Abstract
Facet joint capsules (FJC) may experience large mechanical deformation under spine motion. There has been no previous quantitative study of the relationship between capsular strain and sensory nerve activation in spine FJC in vivo. Space limitation in the cervical spine makes such a study difficult, as the facet joint must be loaded while simultaneously monitoring nerve discharge from nerve roots immediately adjacent to the loaded tissue. A new methodology was developed to investigate biomechanical and neurophysiological properties of spine facet joint capsules in vivo. The method incorporated a custom-fabricated testing frame for facet joint loading, a stereoimaging system, and a template-matching technique to obtain single afferent response. It was tested by loading goat C5–C6 FJC in vivo with simultaneous nerve root recordings and 3D strain tracking of the capsules. Preliminary data showed that 18 of 23 afferents (78.3%) were found to be mechanosensitive to tensile stretch, and five were not responsive, even under tensile load as high as 27.5 N. Mechanosensitive afferents in goat capsules had tensile strain thresholds of 0.119±0.080. Neural responses of all mechanosensitive units showed statistically significant correlations (all P<<0.05) with both capsular load (r2=0.744±0.109) and local strain (r2=0.868±0.088). This method enables the investigation of the correlation between tissue load, deformation and neural responses of mechanoreceptors in spine facet joint capsules, and can be adapted to investigate tissue loading and neural response of other soft tissues.
Keywords: Mechanosensitive, Strain, Facet joint capsule
Introduction
The human spine provides substantial load-bearing capability and range of motion due to important properties of its tissues and structures. Among various spinal soft tissues, facet joint capsules (FJC) have been the focus of several research studies due to their important roles in physical activities and spine pathology. The vertebral column has a greater range of motion in the cervical and lumbar regions than in the thoracic region for flexion and extension, and the FJC may experience large mechanical deformation in these regions. Various biomechanical studies have implicated that the lower cervical FJC can stretch beyond their physiologic range under whiplash loading conditions [7, 18, 28]. Yang and King [30] observed that the lumbar facet joint under compression could cause the capsule to stretch. Clinically, facet denervation has long been employed in the management of cervical, thoracic and low-back pain [4, 17, 22, 24], indicating the neuro-responsive nature of FJC. However, neurophysiologic assessment of FJC to stretch has only been performed in the lumbar region and in very few studies [19, 29].
Additionally, there is no quantitative study of the biomechanical basis for sensory activation in spine FJC in vivo, as opposed to other joint capsules, such as knee [10] and hip [20]. Yamashita et al. [29] reported the response of lumbar FJC mechanoreceptors to stretch, but did not quantify the strain or loading. The first attempt to evaluate nerve response to controlled mechanical loading in lumbar spine was conducted in an in vitro model using an isolated preparation of a rabbit spine column [1]. As its anterior spine elements were retained and some of the applied load was transferred through vertebral column and disk, the mechanosensitive response of the capsule per se was not characterized. Pickar and McLain [19] used an in vivo setup, where upper lumbar articular pillars in cats were isolated and pulled to simulate tension, compression, rotation and lateral bending specifically on facet joints, while joint and muscle afferents were evaluated for mechanoresponse. Their mechanoresponse, however, was not quantitatively characterized in terms of joint deformation, strain or loading.
One reason why there have been so few neurophysiological studies of spine FJC in vivo is that many challenges are involved, including: (1) Spinal nerve roots are rather short in cervical and thoracic regions, and are not conducive to placing conventional hook electrodes for root recording. Many neurophysiological studies have been conducted on lumbar nerve roots, which are long and easy to work with, while virtually none has been done on cervical or thoracic nerve roots. In addition, short roots (2–6 mm length in goat cervical region) do not allow for conventional nerve root splitting to obtain single afferent activity. (2) Spine FJCs are innervated by medial branches of dorsal rami of spinal nerves [3]. Their nerve trunks are underneath articular pillars and difficult to access in an in vivo setting. This does not allow for recording from the peripheral nerve, which is the most common way to record sensory neural activity from tissues of interest. (3) Spine FJCs are small in size at all levels. This workspace limitation makes it difficult to anchor and apply controlled loading on facet joints without involving the surrounding tissues and structures. Another concern is the presence of motion artifact during neural recording especially when the recording site and mechanical stimulus site are so close together. (4) Spine FJCs have approximately even aspect ratios of length and width, and do not have planar surfaces in an in vivo setting. Thus, they cannot be treated as one-dimensional (1D) tissue such as ligaments [20] or two-dimensional (2D) tissue, such as knee joint capsules [13]. It is necessary to quantify three-dimensional (3D) deformation of the capsules during mechanical testing.
