characteristics of contraction induced by TsTX-I in rat vas deferens
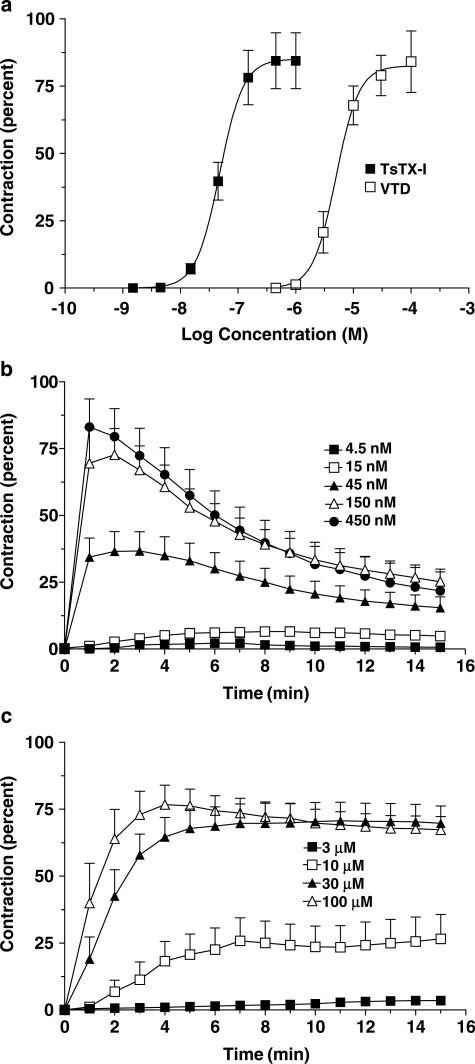
Figure 1a shows concentration–response curves for TsTX-I. The toxin induced contractions in a nanomolar range with a maximum effect at about 450 nM. Maximum contraction induced by TsTX-I represented 84.4±10.4% (mean±s.e.mean) of BaCl2 contraction, which can be expressed by a ρ value of 0.84, as described in Methods (Jurkiewicz et al., 1969; 1976). Calculated EC50 value for TsTX-I was 47.8±0.1 nM. VTD induced similar curves, although when used at about 100 times higher doses. In this case, a maximum contraction of 79.1±7.4% in relation to BaCl2 (thus, ρ=0.79), with an EC50 of 5.0±0.1 μM, was obtained.
Figure 1.
Concentration–response and time–response curves for toxin TsTX-I or VTD in the rat vas deferens. Each point represents the mean±s.e.mean (n=7–8) of the contraction induced by TsTX-I or VTD. Concentration–response curves are shown in panel a and the temporal course of contractions for each dose (time–response curves) is represented in panels b (TsTX-I) and c (VTD). Data are percent values in relation to maximum contraction induced by BaCl2 (10 mM) applied at the beginning of experiments.
Time–response curves of TsTX-I and VTD exhibited a distinct temporal course. Contractions induced by TsTX-I reached maximum values in 1–2 min followed by a fast decay (Figure 1b). In contrast, contractions induced by VTD reached a maximum much more slowly. In addition, no decay, or fade, was observed (Figure 1c), contrary to the effects of TsTX-I (Figure 1b).
Effects of denervation and tetrodotoxin on contractions induced by TsTX-I
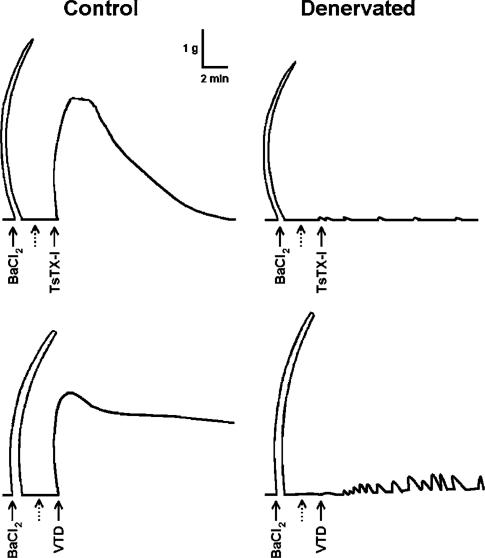
To verify if contractions induced by TsTX-I are pre- or postsynaptic, we tested its effects in denervated preparations. Neither TsTX-I nor VTD was able to induce contractions in denervated preparations (Figure 2). Furthermore, tetrodotoxin (0.1 μM, 30 min) completely blocked the action of these toxins in nondenervated preparations (not shown). These results clearly show that the action of both agents depends on the integrity of sympathetic nerve terminals, and is therefore presynaptic.
Figure 2.
Effects of denervation on contraction induced by toxin TsTX-I or VTD in the rat vas deferens. Typical original traces of contractions induced by TsTX-I (150 nM) or VTD (100 μM) in normal or denervated vas deferens. At the beginning of the experiments, a single dose of BaCl2 (10 mM) was applied to verify the integrity of the organ. In denervated tissue, tyramine (0.1 mM) was added before BaCl2 to ensure that the organs were denervated (not shown). Organs that responded to tyramine were discarded. Dotted arrows indicate that recording was stopped after achieving the maximum effect of barium, followed by washout, and that recording was restarted before adding TsTX-I or VTD. Six experiments were performed with similar results.
