Abstract
Fusion proteins with an alpha-hemolysin (HlyA) C-terminal signal sequence are known to be secreted by the HlyB-HlyD-TolC translocator in Escherichia coli. We aimed to establish an efficient Hly secretory expression system by random mutagenesis of hlyB and hlyD. The fusion protein of subtilisin E and the HlyA signal sequence (HlyA218) was used as a marker protein for evaluating secretion efficiency. Through screening of more than 1.5 × 104 E. coli JM109 transformants, whose hlyB and hlyD genes had been mutagenized by error-prone PCR, we succeeded in isolating two mutants that had 27- and 15-fold-higher levels of subtilisin E secretion activity than the wild type did at 23°C. These mutants also exhibited increased activity levels for secretion of a single-chain antibody-HlyA218 fusion protein at 23 and 30°C but unexpectedly not at 37°C, suggesting that this improvement seems to be dependent on low temperature. One mutant (AE104) was found to have seven point mutations in both HlyB and HlyD, and an L448F substitution in HlyB was responsible for the improved secretion activity. Another mutant (AE129) underwent a single amino acid substitution (G654S) in HlyB. Secretion of c-Myc-HlyA218 was detected only in the L448F mutant (AE104F) at 23°C, whereas no secretion was observed in the wild type at any temperature. Furthermore, for the PTEN-HlyA218 fusion protein, AE104F showed a 10-fold-higher level of secretion activity than the wild type did at 37°C. This result indicates that the improved secretion activity of AE104F is not always dependent on low temperature.
Production of recombinant proteins by Escherichia coli is probably the simplest system and has a wide range of applications when posttranslational modifications are not required for the functions of target proteins. Recombinant proteins are usually expressed in the cytoplasm or periplasm in E. coli because conventional strains do not transport their own proteins extracellularly.
However, when recombinant proteins are overexpressed in the cytoplasm of E. coli, formation of inclusion bodies, which makes it difficult to purify active proteins, frequently occurs. On the other hand, when recombinant proteins are expressed in the periplasm, accumulation of the proteins in the periplasmic space often adversely affects the growth of the cell. One reason for this may be the competition with the SecA-SecY machinery for the transport of essential endogenous periplasmic and outer membrane proteins (20).
Extracellular expression in E. coli has some advantages over cytoplasmic or periplasmic expression. For example, addition of peptide tags to the target proteins can facilitate direct purification from culture medium by affinity chromatography. It can also minimize the effect of intracellular or membrane-type proteases (28). Moreover, some proteins may be correctly folded with an appropriate disulfide bond in extracellular (and also periplasmic) spaces since they are in an oxidative environment (9, 21).
Alpha-hemolysin (HlyA; 110 kDa) is one of several hemolytic toxins that is produced extracellularly through a type I secretion system by uropathogenic E. coli strains (4, 5, 11). The type I system does not require a sec-dependent secretion pathway that is crucial for the secretion of periplasmic proteins. Instead, secretion of HlyA involves a carboxy-terminal targeting signal of 50 to 60 amino acids and is thought to occur directly, without a periplasmic intermediate (5, 12). The HlyA export machinery consists of three components. One is HlyB, an inner membrane protein with a cytoplasmic ATP-binding cassette (ABC) domain that is thought to pump out HlyA protein via interaction with the C terminus of HlyA (2, 3, 19). The second is HlyD, a membrane fusion protein that triggers recruitment of TolC through HlyA binding (1, 25). The other is TolC, an outer membrane protein that constitutes a trimeric exit pore (31). Importantly, foreign proteins with the HlyA signal sequence are also recognized and secreted by the HlyB-HlyD-TolC translocator (5, 30), and secretion efficiency significantly increases with longer C-terminal fragments, up to at least 218 amino acids (17).
Several attempts have been made to identify functional elements of the Hly components by introducing mutations into the HlyA signal sequence, the HlyB ABC domain, or HlyD (6, 8, 13, 14, 18, 19, 26). However, there has been no study of functionally evolved Hly components.
In this study, we tried to establish an efficient protein secretion system by genetically improving the HlyA secretion system. By mutagenizing hlyB and hlyD, we successfully obtained two HlyB mutants that have higher levels of secretion activity than the wild type does.
MATERIALS AND METHODS
Materials, bacterial strains, and growth and induction conditions.