In this study, a goat animal model was used to study the neuroresponse of cervical capsular receptors to tensile stimuli. As surrogates for human spine studies, goats and sheep have been used in many spine studies, such as disk healing and spine fusion [5, 12], particularly in cervical regions [32]. Comparative anatomical studies between sheep and human cervical spine indicated that, although the morphology was not identical, with the most manifest difference in vertebral body and pedicle height, a strong similarity was observed in all other anatomical parameters including facet joint height, width and orientation [26]. In addition, biomechanical data indicated that intervertebral angles and bone mineral density were not significantly different between the two species [14] and the craniocaudal variation in range of motion in all load directions was qualitatively similar [27]. Furthermore, the upright posture of the goat neck loads the cervical spine in a manner physiologically similar to that of the human. Thus, a goat model could potentially serve as an appropriate model for studying mechaosensation of cervical facet joints.
Therefore, in order to understand biomechanical and neurophysiological properties of FJC and how FJC may be involved in whiplash pain, thoracic pain and mechanical low-back pain, a new in vivo experimental method was developed to address these issues in goat. The technique was designed to perform mechanical deformation on the capsules in vivo, with simultaneous neurophysiological recording and 3D mechanical strain mapping. This method enabled the investigation of the correlation between capsular load, deformation and neural responses of mechanoreceptors in spine FJC.
Methods
Test apparatus for biomechanical studies on spine FJC
A fixture was fabricated that incorporated a spine fixator, an actuator with a load cell and a 3D imaging system (Fig. 1). A steel frame anchored to the floor served as the stationary base for the whole test apparatus. An inverted “T” frame constructed from two 5.08×5.08-cm T-slotted extruded aluminum bars (80/20, Columbia City, Ind., USA) was positioned upright and bolted onto the base frame. Gusset corner brackets were used in all rigid connections in the setup. A stereoimaging system was incorporated to quantify and track capsular deformation during mechanical tests. To do so, a 5×5 array of tantalum spheres approximately 0.5 mm in diameter and 2–3 mm apart were placed on the C5–C6 FJC using a minimal amount of acrylic paint as adhesive, which allowed no relative motion of tantalum balls on the capsule (Fig. 1inset). Two Kodak Motion Corder Analyzer SR-500 cameras (Kodak, San Diego, Calif., USA) were oriented to maximize the accuracy of 3D digital reconstruction of deformed capsule by maintaining two camera views in different planes.
Fig. 1.

Testing apparatus design. The fixture frame accommodates a computer-controlled actuator, a spine fixator and two synchronized stereoimaging CCD cameras. The inset displays detailed setup for nerve root recording and visual marker application on a C5–C6 facet joint capsule. Box A in the diagram and box B in the inset are further illustrated in Fig. 2a, b
The spine fixator was composed of a 2.54×2.54-cm extruded aluminum bar attached to the inverted “T” frame by a 180° hinge. Its other end was fixed to the goat spine by a fixation apparatus (Fig. 2a). A machine screw was threaded through the T1 spinal process and tightened to prevent whole body movement during stretch. Once an appropriate position and angle of the bar was identified, the 180° hinge was locked to securely anchor the goat spine for mechanical tests on FJC.
Fig. 2.

Illustration of the spine fixator (a) and the actuator adaptor setup (b). a The fixator was fixed on the spinous process by a screw (illustrated) between the beam bracket and the gusset bracket. b The actuator shaft was coupled to the load cell by adaptor 1, while adaptor 2 connected the anchoring hook with the load cell. Thick stainless steel wires (2.38 mm diameter) were used to make a 75° hook to assure that the hook did not bend under loading up to 89 N
A 5.08×5.08-cm aluminum bar was rigidly fastened perpendicularly on the inverted “T” frame serving as the support for the Gemini GV6 digital servo actuator (Parker Hannifin, Roherk Park, Calif., USA), whose position and angle could be adjusted via slotted plates. The setup from the actuator to the hook on C5 inferior articular process is shown in Fig. 2b. The actuator was connected to a miniature 50 lb (222 N) load cell (Entran, Fairfield, N.J., USA) through an aluminum adaptor while the load cell joined a 75° stainless steel hook (2.38 mm diameter) through another aluminum adaptor. Both adaptors were lightweight (less than 20 g) and did not interfere with the performances of the actuator (maximum load, 600 N) and the load cell (safety limit, 334 N). The hook was inserted into holes drilled into the freed C5 inferior articular process, 8–10 mm rostral to the insertion of the capsule. The C5 superior articular process was removed to allow space for the actuator to anchor the inferior process and for the C5–C6 FJC to be able to undergo tensile stretch in vivo without obstruction from upper levels of vertebrae.