Participation of purinergic and noradrenergic components on the contraction induced by TsTX-I
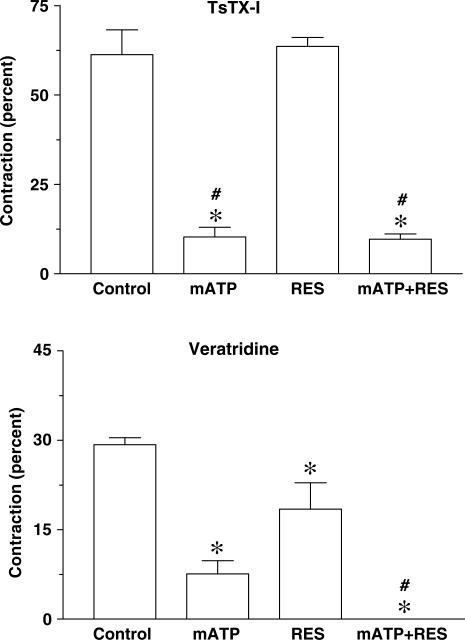
To verify the purinergic and noradrenergic participation on the contraction induced by TsTX-I, the effects of this toxin were evaluated in two different experimental conditions (Figures 3 and 4). In the first, the effects were studied in reserpinized animals or after purinergic receptor desensitization by α,β-m-ATP (Figure 3). We observed that reserpinization did not modify contractions induced by TsTX-I. On the other hand, purinergic receptor desensitization produced a remarkable antagonism of about 83% (P<0.001) of TsTX-I contraction. No additional antagonism was observed when purinergic desensitization was studied in reserpinized animals (Figure 3).
Figure 3.
Effects of reserpinization or desensitization of purinergic receptors on the contraction induced by toxin TsTX-I or VTD in the rat vas deferens. Each bar represents the mean±s.e.mean (n=7) of the maximum contraction induced by TsTX-I (45 nM) or VTD (10 μM). The drugs were added to the organs of rats without (control), or with injection of RES (10 mg kg−1, 24 h before experiments), or to organs whose purinergic receptors were desensitized with α,β-mATP (mATP, 30 μM, 15 min before). The mATP+RES group represents organs of reserpinized animals with purinergic desensitization. Data are percent values in relation to the maximum contraction induced by BaCl2 (10 mM applied at the beginning of experiment). *P<0.01 in relation to control group, #P<0.001 in relation to RES group.
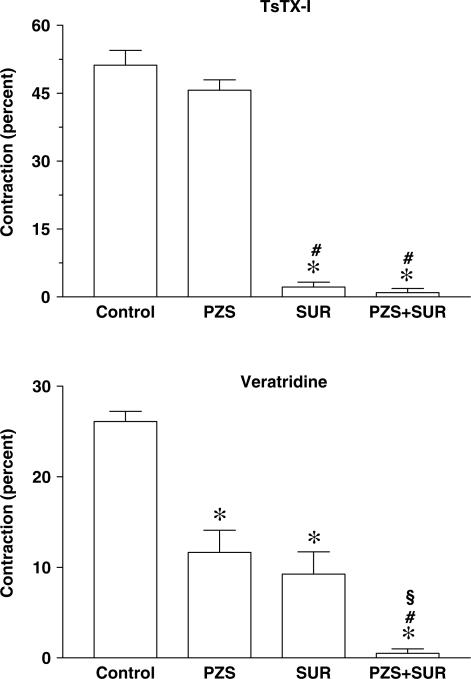
Figure 4.
Effects of PZS or SUR on the contraction induced by toxin TsTX-I or VTD in the rat vas deferens. Each bar represents the mean±s.e.mean (n=7) of the maximum contraction induced by TsTX-I (45 nM) or VTD (10 μM). Effects were obtained in the absence (Control) or presence of PZS (30 μM), SUR (0.1 mM) or both (PZS+SUR), applied 30 min before. Data are percent values in relation to maximum contraction induced by 10 mM of BaCl2, applied at the beginning of the experiment. *P<0.001 in relation to control group, #P<0.001 in relation to PZS group, §P<0.01 in relation to SUR group.
In the second experimental condition, the effects of TsTX-I were evaluated after a block of noradrenergic or purinergic receptors (Figure 4). A striking decrease was observed when toxin TsTX-I was applied in preparations with SUR (decrease of 96% if compared with control, P<0.001), without a significant decrease in preparations pretreated with PZS. The combined blockade of both purinoceptors and adrenoceptors produced no further reduction in the response to TsTX-I (Figure 4).