The E. coli strains used in this study are shown in Table 1. DH5α was used for cloning and plasmid preparation. JM109 was used for the expression of the Hly components. J96 was used for the isolation of the genomic DNA. These strains were grown with aeration at 37°C in Luria-Bertani (LB) broth with ampicillin (100 μg/ml) or chloramphenicol (30 μg/ml) if necessary. To induce the gene expression under trc or lac promoters, IPTG (isopropyl-β-d-thiogalactopyranoside) was added to a final concentration of 0.4 mM.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristicsa | Source or referenceb |
|---|---|---|
| Strains | ||
| DH5α | deoR endA1 gyrA96 hsdR17 (rK− mK+) recA1 relA1 supE44 thi-I, Δ(lacZYA-argF)U169 Φ80lacZ ΔM15 F− λ− | Toyobo |
| JM109 | e14− (McrA−) recA1, endA1 gyrA96 thi-I hsdR17 (rK mK+) supE44 relA1 Δ(lac-pro AB) (F′ traD36 proAB lacIq ZΔM15) | Toyobo |
| J96 | Serotype O4:K6 | ATCC |
| Plasmids | ||
| pTrcHis2C | Apr; vector for expression of recombinant proteins from trc promoter | Invitrogen |
| pSub-HlyA218 | Apr; subtilisin E-HlyA218 fusion protein cloned between NcoI-SalI sites of pTrcHis2C | This work |
| pCANTAB 5 E | Apr; vector for expression of scFv proteins with E tag at the C terminus | Amersham |
| pCANTAB-HSA14-1 | Apr; HSA14-1 scFv cloned between SfiI-NotI sites of pCANTAB 5 E | Y. Yatabe |
| pHSA14-1-HlyA218 | Apr; anti-HSA scFv-HlyA218 cloned between NcoI-Sac I site of pSub-HlyA218 in place of subtilisin E gene; in frame with the C-terminal E tag | This work |
| pSTV28 | Cmr; vector for expression of recombinant proteins from lac promoter | Takara |
| pSTV-HlyBD | Cmr; hlyB and hlyD cloned between BamHI-SphI sites of pSTV28 | This work |
| pGEM-5Zf (+) | Apr; cloning vector | Promega |
| pGEM-104NE | Apr; NdeI-Eco521 fragment of hlyB and hlyD cloned into pGEM-5Zf(+) | This work |
| pAE104 | Cmr; pSTV-HlyBD derivative with HlyB (L448F, A604T, V682A, and Q705L) and HlyD (A-to-G change at nt −14 and F41Y) | This work |
| pAE104A | Cmr; pSTV-HlyBD derivative with HlyB (A604T, V682A, and Q705L) and HlyD (A-to-G change at nt −14 and F41Y) | This work |
| pAE104B | Cmr; pSTV-HlyBD derivative with HlyB (L448F, V682A, and Q705L) and HlyD (A-to-G change at nt −14 and F41Y) | This work |
| pAE104C | Cmr; pSTV-HlyBD derivative with HlyB (L448F, A604T, and Q705L) and HlyD (A-to-G change at nt −14 and F41Y) | This work |
| pAE104D | Cmr; pSTV-HlyBD derivative with HlyB (L448F, A604T, and V682A) and HlyD (F41Y) | This work |
| pAE104E | Cmr; pSTV-HlyBD derivative with HlyB (L448F, A604T, V682A, and Q705L) and HlyD (A-to-G change at nt −14) | This work |
| pAE104F | Cmr; pSTV-HlyBD derivative with HlyB (L448F) | This work |
| pAE129 | Cmr; pSTV-HlyBD derivative with HlyB (G654S) | This work |
| pTriEX-4 Neo | Apr; cloning vector | Novagen |
| pTriEX-myc | Apr; c-myc cDNA cloned into EcoRI-NotI sites of pTriEX-4 Neo | N. Ise |
| pTriEX-PTEN | Apr; PTEN cDNA cloned into SacI-EcoRI sites of pTriEX-4 Neo | N. Ise |
| pMyc-HlyA218 | Apr; c-myc cDNA cloned into NcoI-NotI sites of pHSA14-1-HlyA218 in place of HSA14-1 scFv gene; in frame with the C-terminal E tag | This work |
| pTEN-HlyA218 | Apr; PTEN cDNA cloned into NcoI-NotI sites of pHSA14-1-HlyA218 in place of HSA14-1 scFv gene; in frame with the C-terminal E tag | This work |
nt, nucleotide.
ATCC, American Type Culture Collection.
The sequence of HSA14-1 single-chain variable fragment (scFv) is composed of a heavy chain and light-chain variable regions linked by a 15-amino-acid (GGGGS)3 linker in the orientation VH-linker-VL. The HSA14-1 scFv DNA, purified HSA14-1 scFv, anti-E tag monoclonal antibody (anti-E tag MAb 121), and its alkaline phosphatase (AP) conjugate were from Y. Yatabe (Fujirebio, Inc.).
Plasmid construction.
The plasmids constructed and used in this study are shown in Table 1. DNA manipulations and PCR were performed as described by Sambrook and Russell (23). All oligonucleotides were synthesized by QIAGEN (Hilden, Germany). A DNA fragment containing a truncated subtilisin E gene whose product lacks 19 amino acids of its N-terminal signal peptide was amplified by PCR using Bacillus subtilis 168 genomic DNA as a template. NcoI and SacI sites were generated at each end of the amplified DNA fragment by using the following two primers: SubNcF (5′-CATGCCATGGTGTCTGTGCAGGCTGCCGGA-3′) and SubScR (5′-ACCGCTCGAGCTCTTGTGCAGCTGCTTGTACGT-3′). The underlined sequences are NcoI and SacI restriction sites, respectively.