New approach for neurophysiological studies on spine FJC
Neural activity from C6 dorsal rootlet was recorded by a custom-designed dual bipolar microelectrode described by Chen et al. [6]. This specially fabricated electrode allowed stable recordings from relatively short C6 dorsal nerve rootlets (2–6 mm). Unrelated neural activity was prevented by cutting the ventral ramus at the same level. However, robust spontaneous activity was still observed from naturally split rootlets (usually six or seven rootlets at goat C5–C6 level). Manual splitting of these rootlets was difficult to perform due to short root lengths (2–6 mm).
An alternative approach to manual splitting of these rootlets was taken to obtain and analyze single afferent activities. Nerve discharge data were analyzed using Enhanced Graphics Acquisition and Analysis (EGAA) (RC Electronics, Santa Barbara, Calif., USA) window discrimination software. Single units were identified and isolated by their response to electrical stimulation of receptive fields (8 or 15 V, 0.1 ms duration, 1 Hz) on the capsular surface (Fig. 3). Peak electrical stimulus artifact (not shown) was set as the time trigger for data sampling so that sampled data were synchronized in reference to electrical stimulation. At least three superimpositions of a waveform with the same waveshape determined the presence of a single afferent unit (Fig. 3inset) and its conduction velocity (CV). Using the waveshape recognition function in the EGAA system, the characteristic shape of the mean waveform of these superimposed action potentials was saved as a template and each template was applied to multiunit nerve recordings to isolate the discharge rate histogram of this particular unit. An example is shown in Fig. 4. The action potential spikes that matched the template shape within a margin of error of 30% were considered acceptable. The formulation of margin of error in EGAA software is a combined measure of proximity of all data points of the waveform compared with the template. Using the A/D sampling period of 15 μs, unit templates obtained often had 25–33 data points with a peak-to-valley amplitude of 50–300 mV under the same amplification in the recording system. Under these conditions, a margin of error of 30% corresponded to a mean error percentage of 1–6% of the peak-to-valley amplitude of the waveform for each data point. Figure 4 shows a template pattern applied to multiunit nerve discharge in a tensile test. Even though the template pattern and the source unit looked almost identical, the error percentage was still 29.4%, close to the limit of the 30% error margin. It was shown that the template matching technique in current study was a rather strict process, which was capable of faithfully isolating the single afferent unit of interest.
Fig. 3.
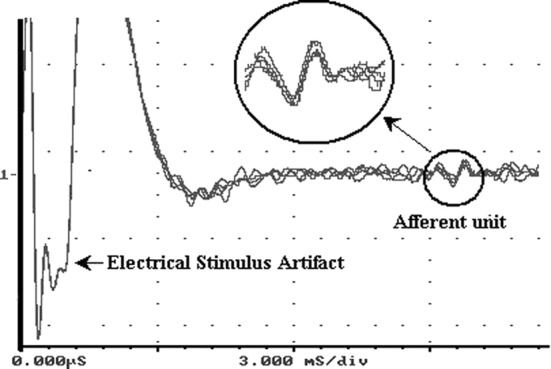
An example of identification of a single afferent unit. Five electrical stimuli of the same voltage, duration and frequency are shown superimposed. This train of electrical stimuli on joint capsules was reflected in root recordings as stimulus artifact. Due to their large amplitude, positive peaks of stimulus artifact are not shown. The inset shows that a single afferent was provoked by these stimuli because all five responses had the same waveshape and the same travel time from stimulated spot to recording site, indicating the same CV. Nerve activities are small in magnitude compared to the large stimulus artifact
Fig. 4.