In VTD-induced contraction, the pattern of effects was very distinct. Reserpinization induced a significant fall in maximum contraction of approximately 37% (P<0.01). In control tissues, desensitization of P2X purinoceptors with α,β-mATP decreased the contraction by around 74% of maximum contraction (P<0.001), and in reserpinized tissues, this treatment entirely blocked contraction (Figure 3). In contrast, contractions induced by VTD were reduced by both PZS and SUR, by 55 and 65%, respectively (P<0.001). Simultaneous antagonism of purinergic and noradrenergic receptors promoted a reduction of 98% in relation to contractions induced by VTD in control conditions (Figure 4).
Overflow of noradrenaline and purines induced by toxin TsTX-I
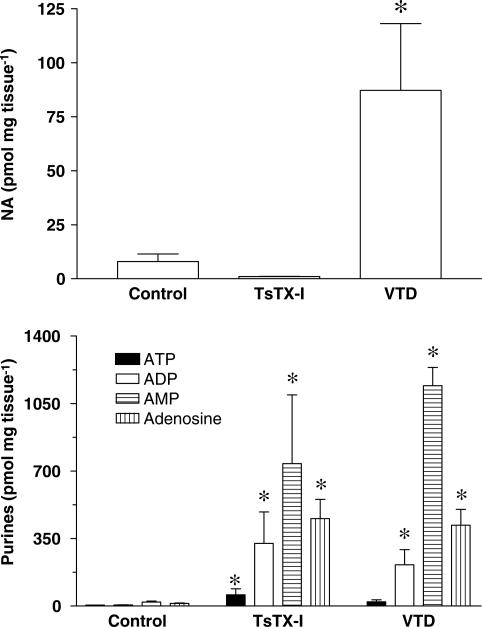
Figure 5 illustrates the effects of toxin TsTX-I and VTD on the overflow of noradrenaline and purines. The overflow of all the purines measured was markedly increased by 150 nM TsTX-I (Figure 5). Together, the total release of purines in the presence of TsTX-I was about 42 times higher than in control group. In contrast, this concentration of TsTX-I did not modify significantly the overflow of noradrenaline, showing that the release of neurotransmitters by the toxin was selective for purines.
Figure 5.
Effects of toxin TsTX-I and VTD on neurotransmitter overflow in the rat vas deferens. Each bar represents the mean±s.e.mean (n=3–8) of the release of noradrenaline or purines (ATP, ADP, AMP or adenosine) in the absence (control) or presence of TsTX-I (150 nM) or VTD (30 μM). Note that since ATP is rapidly degraded by metabolic enzymes into ADP, AMP and adenosine, the total sum of the released nucleotides can be used as an estimate of ATP released, instead of the actual value for ATP alone (Todorov et al., 1996). Data are presented as pmol mg tissue−1. *P<0.05 in relation to control group.
In a group of experiments with calcium channel blockers, it was shown that the release of total purines by TsTX-I (45 nM) was reduced by the N-type channel blocker ω-conotoxin GVIA by about 56% (from 45.2±5.2 (control) to 19.7±5.1 pmol mg tissue−1), or by the P/Q-type channel blocker ω-conotoxin MVIIC by about 65% (from 45.2±5.2 (control) to 15.9±6.0 pmol mg tissue−1). This result clearly indicates the involvement of calcium in the effect of TsTX-I.
With respect to VTD, Figure 5 shows that it induced a significant increase of purine overflow (except ATP) with an enhancement of 52 times (P<0.01), compared with basal overflow (control group). However, in contrast to TsTX-I, VTD also increased the overflow of noradrenaline by about 11 times.
Discussion
Our results indicate that toxin TsTX-I from the Brazilian scorpion T. serrulatus causes a selective release of purinergic agents from the rat vas deferens, without influencing noradrenaline overflow.
The contraction induced by TsTX-I in the rat vas deferens is due to an exclusive action in presynaptic membranes, as tetrodotoxin and denervation were able to block its effects. In addition, the effects of TsTX-I are clearly calcium-dependent as judged from the partial inhibition by N- and P/Q-type calcium channel blockers. Furthermore, the involvement of calcium supports the argument of presynaptic origin of the released ATP (Zucker, 1996). TsTX-I is a β-type scorpion toxin that acts in the sodium channel by enhancing its activation, so we can propose that this toxin acts by inducing nerve cell depolarization, which is followed by neurotransmitter release. In fact, Conceição et al. (1998) demonstrated in bovine chromaffin cells that toxin TsTX-I can open or at least enhance the probability of opening sodium channels at resting potentials. Similar results were obtained previously by other authors in different preparations, such as neuroblastoma cells (Barhanin et al., 1983), nerve fibers of Xenopus laevis (Jonas et al., 1986) and cardiac myocardial cells (Yatani et al., 1988). This depolarization in chromaffin cells is followed by enhancement of 45Ca2+ uptake and increase of intracellular calcium, which is known to induce neurotransmitter release (Conceição et al., 1998).