A DNA fragment containing E. coli J96 hlyA encoding 218 amino acids of the C-terminal region of HlyA (HlyA218) was amplified by PCR using primers HlyA218-ScF (5′-AACGAGCTCGGAAATTCTCTTGCAAAAAATGTATTATC-3′) and HASLR (5′-TGAATGGTCGACTTATGCTGATGCTGTCAAAGTTATTG-3′), with SacI and SalI restriction sites also introduced at each end of the amplified DNA fragment (indicated by italics). The two resultant DNA fragments (subtilisin E gene and hlyA218) were ligated and cloned into the pTrcHis2C expression vector in frame, resulting in pSub-HlyA218. The DNA fragment encoding HSA14-1 scFv fused with an E tag at the C-terminal region was amplified by PCR using primers HSA-NcF1 (5′-CCGGCCATGGCCCAGGTGCAG-3′) and HSA-ScR1 (5′-AACGAGCTCTGCGGCACGCGGTTCCAGCGG-3′) from pCANTAB-HSA14-1. NcoI and SacI sites, shown in italics, were generated at each end of the amplified DNA fragment and then substituted for the NcoI-SacI fragment of subtilisin E in pSub-HlyA218, resulting in pHSA14-1-HlyA218. The DNA fragment encoding human c-myc cDNA was amplified by PCR using primers Myc-NcF (5′-CATGCCATGGCACCCCTCAACGTTAGCTTCACCA-3′) and Myc-NtR (5′-ATAGTTTAGCGGCCGCACAAGAGTTCCGTAGCTGT-3′) from pTriEX-myc. The DNA fragment encoding human PTEN cDNA was also amplified by PCR using primers PTEN-NcF (5′-CATGCCATGGCAACAGCCATCATCAAAGAGATCGTT-3′) and PTEN-NtR (5′-ATAGTTTAGCGGCCGCGACTTTTGTAATTTGTGTATGCTGATC-3′) from pTriEX-PTEN. NcoI and NotI sites, shown in italics, were generated at each end of the amplified DNA fragments and then substituted for the NcoI-NotI fragment of HSA14-1 scFv in pHSA14-1-HlyA218, resulting in pMyc-HlyA218 and pPTEN-HlyA218, respectively.
hlyB and hlyD genes were also cloned by PCR from E. coli J96 genomic DNA by using primers HBBaF1 (5′-CGCGGATCCGGATTCTTGTCATAAAATTGATTATG-3′) and HDSpR (5′-TGTAAGCATGCTTAACGCTCATGTAAACTTTCTG-3′). BamHI and SphI sites, shown in italics, were generated at each end of the amplified DNA fragment and then cloned into the pSTV28 expression vector (Takara, Shiga, Japan), resulting in pSTV-HlyBD.
Plasmids pAE104 and pAE129, which encode mutated HlyB and HlyD, were isolated from clones AE104 and AE129, respectively (see Results). pAE104A was constructed by introducing a BspHI-SphI DNA fragment (1,829 bp) of pAE104 into pSTV-HlyBD (see Fig. 1). pAE104F was constructed by insertion of a BspHI-SphI DNA fragment of pSTV-HlyBD into pAE104 (see Fig. 1). The plasmids containing other mutants (pAE104B, pAE104C, pAE104D, and pAE104E) were constructed by site-directed mutagenesis of hlyB and hlyD genes (see Fig. 6). Site-directed mutagenesis was carried out by PCR using pGEM-104NE, which contains the 1.2-kb NdeI-Eco52I DNA fragment of hlyB and hlyD based on the inverse PCR method described by Imai et al. (16). The following oligonucleotides were designed to insert codon substitutions in the template: 104B1 (5′-GCAGGATTATCCGGAGGTCAACGT-3′) and 104B2 (5′-CCCCTGTTCCCCGACAATGGTGT-3′) for pAE104B, 104C1 (5′-TGAACAGGGTAAACATAAGGAGCTGC-3′) and 104C2 (5′-ACAATTTTCCCTTTTTCCATGACAATAATG-3′) for pAE104C, 104D1 (5′-GTCAGACTAACAGAAAGAACAGAAGAATATG-3′) and 104D2 (5′-TGTAACTGATATAAGTAACTGTATAAACTTTCCGG-3′) for pAE104D, and 104E1 (5′-CTTACCCGCTCATCTGGAATTAATTG-3′) and 104E2 (5′-AATTCATTTTCGTCCTTTTCACGTACC-3′) for pAE104E.
FIG. 1.
Structure of hlyB and hlyD genes on pSTV-HlyBD as a target for mutagenesis. Primers for random mutagenesis, including restriction enzyme recognition sites, are indicated by arrows. The ATPase region of HlyB is shown by a gray box.
FIG. 6.