An example of template matching technique used in current study. Source action potentials were screened to match the specific pattern of a single afferent. If the match error of a source action potential fell within a margin of error of 30%, it was considered as one impulse of this afferent. A margin of error of 30% corresponded to a mean error percentage of 1–6% of the peak-to-valley amplitude of the waveform for each data point. Histograms could then be obtained for this afferent from multiunit nerve root recordings
Method evaluation
Goat cervical FJCs were used to evaluate this method. As indicated in the Fig. 1inset, a C5–C6 laminectomy was performed on an adult Lamancha or Alpine female goat (34–55 kg) under general anesthesia. Initial anesthesia was induced with diazepam (0.5 mg/kg, i.m.), pentothal (15 mg/kg, i.v.), butorphenol (0.22 mg/kg, i.m.) and atropine (0.066 mg/kg, i.m.) and anesthesia was maintained by isoflurane inhalation (2.5–3%). All surgical procedures were approved by the institutional Animal Investigation Committee. The C5–C6 FJC had original measurements of a 10–15 mm length and a 10–16 mm width. The capsule underwent a series of quasi-static stretch tests at a rate of 0.5 mm/s until the load trace indicated a failure of the capsule. Nerve activity was amplified with a differential AC amplifier (Model 1700, A-M Systems, Carlsborg, Wash., USA), displayed on an oscilloscope (Model R5103N, Tektronix, Beaverton, Ore., USA), and then recorded on an analogue tape recorder (Model MR-30, TEAC, Montebello, Calif., USA) along with capsular load, which was amplified through another differential amplifier (Transbridge TBM4, WPI, Sarasota, Fla., USA). Image capturing (480×512 pixels, three frames per second) was synchronized with nerve activity and load recordings. The 3D strain analysis was conducted using a finite element method (FEM) after each experiment. Digital video images of a 3D calibration frame were taken to enclose the capsule location into the frame’s 3D volume in order to maximize the accuracy of digital reconstruction of the capsular markers. The calibration frame had 15 markers with known coordinates with a volume of 30×20×16 mm. Similar techniques have been used in kinematics studies [20, 23]. FEM strain analysis was comprised of the following steps: (1) ImageExpress 3D Wizard (SAI, Utica, N.Y., USA) was used to track the displacements of the tantalum spheres to obtain their 3D coordinates using the images of the calibration frame as the reference system; (2) linear membrane elements were developed using Hypermesh (Altair, Troy, Mich., USA) by interpolating the array of markers to an element mesh using the original positions of the markers, each marker as a node; (3) digitized marker displacements were imposed on the nodes of the mesh to reconstruct the capsule deformation; (4) LS-DYNA (LSTC, Livermore, Calif., USA) was then used to perform strain analysis. The mean error of marker digitization and FEM strain reconstruction was determined to be less than 1% by performing the same procedures on a calibration frame.
Maximum principal Lagrangian strains were obtained for each identified mechanosensitive units on their receptive fields from FEM analysis. Linear regression was performed to evaluate the correlation between neural response and local strain, and the correlation between neural response and capsular load. A P value of less than 0.05 in t-test and one-way ANOVA was considered statistically significant.
Results
Four goats were used to develop the methodology, and four additional goat experiments were successfully conducted using this method. During development, failures sometimes occurred in the setup of mechanical testing, mainly due to hook slippage out of the C5 articular process. To resolve this problem, thicker and steeper steel hooks were utilized to prevent yielding at the curvature of the hooks. There was no slippage in the adaptor (Fig. 2a) between spinous process and the spine fixator, and the spine fixator did not bend under loads. The mechanical testing system proved to be efficient and stable.
Spine FJCs are innervated by the articular branches of the medial branch of the dorsal ramus in human [3] and the same was observed in goat neck dissection in this study. As peripheral nerves, articular branches were embedded in the capsule and muscles, and entered the capsule from the lateral face of the articular process immediately after exiting the intervertebral foramen.
Twenty-three single afferents were identified using the template-matching method in four goats. Eighteen of 23 units (78.3%) were found to be mechanosensitive to tensile stretch, and five were not responsive, even under tensile loads as high as 27.5 N. Based on their CVs, 18 mechanosensitive units were categorized into four Aβ, six Aδ and eight C afferent units. Afferents with CVs of greater than 20 m/s, 2.5–20 m/s or less than 2.5 m/s, are considered as Aβ, Aδ or C units, respectively [21]. One example is shown in Fig. 5, which presents the response of an identified C fiber (CV=2.48 m/s) in a 4-mm stretch test. Multiunit nerve recordings displayed increased activities over tensile stretch (Fig. 5b). Using its waveshape (Fig. 5c inset) identified from electrical stimulation on its receptive field (Fig. 4), which was located at the lateral region of the capsule just on the joint gap, the multiunit data underwent waveshape discrimination and the response of this unit to joint loading was obtained (Fig. 5c).