In the rat vas deferens, our results show that toxin TsTX-I promotes a selective release of ATP, while VTD induced the release of noradrenaline plus ATP. This observation is supported by the various experiments shown here. First of all, in overflow experiments, toxin TsTX-I induced a large increase of released purines, but was unable to promote release of noradrenaline. Furthermore, these observations are in full agreement with functional studies where we observed that pharmacological removal of the endogenous neurotransmitter noradrenaline (by reserpinization) or α1 receptor blockade (by PZS) did not modify the response to TsTX-I, while blockade of P2X receptors by SUR or its desensitization by α,β-mATP virtually abolished the contractile response to the toxin. The distinct time courses of contraction induced by TsTX-I or VTD could also suggest a differential release of neurotransmitters. Contraction induced by TsTX-I reached maximum values very fast, and was rapidly followed by relaxation, resembling a phasic contraction of purinergic origin, when the organ is electrically stimulated (not shown). In contrast, contractions induced by VTD were slower and persisted throughout the observation period like a tonic noradrenergic contraction.
Toxin TsTX-I was about 125-fold more potent than VTD, considering the EC50 values. This result is in agreement with data obtained in bovine adrenal chromaffin cells where the potency of TsTX-I was about 200-fold higher (Conceição et al., 1998). This difference reflects the affinity of both agents for their respective binding sites on Na+ channels. In fact, toxin TsTX-I is the agent with the highest affinity for Na+ channel (Vijverberg et al., 1984), with a dissociation constant of 0.1 nM (Barhanin et al., 1982; 1983; 1984; Vijverberg et al., 1984), while the estimated dissociation constant for VTD is 7 μM (Catterall & Coppersmith, 1981).
Nothwithstanding the different affinity values, both TsTX-I and VTD were equally effective in inducing maximal contractions of about 80% in relation to the maximal contraction induced by barium chloride (10 mM). Thus, the ρ value for TsTX-I (0.84) shows that it is one of the most effective agonists in the vas deferens, with a ρ value even higher than that for other agonists in this organ, such as acetylcholine (ρ=0.50) and noradrenaline (ρ=0.69) (Jurkiewicz et al., 1969).
The similar effectiveness of TsTX-I and VTD to induce contractions of the rat vas deferens contrasts with the ability of these toxins to release catecholamines in bovine adrenal chromaffin cells. Toxin TsTX-I only produced a slight increase of basal secretion of catecholamines from chromaffin cells, with significant enhancements only when high concentrations were used. VTD, in contrast, produced a large increment of catecholamine release (Conceição et al., 1998).
The time course of the response to TsTX-I was relatively slow (Figure 1), while that to ATP was rapid and not maintained for more than a few seconds (not shown). This could be due to the fact that exogenous ATP is added at once and undergoes rapid metabolism, while TsTX-I induces an indirect effect consisting of a complex chain of events involving sodium channel activation, cell depolarization and calcium translocation, leading to the release of endogenous ATP. A similar condition has been described for ciguatoxin-1, a neurotoxin that interacts with sodium channels and induces neurotransmitter release (Brock et al., 1995; 1997). Ciguatoxin-1 evokes continuous asynchronous sympathetic nerve activity in rat smooth muscle that can be maintained for 40 min or longer. Since TsTX-I shares various similarities with ciguatoxin-1 in relation to sodium channel activation (Catterall et al., 2003), it can be advanced that TsTX-I could also induce continuous asynchronous discharges in nerve terminals of the rat vas deferens, leading to a sustained release of ATP and to a consequent maintained contraction.
In different tissues innervated by the sympathetic nervous system, like the vascular tree and the vas deferens, there is considerable evidence that noradrenaline and ATP coexist in the same nerve terminal, but it is not clear if these neurotransmitters are stored in the same vesicle or not (Stjärne, 2001). There are many suggestions that they may be stored in separate vesicles, with different proportions of noradrenaline and ATP, and these vesicles could be released by independent mechanisms (Ellis & Burnstock, 1989; Todorov et al., 1996; 1999; Brock et al., 2000; Stjärne, 2001). As a matter of fact, it was shown here that RES, which is known to deplete noradrenaline stores, was ineffective in relation to endogenous ATP, corroborating the previous data of Kirkpatrick & Burnstock (1987). Evidence for the differential release of neurotransmitters comes from studies with drugs that modulate neurotransmission. Drugs like angiotensin II (Ellis & Burnstock, 1989), α2-adrenoceptor agonists or antagonists (Todorov et al., 1994; 1996) and low doses of tetrodotoxin (Yang & Chiba, 2000) affect the release of noradrenaline to a greater extent than the release of ATP in the vas deferens and some vascular smooth muscles. On the other hand, Prostaglandin E2 (Trachte et al., 1989) and α-latrotoxine (Brock et al., 2000) are examples of drugs that modulate preferentially ATP release. Finally, there is also the observation of a frequency-dependent release of noradrenaline or ATP. At low frequency, the response to electrical stimulation is mediated primarily by ATP and at higher frequency, there is also noradrenergic participation (Todorov et al., 1999). The finding that, in our experiments, the estimation of ATP was lower than that of the other purines (Figure 5) can be explained by the immediate transformation of released ATP by coreleased metabolic enzymes (Todorov et al., 1996).