Mutation sites in HlyB and HlyD mutants. The ATP-binding domain or NBD in HlyB is shown by a gray box. Putative TMDs in HlyB and HlyD are shown by stippled boxes. The conserved switch II region is shown by a hatched box. The mutation site in pAE129 (G654S) is shown by an asterisk.
All of the sequences amplified by PCR were confirmed by nucleotide sequencing.
Random mutagenesis of HlyBD.
Random mutagenesis of hlyB and hlyD genes was performed with error-prone PCR using a GeneMorph PCR mutagenesis kit (Stratagene, La Jolla, Calif.) according to the manufacturer's instructions. As shown in Fig. 1, pSTV-HlyBD (0.5 to 50 ng) was used as a template for the error-prone PCR, and the following five primers were designed: Bam-F (5′-AGCTCGGTACCCGGGGATCC-3′), Bsp-R (5′-CACGCAATTCAGAAATAAAATCATGA-3′), Bsp-F (5′-GCGAAATTAGCAGGTGCTCATGA-3′), Sph-R (5′-CCAGTGCCAAGCTTGCATGC-3′), AL-F (5′-CAACCTGTGGTTGGGTGCAC-3′), and E52-R (5′-TAAGCAACCAGACGCGGCCG-3′). The italicized sequences are BamHI, BspHI, BspHI, SphI, ApaLI, and Eco52I restriction enzyme sites, respectively.
Screening for improved secretion activity.
E. coli JM109 harboring pSub-HlyA218 and mutagenized pSTV-HlyBD was cultured on LB plates containing 2% skim milk, 0.4 mM IPTG, 200 μg of ampicillin/ml and 30 μg of chloramphenicol/ml. Since prepro-subtilisin E could be efficiently folded and autoprocessed at temperatures lower than 37°C (15), screening of HlyB and HlyD mutants was performed at 23°C. The secretion efficiency of subtilisin E-HlyA218 was estimated on the basis of the diameters of halos that appeared by degradation of skim milk that was dependent on the proteolytic activity of subtilisin E.
Quantitative assay for subtilisin activity.
Subtilisin activity was assayed by monitoring at 410 nm the release of p-nitroaniline due to enzymatic hydrolysis of N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide (s-AAPF-pNa) (Sigma, St. Louis, Mo.) as described previously (29).
Quantitation of HSA14-1 scFv.
The amount of secreted HSA14-1 scFv was measured by enzyme-linked immunosorbent assay (ELISA). Human serum albumin (HSA;10 μg/ml) (Sigma) was adsorbed onto the ELISA plates (Maxisorb; Nunc, Rochester, N.Y.) overnight at 4°C in 0.1 M NaHCO3 (100 μl per well). After blocking with 2% skim milk in Tris-buffered saline (TBS) (200 μl per well) at room temperature (RT) for 1 h, appropriately diluted culture supernatants or serially diluted purified scFv was added to the wells (100 μl per well), and the plates were incubated at RT for 1 h. The plates were then washed three times with 0.05% Tween 20 in TBS (TBS-T), and the anti-E tag MAb 121-AP was added to TBS-T (diluted 1/2,000; 100 μl per well). After 1 h of further incubation, the bound anti-E tag MAb 121-AP was developed using p-nitrophenyl phosphate (Bio-Rad, Hercules, Calif.) as a substrate for AP. The reaction was allowed to proceed for 5 min and then stopped with 0.4 N NaOH, and the optical density at 405 nm (OD405) was measured.
Preparation of subcellular fractions.
Culture supernatants and whole-cell protein extracts were separated by centrifugation at 10,000 × g for 15 min at 4°C. The spheroplast and periplasmic fractions were prepared from the whole-cell protein extracts by osmotic shock (22).
SDS-PAGE and Western blotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed with 8 to 16% polyacrylamide gradient gels as described previously (23). For immunoblotting, the proteins separated by SDS-PAGE were transferred to a polyvinylidene difluoride membrane (Immobilon-P; Bio-Rad). Membranes were soaked in 5% skim milk for 30 min at RT. The following antibodies were used as primary antibodies: anti-E tag MAb 121 for the detection of scFv, c-Myc, and PTEN; anti-β-lactamase MAb (QED, San Diego, Calif.) for the detection of β-lactamase; and anti-σ70 MAb (NeoClone, Madison, Wis.) for the detection of σ70. Rabbit anti-mouse immunoglobulin G-AP (Zymed, San Francisco, Calif.) was used as a secondary antibody.
RESULTS
Isolation of HlyB and HlyD mutants with higher levels of secretion activity.
Three pairs of primers were used for random mutagenesis of hlyB and hlyD genes: Bam-F-Bsp-R, Bsp-F-Sph-R, and AL-F-E52-R (Fig. 1). Skim milk plates were screened for mutated HlyB or HlyD candidates with higher levels of subtilisin E secretion activity (see Materials and Methods). Two candidates (mutants AE104 and AE129) that formed larger halos by efficient degradation of skim milk were obtained from approximately 4,500 clones mutagenized with the primer pair AL-F-E52-R (Fig. 1 and 2). Another three candidates were also obtained from approximately 5,500 clones by use of the primer pair Bam-F-Bsp-R. However, the halos were much smaller than those of the two clones described above, and they were therefore not studied further. On the other hand, no significant candidates were obtained from approximately 5,600 clones by use of the primer pair Bsp-F-Sph-R.