Fig. 5a–d.

Data summary for a representative 4-mm tensile test. a Multiunit impulses of action potentials. T on the voltage axis indicates a threshold level used to obtain multiunit histogram of discharge rates in b. b Multiunit discharge rate increased during stretch. c An identified C afferent (CV=2.48 m/s) with the waveshape shown in the inset exhibited increased discharge rate after a specific threshold. d Capsular load increased nonlinearly with time during stretch, and showed stress relaxation during the 10 s hold
The 18 afferents in three different categories responded to mechanical stretch with various strain thresholds. The strain definition used in the current study was the first principal Lagrangian strain found at the element where the receptive field of a unit was located. The mean tensile strain threshold of all units was 0.119±0.080, while it was 0.124±0.066, 0.098±0.065 and 0.132±0.104 for Aβ, Aδ and C categories, respectively. No significance was observed between groups. The mechanoresponse of sensory units was analyzed by linear regression against capsular load and local strain. Neural responses above threshold and below saturation for each unit have statistically significant correlations with both factors (P<<0.05), and were shown to have a mean coefficient r2 of 0.744±0.109 with capsular load, and a mean r2 of 0.868±0.088 with local strain. Load correlation was significantly less than strain correlation (P=0.00). Figure 6 is a sample plot of the two correlations in a representative unit.
Fig. 6a,b.

Neural response of a representative mechanosensitive C afferent (CV=2.48 m/s) to tensile stretch. Nerve discharge increases above thresholds were linearly regressed against capsular load (a), and maximum principal Lagrangian strain (b). Both correlations were statistically significant
Discussion
This study is the first to provide an experimental platform for simultaneously studying biomechanics and neurophysiology on spine FJC in vivo. This methodology presented several advantages in the investigation of the mechanosensitivity in spinal soft tissues under tensile loading. First, the in vivo setting maintained the capsule and its innervation viable for a much longer period of time compared with the in vitro lumbar facet joint study of Avramov et al. [1]. Secondly, it provided an effective way to isolate the facet joint for mechanical testing on capsules alone. This allowed minimal load sharing with other spinal soft tissues in a limited working space. Finally, the test apparatus can be inexpensively fabricated in customized sizes and shapes, which allows applications in other animal models for in vivo spinal soft tissue studies.
The application of template matching technique to cervical nerve root recordings proved to be successful for isolating single afferent responses when splitting nerve roots was not an option while working with extremely short roots such as those in cervical and thoracic regions. Spinal nerve roots have been reported to have failure tensile strains of 8–46% [2, 25]. Short nerve roots of 2–6 mm length are more susceptible to injury if being manually split, as short displacements can produce high strains. Using the template matching method instead, it was found in our preliminary data that a mean of 5.8 single afferents were identified in each goat experiment, and 78.3% of single afferents provoked by electrical stimulation showed mechanical response to tensile stretch with various strain thresholds.
A 2D stress measurement of spine FJC under loading is impractical in the spine setting. All 2D stress studies with neurophysiology have been performed in vitro on clearly isolated and easily accessible tissues, such as skin [16] and limb muscle [9]. However, because spine FJCs are small and hard to access in vivo, it is rather difficult to accommodate multiple load cells, which are required to measure 2D stress during tensile tests. In addition, a FJC cannot be anchored to load cells, especially on lateral sides, without destroying its structural integrity and innervation. Therefore, direct 2D stress measurement cannot possibly be done in the spine setting.
Considering all the practical limits, 3D strain is the only measurable mechanical state for characterizing the mechanosensitivity of spine FJC. Similar 3D strain tracking techniques were used in several kinematics studies of cervical facet joints [23, 28]. In our study, 3D strain tracking was incorporated with neurophysiological recordings. Other studies on mechanosensitive afferents have suggested that the correlation between nerve response and local stress was better than local strain in skin [11, 16] and knee joint [8, 15]. High coefficients were observed in both strain correlation and load correlation with neural responses in current study. This suggests that the capsules may behave somewhat like a linear elastic material in a certain range under quastistatic loading. Thus, under quastistatic loading, strain can be directly used as an indicator of mechanical state. Under dynamic conditions, stress values in deformed soft tissues can only be obtained indirectly by FEM, which can numerically calculate local stresses, based on known material properties and boundary conditions (local strains in our case). Systematic evaluation of the viscoelastic properties is crucial in providing a realistic basis for numerical simulations of soft tissues under various strain rates. Appropriate viscoelastic material models are needed for this FEM approach. To date, material properties of human cervical FJC have been studied under several different loading rates ranging from 0.0083 to 100 mm/s [23, 28, 31]. Further systematic studies on material properties are needed.