To our knowledge, this is the first report about an agent inducing selective release of purinergic neurotransmitters in the vas deferens. It is reasonable to suppose that, in this preparation, TsTX-I induces a pattern of depolarization of postganglionic sympathetic nerves that resembles that of low-frequency field stimulation. It is still a matter of speculation as to whether or not TsTX-I has this action in all neurons of systems that possess ATP as cotransmitter. In fact, as mentioned in Introduction this toxin has been implied with acetylcholine release from parasympathetic nerves from the heart and ileum, although parasympathetic nerves may also possess ATP as cotransmitter (Hoyle & Burnstock, 1985; Burnstock, 2004). Furthermore, Langer et al. (1975) have reported that TsTX-I promotes the release of 3H-noradrenaline from the sympathetic nerves from the heart.
It is presently difficult to explain why a depolarizing agent that acts at a given molecular target, such as TsTX-I, releases a single neurotransmitter. It is probable that these characteristics are due to how the depolarization pattern affects each cotransmitter in different neurons (Burnstock, 2004). If this is true, it is possible that nerve cells also use different patterns of depolarization to change quantitative and qualitatively the released neurotransmitters, providing more than one mechanism to control the activity of postsynaptic cells. This hypothesis although interesting is only speculative, indicating the need of more experiments.
Acknowledgments
This paper was supported by FAPESP, CNPq and the Butantan Foundation, Brazil.
Abbreviations
- ADP
adenosine diphosphate
- AMP
adenosine monophosphate
- ATP
adenosine triphosphate
- α,β-mATP
α,β-methylene-ATP
- EC50
effective concentration 50% of maximum response
- HPLC
high-performance liquid chromatography
- i.p.
intraperitoneal
- PZS
prazosin
- ρ
relative responsiveness ratio
- RES
reserpine
- s.e.mean
standard error (of estimate mean value)
- SUR
suramin
- VTD
veratridine
References
- ARISPE N., JAIMOVICH E., LIBERONA J.L., ROJAS E. Use of selective toxins to separate surface and tubular sodium currents in frog skeletal muscle fibers. Pflugers Arch. 1988;411:1–7. doi: 10.1007/BF00581639. [DOI] [PubMed] [Google Scholar]
- BARHANIN J., GIGLIO J.R., LEOPOLD P., SCHMID A., SAMPAIO S.V., LAZDUNSKI M. Tityus serrulatus venom contains two classes of toxins. Tityus gamma toxin is a new tool with a very high affinity for studying the Na+ channel. J. Biol. Chem. 1982;257:12553–12558. [PubMed] [Google Scholar]
- BARHANIN J., ILDEFONSE M., ROUGIER O., SAMPAIO S.V., GIGLIO J.R., LAZDUNSKI M. Tityus gamma toxin, a high affinity effector of the Na+ channel in muscle, with a selectivity for channels in the surface membrane. Pflugers Arch. 1984;400:22–27. doi: 10.1007/BF00670531. [DOI] [PubMed] [Google Scholar]
- BARHANIN J., PAURON D., LOMBET A., NORMAN R.I., VIJVERBERG H.P., GIGLIO J.R., LAZDUNSKI M. Electrophysiological characterization, solubilization and purification of the Tityus gamma toxin receptor associated with the gating component of the Na+ channel from rat brain. EMBO J. 1983;2:915–920. doi: 10.1002/j.1460-2075.1983.tb01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK J.A., CUNNANE T.C. Effects of Ca2+ concentration and Ca2+ channel blockers on noradrenaline release and purinergic neuroeffector transmission in rat tail artery. Br. J. Pharmacol. 1999;126:11–18. doi: 10.1038/sj.bjp.0702256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK J.A., DUNN W.R., BOYD N.S., WONG D.K. Spontaneous release of large packets of noradrenaline from sympathetic nerve terminals in rat mesenteric arteries in vitro. Br. J. Pharmacol. 2000;131:1507–1511. doi: 10.1038/sj.bjp.0703733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK J.A., MCLACHLAN E.M., JOBLING P., LEWIS R.J. Electrical activity in rat tail artery during asynchronous activation of postganglionic nerve terminals by ciguatoxin-1. Br. J. Pharmacol. 1995;116:2213–2220. doi: 10.1111/j.1476-5381.1995.tb15056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK J.A., MCLACHLAN E.M., RAYNER S.E. Contribution of alpha-adrenoceptors to depolarization and contraction evoked by continuous asynchronous sympathetic nerve activity in rat tail artery. Br J Pharmacol. 1997;120:1513–1521. doi: 10.1038/sj.bjp.0701055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G. Cotransmission. Curr. Opin. Pharmacol. 2004;4:47–52. doi: 10.1016/j.coph.2003.08.001. [DOI] [PubMed] [Google Scholar]