FIG. 2.
Proteolytic degradation of skim milk by secretion of subtilisin E-HlyA218. E. coli JM109 strains carrying pSub-HlyA218 with pSTV-HlyBD (wild type [WT]), pSTV28 (negative control [NC]), pAE104 (AE104), and pAE129 (AE129) were selected and grown at 23°C on an LB agar plate containing 2% skim milk, 0.4 mM IPTG, 100 μg of ampicillin/ml, and 30 μg of chloramphenicol/ml.
The levels of subtilisin E secreted by the two mutants were measured using s-AAPF-pNa as a substrate. The relative activities of the two mutants are shown in Fig. 3. The activity level of AE129 was approximately 15-fold higher than that of the wild type at 23°C, and AE104 exhibited an approximately 27-fold-higher activity level than the wild type did at 23°C.
FIG. 3.
Relative secretion levels of subtilisin E-HlyA218 in HlyB and HlyD mutants. Activities of subtilisin E in the culture supernatants were measured 24 h after induction with IPTG at 23°C. Each bar represents the mean ± standard deviation (n = 3). WT, wild type.
Identification of mutations in HlyB and HlyD mutants.
As shown in Fig. 1 and Table 2, DNA sequencing revealed that AE104 had seven mutations, including one silent mutation of L416 (encoded by CTT→CTA) and four amino acid substitutions (L448F, A604T, V682A, and Q705L) in HlyB, one base substitution in the 5′ untranslated region of hlyD (A to G at nucleotide position −14), and one amino acid substitution in the HlyD coding sequence (F41Y). AE129 had a single amino acid substitution in HlyB (G654S).
TABLE 2.
DNA and amino acid substitutions in HlyBD mutants
| Mutant | Gene | Nucleotide position | Base substitution | Position in codon | Amino acid position | Amino acid substitution |
|---|---|---|---|---|---|---|
| AE104 | hlyB | 1248 | T→A | 3 | 416 | Silent |
| 1342 | C→T | 1 | 448 | Leu→Phe | ||
| 1810 | G→A | 1 | 604 | Ara→Thr | ||
| 2045 | T→C | 2 | 682 | Val→Ala | ||
| 2114 | A→T | 2 | 705 | Gln→Leu | ||
| hlyD | −14 | A→G | ||||
| 122 | T→A | 2 | 41 | Phe→Tyr | ||
| AE129 | hlyB | 1960 | G→A | 1 | 654 | Gly→Ser |
HSA14-1 scFv secretion activity.
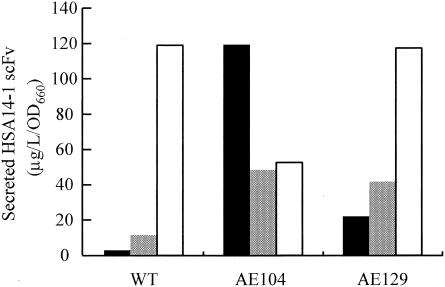
To determine whether the two mutants had enhanced activities for secretion of not only subtilisin E but also other proteins, an anti-HSA single-chain antibody (HSA14-1 scFv) was employed as another candidate protein for the evaluation of secretion activity. The level of secreted HSA14-1 scFv was measured at 5 h after induction at 30 and 37°C and also at 24 h after induction at 23°C. As shown in Fig. 4, the secretion activity level of the wild type dramatically decreased with a decrease in temperature. AE129 exhibited the same secretion activity level the wild type did at 37°C, and this level was also decreased with a decrease in temperature. However, the secretion activity level was much higher than that of the wild type at 23 and 30°C. AE104 exhibited the highest secretion activity at 23°C, almost the same level as that of the wild type and AE129 at 37°C. The secretion activity level of AE104 was still higher at 30°C than that of the wild type. However, at 37°C, it was lower than that of the wild type. This result may be due partly to growth inhibition of AE104 after IPTG induction at 37°C (data not shown). From these results, we conclude that mutants AE104 and AE129 have increased secretion activity of heterologous proteins at low temperatures.
FIG. 4.
Secretion activities of HSA14-1 scFv-HlyA218 in HlyB and HlyD mutants. The amount of HSA14-1 scFv-HlyA218 in the culture supernatant was measured by ELISA. HSA14-1 scFv-HlyA218 expression was induced by addition of 0.4 mM IPTG at 37°C for 5 h (white bars), at 30°C for 5 h (gray bars), and at 23°C for 24 h (black bars). Secretion efficiency is shown as micrograms of secreted protein per liter per OD660 unit. WT, wild type.