In conclusion, the method presented here proved to be successful in evaluating neural and mechanical response in goat cervical FJC. The method can be adapted to investigate tissue loading and neural response of other soft tissues.
Acknowledgements
This work was supported by CDC Grant no. R49-CCR519751 and a Ford Fellowship provided by Ford Motor Company to the first author. The technical assistance of Anita Singh is gratefully acknowledged.
References
- 1.Avramov AI, Cavanaugh JM, Ozaktay CA, Getchell TV, King AI. The effects of controlled mechanical loading on group-II, III, and IV afferent units from the lumbar facet joint and surrounding tissue. An in vitro study. J Bone Joint Surg Am. 1992;74:1464–1471. [PubMed] [Google Scholar]
- 2.Beel JA, Stodieck LS, Luttges MW. Structural properties of spinal nerve roots: biomechanics. Exp Neurol. 1986;91:30–40. doi: 10.1016/0014-4886(86)90023-3. [DOI] [PubMed] [Google Scholar]
- 3.Bogduk N. The clinical anatomy of the cervical dorsal rami. Spine. 1982;7:319–330. doi: 10.1097/00007632-198207000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Bogduk N, Marsland A. The cervical zygapophysial joints as a source of neck pain. Spine. 1988;13:610–617. [PubMed] [Google Scholar]
- 5.Brantigan JW, McAfee PC, Cunningham BW, Wang H, Orbegoso CM. Interbody lumbar fusion using a carbon fiber cage implant versus allograft bone. An investigational study in the Spanish goat. Spine. 1994;19:1436–1444. doi: 10.1097/00007632-199407000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Chen C, Lu Y, Cavanaugh JM, Kallakuri S, Patwardhan A (2004) Neurophysiologic studies of cervical facet joint capsule—experimental setup and characterization of sensory receptors. In: Transactions of the ORS 50th annual meeting
- 7.Deng B, Begeman PC, Yang KH, Tashman S, King AI (2000) Kinematics of human cadaver cervical spine during low speed rear-end impacts. Proceedings of the 44th Stapp Car Crash Conference, pp 171–188 [DOI] [PubMed]
- 8.Fuller MS, Grigg P, Hoffman AH. Response of joint capsule neurons to axial stress and strain during dynamic loading in cat. J Neurophysiol. 1991;65:1321–1328. doi: 10.1152/jn.1991.65.6.1321. [DOI] [PubMed] [Google Scholar]
- 9.Ge W, Khalsa PS. Encoding of compressive stress during indentation by group III and IV muscle mechano-nociceptors in rat gracilis muscle. J Neurophysiol. 2003;89:785–792. doi: 10.1152/jn.00624.2002. [DOI] [PubMed] [Google Scholar]
- 10.Grigg P, Hoffman AH. Loading and deformation of the cat posterior knee joint capsule in axial and extension rotations. J Biomech. 1993;26:1283–1290. doi: 10.1016/0021-9290(93)90352-F. [DOI] [PubMed] [Google Scholar]
- 11.Grigg P. Stretch sensitivity of mechanoreceptor neurons in rat hairy skin. J Neurophysiol. 1996;76:2886–2895. doi: 10.1152/jn.1996.76.5.2886. [DOI] [PubMed] [Google Scholar]
- 12.Gunzburg R, Fraser RD, Moore R, Vernon-Roberts B. An experimental study comparing percutaneous discectomy with chemonucleolysis. Spine. 1993;18:218–226. doi: 10.1097/00007632-199302000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman AH, Grigg P. A method for measuring strains in soft tissue. J Biomech. 1984;17:795–800. doi: 10.1016/0021-9290(84)90110-6. [DOI] [PubMed] [Google Scholar]
- 14.Kandziora F, Pflugmacher R, Scholz M, et al. Comparison between sheep and human cervical spines: an anatomic, radiographic, bone mineral density, and biomechanical study. Spine. 2001;26:1028–1037. doi: 10.1097/00007632-200105010-00008. [DOI] [PubMed] [Google Scholar]