- CARVALHO F.F., NENCIONI A.L., LEBRUN I., SANDOVAL M.R., DORCE V.A. Behavioral, electroencephalographic, and histopathologic effects of a neuropeptide isolated from Tityus serrulatus scorpion venom in rats. Pharmacol. Biochem. Behav. 1998;60:7–14. doi: 10.1016/s0091-3057(97)00407-3. [DOI] [PubMed] [Google Scholar]
- CATTERALL W.A., COPPERSMITH J. Pharmacological properties of sodium channels in cultured rat heart cells. Mol. Pharmacol. 1981;20:533–542. [PubMed] [Google Scholar]
- CATTERALL W.A., STRIESSNIG J., SNUTCH T.P., PEREZ-REYES E. International Union of Pharmacology. XL. Compendium of voltage-gated ion channels: calcium channels. Pharmacol. Rev. 2003;55:579–581. doi: 10.1124/pr.55.4.8. [DOI] [PubMed] [Google Scholar]
- CONCEIÇÃO I.M., LEBRUN I., CANO-ABAD M., GANDIA L., HERNANDEZ-GUIJO J.M., LOPEZ M.G., VILLARROYA M., JURKIEWICZ A., GARCIA A.G. Synergism between toxin-gamma from Brazilian scorpion Tityus serrulatus and veratridine in chromaffin cells. Am. J. Physiol. 1998;274:C1745–C1754. doi: 10.1152/ajpcell.1998.274.6.C1745. [DOI] [PubMed] [Google Scholar]
- COURAUD F., JOVER E.Mechanism of action of scorpion toxins Handbook of Natural Toxins 1984New York: Marcel Dekker; 659–678.ed. Tu, A.T. pp [Google Scholar]
- COUTO A.S., MORAES-SANTOS T., AZEVEDO A.D., ALMEIDA A.P., FREIRE-MAIA L. Effects of toxin Ts-gamma, purified from Tityus serrulatus scorpion venom, on the isolated rat atria. Toxicon. 1992;30:339–343. doi: 10.1016/0041-0101(92)90874-5. [DOI] [PubMed] [Google Scholar]
- DEBIN J.A., MAGGIO J.E., STRICHARTZ G.R. Purification and characterization of chlorotoxin, a chloride channel ligand from the venom of the scorpion. Am. J. Physiol. 1993;264:C361–C369. doi: 10.1152/ajpcell.1993.264.2.C361. [DOI] [PubMed] [Google Scholar]
- DORCE V.A., SANDOVAL M.R. Effects of Tityus serrulatus crude venom on the GABAergic and dopaminergic systems of the rat brain. Toxicon. 1994;32:1641–1647. doi: 10.1016/0041-0101(94)90322-0. [DOI] [PubMed] [Google Scholar]
- DRUMOND Y.A., COUTO A.S., MORAES-SANTOS T., ALMEIDA A.P., FREIRE-MAIA L. Effects of toxin Ts-gamma and tityustoxin purified from Tityus serrulatus scorpion venom on isolated rat atria. Comp. Biochem. Physiol. C. 1995;111:183–190. doi: 10.1016/0742-8413(95)00026-k. [DOI] [PubMed] [Google Scholar]
- ELLIS J.L., BURNSTOCK G. Angiotensin neuromodulation of adrenergic and purinergic co-transmission in the guinea-pig vas deferens. Br. J. Pharmacol. 1989;97:1157–1164. doi: 10.1111/j.1476-5381.1989.tb12574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEDAN J.S., BESSE J.C. Distensibility and responsiveness of the rat seminal vesicle: effects of denervation and pretreatment of animals with 6-hydroxydopamine and reserpine. J. Pharmacol. Exp. Ther. 1980;214:472–477. [PubMed] [Google Scholar]
- FERNANDES DE OLIVEIRA J., TEIXEIRA C.E., ARANTES E.C., DE NUCCI G., ANTUNES E. Relaxation of rabbit corpus cavernosum by selective activators of voltage-gated sodium channels: role of nitric oxide-cyclic guanosine monophosphate pathway. Urology. 2003;62:581–588. doi: 10.1016/s0090-4295(03)00462-x. [DOI] [PubMed] [Google Scholar]
- GARCIA M.L., HANNER M., KNAUS H.G., SLAUGHTER R., KACZOROWSKI G.J. Scorpion toxins as tools for studying potassium channels. Methods Enzymol. 1999;294:624–639. doi: 10.1016/s0076-6879(99)94035-1. [DOI] [PubMed] [Google Scholar]
- HIRATA H., ALBILLOS A., FERNÁNDEZ F., MEDRANO J., JURKIEWICZ A., GARCÍA A.G. Conotoxins block neurotransmission in the rat vas deferens by binding to different presynaptic sites on the N-type Ca2+ channel. Eur. J. Pharmacol. 1997;321:217–223. doi: 10.1016/s0014-2999(96)00951-x. [DOI] [PubMed] [Google Scholar]