In order to rule out the possibility that the increase in the amount of scFv-HlyA218 protein in the culture media of AE104 and AE129 was caused by cell lysis, the leakage of cellular marker proteins into culture media was evaluated. As shown in Fig. 5, during a 24-h period after induction at 23°C, intracellular σ70 protein was not detected in either the medium or the periplasmic fractions in any of the samples. In the case of the periplasmic protein β-lactamase, although it leaked slightly into the supernatant, the expression levels in the wild type and the mutants were comparable (Fig. 5). These results indicate that in these mutants the hybrid protein does not leak by cell lysis. Therefore, increased secretion of scFv-HlyA218 into culture media was found to be dependent on the HlyA system.
FIG. 5.
Specific secretion of HSA14-1 scFv-HlyA218. After IPTG induction at 23°C for 24 h, fractions of periplasm, spheroplast, and culture supernatants of equal amounts of cells (0.05 OD660 equivalents of periplasm and spheroplast samples and 0.02 OD660 equivalents of supernatants) were detected with anti-σ70 MAb (row 1), anti-E tag MAb (row 2), and anti-β-lactamase MAb (row 3) by Western blotting. Anti-mouse immunoglobulin G-AP was used as a secondary antibody. NC, negative control; WT, wild type.
Determination of mutation sites that are critical for the improvement of secretion activity in AE104.
To determine which amino acid residues are responsible for improvement of secretion activity in AE104, several mutants of pAE104 (Fig. 6) were constructed and tested for their HSA14-1 scFv secretion activities. Figure 7 shows that the secretion activity level in AE104A, in which HlyB Phe448 in pAE104 was replaced with wild-type Leu, decreased to the level of the wild type, whereas the activity levels in other mutants (AE104B through AE104E) were comparable to that of AE104. These results suggest that the L448F substitution in HlyB is important for increased secretion activity in AE104. To verify this, pAE104F, which has an L448F mutation as the sole amino acid substitution in HlyB, was constructed and tested for secretion activity. As shown in Fig. 8, AE104F restored secretion activity to a level equivalent to that of AE104, indicating that the L448F substitution is crucial for the improvement of secretion activity in HlyB.
FIG. 7.
Secretion activities of HSA14-1 scFv-HlyA218 in AE104-based mutants. E. coli JM109 strains carrying pHSA14-1-HlyA218 with pSTV-HlyBD (WT), pAE104 (AE104), pAE104A (AE104A), pAE104B (AE104B), pAE104C (AE104C), pAE104D (AE104D), or pAE104E (AE104E) were cultured, and protein expression was induced by IPTG at 23°C for 24 h. The level of secreted HSA14-1 scFv was measured by ELISA. Secretion activities were calculated relative to that of AE104 (100%).
FIG. 8.
Comparison of secretion activities of the wild type (WT), AE104, and AE104F. E. coli JM109 strains carrying pHSA14-1-HlyA218 with pSTV-HlyBD (WT), pAE104 (AE104), or pAE104F (AE104F) were cultured, and protein expression was induced by IPTG at 23°C for 24 h. The level of secreted HSA14-1 scFv was measured by ELISA. Secretion activities were calculated relative to that of AE104 (100%).
Secretory expression of intracellular proteins.
By using the AE104F mutant, we tested the secretory expression of intracellular proteins. The human oncogenic protein c-Myc and tumor suppressor PTEN were chosen as candidates for secretory expression by the mutated HlyA system. These proteins formed inclusion bodies when they were overexpressed intracellularly at both 23 and 37°C (data not shown). As shown in Fig. 9A, secretory expression of c-Myc was not detected in the HlyB wild type at either 23 or 37°C but was clearly observed in mutant AE104F after 24 h of induction at 23°C. Furthermore, PTEN secretion was also clearly detected in AE104F at 37°C, and the level of secreted protein increased depending on induction time (Fig. 9B). The secretion levels at 37°C after 2 and 5 h of induction were much higher than that at 23°C. In the wild type, the level of PTEN secretion was much lower than that in mutant AE104F at 37°C. AE104F showed a 10-fold-higher secretion at 37°C (after 5 h of induction) than the wild type did, although some degradation products were observed. These results suggest that AE104F has the potential to efficiently secrete intracellular proteins not only at 23°C but also at 37°C.
FIG. 9.
Secretion of intracellular proteins by mutant HlyB and HlyD strains. (A) Secretion of c-Myc-HlyA218 was evaluated by using E. coli cultures carrying pMyc-HlyA218 with pSTV-HlyBD (wild type [WT]) or pAE104F (AE104F) or carrying pSTV28 with pMyc-HlyA218 (negative control [N]). (B) Secretion of PTEN-HlyA218 was evaluated by using E. coli cultures carrying pPTEN-HlyA218 with pSTV-HlyBD (WT) or pAE104F (AE104F) or carrying pSTV28 with pPTEN-HlyA218. Culture media were taken at 1, 2, and 5 h after IPTG induction at 37°C and at 4, 8, and 24 h after IPTG induction at 23°C as indicated. Then, 0.004 OD660 equivalent was applied to the gel, and the proteins were separated by SDS-PAGE. The secretion levels of target proteins were evaluated by Western blotting. Negative control cells were cultured for 24 h after induction at 23°C.