- 15.Khalsa PS, Hoffman AH, Grigg P. Mechanical states encoded by stretch-sensitive neurons in feline joint capsule. J Neurophysiol. 1996;76:175–187. doi: 10.1152/jn.1996.76.1.175. [DOI] [PubMed] [Google Scholar]
- 16.Khalsa PS, Lamotte RH, Grigg P. Tensile and compressive responses of nociceptors in rat hairy skin. J Neurophysiol. 1997;78:492–505. doi: 10.1152/jn.1997.78.1.492. [DOI] [PubMed] [Google Scholar]
- 17.Lord SM, Barnsley L, Wallis BJ, McDonald GJ, Bogduk N. Percutaneous radio-frequency neurotomy for chronic cervical zygapophyseal-joint pain. N Engl J Med. 1996;335:1721–1726. doi: 10.1056/NEJM199612053352302. [DOI] [PubMed] [Google Scholar]
- 18.Ono K, Kaneoka K, Wittek A, Kajzer J (1997) Cervical injury mechanism based on the analysis of human cervical vertebral motion and head-neck-torso kinematics during low-speed rear impacts. Proceedings of the 41st Stapp Car Crash Conference, pp 339–356
- 19.Pickar JG, McLain RF. Responses of mechanosensitive afferents to manipulation of the lumbar facet in the cat. Spine. 1995;20:2379–2385. doi: 10.1097/00007632-199511001-00002. [DOI] [PubMed] [Google Scholar]
- 20.Rossi A, Rossi B. Characteristics of the receptors in the isolated capsule of the hip in the cat. Int Orthop. 1985;9:123–127. doi: 10.1007/BF00266954. [DOI] [PubMed] [Google Scholar]
- 21.Schaible HG, Schmidt RF. Activation of groups III and IV sensory units in medial articular nerve by local mechanical stimulation of knee joint. J Neurophysiol. 1983;49:35–44. doi: 10.1152/jn.1983.49.1.35. [DOI] [PubMed] [Google Scholar]
- 22.Shealy CN. Facet denervation in the management of back and sciatic pain. Clin Orthop. 1976;Mar–Apr(115):157–164. [PubMed] [Google Scholar]
- 23.Siegmund GP, Myers BS, Davis MB, Bohnet HF, Winkelstein BA (2000) Human cervical motion segment flexibility and facet capsular ligament strain under combined posterior shear, extension and axial compression. Proceedings of the 44th Stapp Car Crash Conference, pp 159–170 [DOI] [PubMed]
- 24.Stolker RJ, Vervest AC, Groen GJ. Percutaneous facet denervation in chronic thoracic spinal pain. Acta Neurochir (Wien) 1993;122:82–90. doi: 10.1007/BF01446991. [DOI] [PubMed] [Google Scholar]
- 25.Sunderland S, Bradley KC. Stress-strain phenomena of human spinal nerve roots. Brain. 1961;84:120–124. [Google Scholar]
- 26.Wilke HJ, Kettler A, Wenger KH, Claes LE. Anatomy of the sheep spine and its comparison to the human spine. Anat Rec. 1997;247:542–555. doi: 10.1002/(SICI)1097-0185(199704)247:4<542::AID-AR13>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 27.Wilke HJ, Kettler A, Claes LE. Are sheep spines a valid biomechanical model for human spines. Spine. 1997;22:2365–2374. doi: 10.1097/00007632-199710150-00009. [DOI] [PubMed] [Google Scholar]
- 28.Winkelstein Cervical facet joint. 1999;mechanics:its. [Google Scholar]
- 29.Yamashita T, Cavanaugh JM, el Bohy AA, Getchell TV, King AI. Mechanosensitive afferent units in the lumbar facet joint. J Bone Joint Surg Am. 1990;72:865–870. [PubMed] [Google Scholar]
- 30.Yang KH, King AI. Mechanism of facet load transmission as a hypothesis for low-back pain. Spine. 1984;9:557–565. doi: 10.1097/00007632-198409000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Yoganandan N, Pintar FA, Kumaresan S, Elhagediab A (1998) Biomechanical assessment of human cervical spine ligaments. Proceedings of the 42nd Stapp Car Crash Conference
- 32.Zdeblick TA, Cooke ME, Wilson D, Kunz DN, Mccabe R. Anterior cervical diskectomy, fusion, and plating—a comparative animal study. Spine. 1993;18:1974–1983. doi: 10.1097/00007632-199310001-00009. [DOI] [PubMed] [Google Scholar]