- HOYLE C.H.V., BURNSTOCK G. Atropine-resistant excitatory junction potentials in rabbit bladder are blocked by α,β-methylene ATP. Eur. J. Pharmacol. 1985;114:239–240. doi: 10.1016/0014-2999(85)90635-1. [DOI] [PubMed] [Google Scholar]
- JONAS P., VOGEL W., ARANTES E.C., GIGLIO J.R. Toxin gamma of the scorpion Tityus serrulatus modifies both activation and inactivation of sodium permeability of nerve membrane. Pflugers Arch. 1986;407:92–99. doi: 10.1007/BF00580727. [DOI] [PubMed] [Google Scholar]
- JURKIEWICZ A., ABDO A.O., JURKIEWICZ N.H., GUEDES A.O., SOUCCAR C. Relative responsiveness (rho): a critical analysis of a new method in receptor differentiation. Gen. Pharmacol. 1976;7:93–101. doi: 10.1016/0306-3623(76)90042-2. [DOI] [PubMed] [Google Scholar]
- JURKIEWICZ A., JURKIEWICZ N.H. Dual effect of alpha-adrenoceptor antagonists in rat isolated vas deferens. Br. J. Pharmacol. 1976;56:169–178. doi: 10.1111/j.1476-5381.1976.tb07439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JURKIEWICZ A., JURKIEWICZ N.H., BARROS G.G., VALLE J.R. Relative responsiveness (rho) of pharmacological receptor systems in the rat vas deferens. Pharmacology. 1969;2:89–99. doi: 10.1159/000136005. [DOI] [PubMed] [Google Scholar]
- KASUYA Y., GOTO K., HASHIMOTO H., WATANABE H., MUNAKATA H., WATANABE M. Nonspecific denervation supersensitivity in the rat vas deferens ‘in vitro'. Eur. J. Pharmacol. 1969;8:177–184. doi: 10.1016/0014-2999(69)90074-0. [DOI] [PubMed] [Google Scholar]
- KIRKPATRICK K., BURNSTOCK G. Sympathetic nerve-mediated release of ATP from the guinea-pig vas deferens is unaffected by reserpine. Eur. J. Pharmacol. 1987;138:207–214. doi: 10.1016/0014-2999(87)90434-1. [DOI] [PubMed] [Google Scholar]
- LANGER S.Z., ADLER-GRASCHINSKY E., ALMEIDA A.P., DINIZ C.R. Prejunctional effects of a purified toxin from the scorpion Tityus serrulatus: release of 3H-noradrenaline and enhancement of transmitter overflow elicited by nerve stimulation. Naunyn Schmiedebergs Arch. Pharmacol. 1975;287:243–259. doi: 10.1007/BF00501471. [DOI] [PubMed] [Google Scholar]
- LEVITT B., HEAD R.J., WESTFALL D.P. High-pressure liquid chromatographic-fluorometric detection of adenosine and adenine nucleotides: application to endogenous content and electrically induced release of adenyl purines in guinea pig vas deferens. Anal. Biochem. 1984;137:93–100. doi: 10.1016/0003-2697(84)90352-x. [DOI] [PubMed] [Google Scholar]
- MARCOTTE P., CHEN L.Q., KALLEN R.G., CHAHINE M. Effects of Tityus serrulatus scorpion toxin gamma on voltage-gated Na+ channels. Circ. Res. 1997;80:363–369. doi: 10.1161/01.res.80.3.363. [DOI] [PubMed] [Google Scholar]
- MATOS I.M., TEIXEIRA M.M., LEITE R., FREIRE-MAIA L. Pharmacological evidence that neuropeptides mediate part of the actions of scorpion venom on the guinea pig ileum. Eur. J. Pharmacol. 1999;368:231–236. doi: 10.1016/s0014-2999(99)00016-3. [DOI] [PubMed] [Google Scholar]
- OHIZUMI Y. Application of physiologically active substances isolated from natural resources to pharmacological studies. Jpn. J. Pharmacol. 1997;73:263–289. doi: 10.1254/jjp.73.263. [DOI] [PubMed] [Google Scholar]
- POSSANI L.D., ALAGON A.C., FLETCHER P.L., JR, ERICKSON B.W. Purification and properties of mammalian toxins from the venom of Brazilian Scorpion Tityus serrulatus Lutz and Mello. Arch. Biochem. Biophys. 1977;180:394–403. doi: 10.1016/0003-9861(77)90053-4. [DOI] [PubMed] [Google Scholar]
- PUPO A.S., CAVENAGHI D.L.C., CAMPOS M., MORAIS P.L., JURKIEWICZ N.H., JURKIEWICZ A. Effects of indoramin in rat vas deferens and aorta: concomitant α1-adrenoceptor and neuronal uptake blockade. Br. J. Pharmacol. 1999;127:1832–1836. doi: 10.1038/sj.bjp.0702735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUPO A.S., JURKIEWICZ N.H., JURKIEWICZ A. Functional change of the balance between alpha 1A and alpha 1B adrenoceptor populations after transplantation of the vas deferens to the intestine. Ann. NY Acad. Sci. 1997;812:193–195. doi: 10.1111/j.1749-6632.1997.tb48171.x. [DOI] [PubMed] [Google Scholar]