DISCUSSION
Since prepro-subtilisin E could not be efficiently folded and autoprocessed at 37°C (15), screening of HlyB and HlyD mutants was performed at 23°C. Using this strategy, we could obtain several HlyB mutants with improved secretion at 23°C, whereas no mutants were isolated by mutagenic PCR covering all of HlyD. The two isolated mutants, AE104 and AE129, were HlyB mutants that were thought to be highly improved for secretion. Unexpectedly, secretion activities of the HSA14-1 scFv in these two mutants at 23°C were comparable to or lower than those in the wild type at 37°C, and their maximal secretion levels at the temperatures tested were almost the same (Fig. 4). These results suggest that the two mutants are temperature sensitive for HSA14-1 scFv secretion and that the basal transport activities may be at the same level among the wild type, AE104, and AE129.
In contrast, c-Myc was detected solely in AE104F at 23°C, suggesting that it was secreted efficiently with the aid of both the L448F mutation and a conformation of the substrate suitable for secretion at 23°C (Fig. 9A). More interestingly, secretion of PTEN was greatly enhanced at 37°C (Fig. 9B). This result is inconsistent with the case of HSA14-1 scFv secretion. Since secretion efficiency would depend on structures of fusion proteins, and since protein structures also change with change in temperature, the secretory pattern may differ among fusion proteins.
Although the secretion efficiencies of the wild-type and mutant strains were completely different, only small amounts of the fusion proteins were detected at the same level in the spheroplast fractions in the wild type and the mutants (Fig. 5). Similar observations have been reported by Gentschev et al. and Schulein et al. (10, 27), and HlyA218 fusion proteins, as they suggested, may cause enhanced proteolytic degradation by inhibiting the folding of the fusion proteins, which in turn favors the secretion competence of the fusion proteins in the presence of HlyB or HlyD.
We found that the L448F substitution in HlyB (resulting in mutant AE104F) is essential for improved secretion activity in strain AE104. Secretion of the natural substrate HlyA was also enhanced at 23°C in the L448F mutant (data not shown), indicating that improved secretion is not specific to heterologous proteins. In addition, the secretory level of intact HlyA at 23°C in AE104F was found to be much higher than that in the wild type at any temperature (data not shown). This finding also indicates that the L448F mutant has the ability to improve secretion activity of HlyB itself.
L448 is highly conserved throughout ABC type transporters for members of the repeats-in-toxin (RTX) family (32), such as Pasteurella aerogenes PaxB, Bordetella pertussis CyaB, and Vibrio cholerae RtxB. It is located in the intracellular domain that is directly involved in the connection between the transmembrane domain (TMD) and the ATP- or nucleotide-binding domain (NBD) (Fig. 6). The intracellular domain has been proposed to be involved in conformational changes between the TMD and the NBD, which is thought to be caused by ATP or substrate binding (7, 24). Therefore, although the mechanism by which the L448F substitution contributes to the improvement of secretion activity is unclear, this mutation may enhance secretion activity by facilitating interaction between the NBD and the HlyA signal, or it may stabilize the mutant HlyB structure at low temperatures.
On the other hand, G654S, the sole amino acid substitution in HlyB in strain AE129, is adjacent to the conserved switch II region (amino acid residues 656 to 663) (Fig. 6) of the NBD of ABC transporters (24). G654 is highly conserved among ABC transporters for the RTX family described above. It is also widely conserved throughout other NBDs of not only bacterial ABC transporters, such as Salmonella enterica serovar Typhimurium histidine importer (HisP), Thermococcus litoralis maltose importer (MalK), and E. coli vitamin B12 importer (BtuD), but also the human multidrug resistance exporter MDR1 (24). However, some diversities are also found in this position: e.g., N529 in E. coli lipid A exporter MsbA, I847 in Pyrococcus furiosus DNA repair enzyme Rad50, and N597 and D1394 in human cystic fibrosis transmembrane conductance regulator (CFTR). Interestingly, human TAP1, the transporter associated with antigen processing, which has 62% homology and 40% identity with HlyB in the NBD region, has Ser instead of Gly at position 693 as does AE129. In spite of its similarity, TAP1 does not interact with HlyA218 and thus would not have the ability to transport HlyA (2). These findings suggest that although G654 is not always crucial for protein transport, some substitutions like Ser may enhance secretion by improving ATPase activity at low temperatures.
In addition to extracellular proteins such as subtilisin protease or antibody fragments, we also succeeded in generating secretory expression of the intracellular proteins c-Myc and PTEN by using AE104F. Intracellular proteins, especially those from mammals, are often difficult to express in E. coli in a soluble form. In such cases, this mutant may be useful. Further analysis will be necessary to elucidate the mechanism by which the secretion levels and profiles are changed by L448F or G654S mutations.