- RANDALL A., TSIEN R.W. Pharmacological dissection of multiple types of Ca2+ channel currents in rat cerebellar granule neurons. J. Neurosci. 1995;15:2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVEIRA N.P., MORAES-SANTOS T., AZEVEDO A.D., FREIRE-MAIA L. Effects of Tityus serrulatus scorpion venom and one of its purified toxins (toxin gamma) on the isolated guinea-pig heart. Comp. Biochem. Physiol. C. 1991;98:329–336. doi: 10.1016/0742-8413(91)90213-d. [DOI] [PubMed] [Google Scholar]
- SNEDDON P., WESTFALL D.P. Pharmacological evidence that adenosine triphosphate and noradrenaline are co-transmitters in the guinea-pig vas deferens. J. Physiol. 1984;347:561–580. doi: 10.1113/jphysiol.1984.sp015083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNEDDON P., WESTFALL D.P., COLBY J., FEDAN J.S. A pharmacological investigation of the biphasic nature of the contractile response of rabbit and rat vas deferens to field stimulation. Life Sci. 1984;35:1903–1912. doi: 10.1016/0024-3205(84)90470-3. [DOI] [PubMed] [Google Scholar]
- STJÄRNE L. Novel dual ‘small' vesicle model of ATP- and noradrenaline-mediated sympathetic neuromuscular transmission. Auton. Neurosci. 2001;87:16–36. doi: 10.1016/S1566-0702(00)00246-0. [DOI] [PubMed] [Google Scholar]
- TODOROV L.D., BJUR R.A., WESTFALL D.P. Temporal dissociation of the release of the sympathetic co-transmitters ATP and noradrenaline. Clin. Exp. Pharmacol. Physiol. 1994;21:931–932. doi: 10.1111/j.1440-1681.1994.tb02469.x. [DOI] [PubMed] [Google Scholar]
- TODOROV L.D., MIHAYLOVA-TODOROVA S.T., BJUR R.A., WESTFALL D.P. Differential cotransmission in sympathetic nerves: role of frequency of stimulation and prejunctional autoreceptors. J. Pharmacol. Exp. Ther. 1999;290:241–246. [PubMed] [Google Scholar]
- TODOROV L.D., MIHAYLOVA-TODOROVA S., CRAVISO G.L., BJUR R.A., WESTFALL D.P. Evidence for the differential release of the cotransmitters ATP and noradrenaline from sympathetic nerves of the guinea-pig vas deferens. J. Physiol. 1996;496 Part 3:731–748. doi: 10.1113/jphysiol.1996.sp021723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODOROV L.D., MIHAYLOVA-TODOROVA S., WESTFALL T.D., SNEDDON P., KENNEDY C., BJUR R.A., WESTFALL D.P. Neuronal release of soluble nucleotidases and their role in neurotransmitter inactivation. Nature. 1997;387:76–79. doi: 10.1038/387076a0. [DOI] [PubMed] [Google Scholar]
- TRACHTE G.J., BINDER S.B., PEACH M.J. Indirect evidence for separate vesicular neuronal origins of norepinephrine and ATP in the rabbit vas deferens. Eur. J. Pharmacol. 1989;164:425–433. doi: 10.1016/0014-2999(89)90250-1. [DOI] [PubMed] [Google Scholar]
- TRIPATHY A., RESCH W., XU L., VALDIVIA H.H., MEISSNER G. Imperatoxin A induces subconductance states in Ca2+ release channels (ryanodine receptors) of cardiac and skeletal muscle. J. Gen. Physiol. 1998;111:679–690. doi: 10.1085/jgp.111.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIJVERBERG H.P., PAURON D., LAZDUNSKI M. The effect of Tityus serrulatus scorpion toxin gamma on Na+ channels in neuroblastoma cells. Pflugers Arch. 1984;401:297–303. doi: 10.1007/BF00582600. [DOI] [PubMed] [Google Scholar]
- YANG X., CHIBA S. Differential effects of omega-conotoxin GVIA, tetrodotoxin and prolonged cold storage on purinergic and adrenergic transmission in isolated canine splenic artery. J. Cardiovasc. Pharmacol. 2000;36:S5–S8. doi: 10.1097/00005344-200000006-00003. [DOI] [PubMed] [Google Scholar]
- YATANI A., KIRSCH G.E., POSSANI L.D., BROWN A.M. Effects of New World scorpion toxins on single-channel and whole cell cardiac sodium currents. Am. J. Physiol. 1988;254:H443–H451. doi: 10.1152/ajpheart.1988.254.3.H443. [DOI] [PubMed] [Google Scholar]
- ZUCKER R.S. Exocytosis: a molecular and physiological perspective. Neuron. 1996;17:1049–1055. doi: 10.1016/s0896-6273(00)80238-x. [DOI] [PubMed] [Google Scholar]