Acknowledgments
We thank Y. Yatabe for generously providing HSA14-1 scFv DNA, purified HSA14-1 scFv, and anti-E tag MAb. We also thank N. Ise for his generous gifts of plasmids containing c-myc and PTEN cDNA.
REFERENCES
- 1.Balakrishnan, L., C. Hughes, and V. Koronakis. 2001. Substrate-triggered recruitment of the TolC channel-tunnel during type I export of hemolysin by Escherichia coli. J. Mol. Biol. 313:501-510. [DOI] [PubMed] [Google Scholar]
- 2.Benabdelhak, H., S. Kiontke, C. Horn, R. Ernst, M. A. Blight, I. B. Holland, and L. Schmitt. 2003. A specific interaction between the NBD of the ABC-transporter HlyB and a C-terminal fragment of its transport substrate haemolysin A. J. Mol. Biol. 327:1169-1179. [DOI] [PubMed] [Google Scholar]
- 3.Blight, M. A., and I. B. Holland. 1990. Structure and function of haemolysin B, P-glycoprotein and other members of a novel family of membrane translocators. Mol. Microbiol. 4:873-880. [DOI] [PubMed] [Google Scholar]
- 4.Blight, M. A., C. Chervaux, and I. B. Holland. 1994. Protein secretion pathway in Escherichia coli. Curr. Opin. Biotechnol. 5:468-474. [DOI] [PubMed] [Google Scholar]
- 5.Blight, M. A., and I. B. Holland. 1994. Heterologous protein secretion and the versatile Escherichia coli haemolysin translocator. Trends Biotechnol. 12:450-455. [DOI] [PubMed] [Google Scholar]
- 6.Blight, M. A., A. L. Pimenta, J. C. Lazzaroni, C. Dando, L. Kotelevets, S. J. Seror, and I. B. Holland. 1994. Identification and preliminary characterization of temperature-sensitive mutations affecting HlyB, the translocator required for the secretion of haemolysin (HlyA) from Escherichia coli. Mol. Gen. Genet. 245:431-440. [DOI] [PubMed] [Google Scholar]
- 7.Chang, G., and C. B. Roth. 2001. Structure of MsbA from E. coli: a homolog of the multidrug resistance ATP binding cassette (ABC) transporters. Science 293:1793-1800. [DOI] [PubMed] [Google Scholar]
- 8.Chervaux, C., and I. B. Holland. 1996. Random and directed mutagenesis to elucidate the functional importance of helix II and F-989 in the C-terminal secretion signal of Escherichia coli hemolysin. J. Bacteriol. 178:1232-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez, L. A., and V. de Lorenzo. 2001. Formation of disulphide bonds during secretion of proteins through the periplasmic-independent type I pathway. Mol. Microbiol. 40:332-346. [DOI] [PubMed] [Google Scholar]
- 10.Gentschev, I., G. Dietrich, H. J. Mollenkopf, Z. Sokolovic, J. Hess, S. H. Kaufmann, and W. Goebel. 1997. The Escherichia coli hemolysin secretion apparatus—a versatile antigen delivery system in attenuated Salmonella. Behring Inst. Mitt. 98:103-113. [PubMed] [Google Scholar]
- 11.Gentschev, I., G. Dietrich, and W. Goebel. 2002. The E. coli alpha-hemolysin secretion system and its use in vaccine development. Trends Microbiol. 10:39-45. [DOI] [PubMed] [Google Scholar]
- 12.Gray, L., K. Baker, B. Kenny, N. Mackman, R. Haigh, and I. B. Holland. 1989. A novel C-terminal signal sequence targets Escherichia coli haemolysin directly to the medium. J. Cell Sci. Suppl. 11:45-57. [DOI] [PubMed] [Google Scholar]
- 13.Hui, D., C. Morden, F. Zhang, and V. Ling. 2000. Combinatorial analysis of the structural requirements of the Escherichia coli hemolysin signal sequence. J. Biol. Chem. 275:2713-2720. [DOI] [PubMed] [Google Scholar]
- 14.Hui, D., and V. Ling. 2002. A combinatorial approach toward analyzing functional elements of the Escherichia coli hemolysin signal sequence. Biochemistry 41:5333-5339. [DOI] [PubMed] [Google Scholar]
- 15.Ikemura, H., H. Takagi, and M. Inouye. 1987. Requirement of pro-sequence for the production of active subtilisin E in Escherichia coli. J. Biol. Chem. 262:7859-7864. [PubMed] [Google Scholar]
- 16.Imai, Y., Y. Matsushima, T. Sugimura, and M. Terada. 1991. A simple and rapid method for generating a deletion by PCR. Nucleic Acids Res. 19:2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenny, B., R. Haigh, and I. B. Holland. 1991. Analysis of the haemolysin transport process through the secretion from Escherichia coli of PCM, CAT or beta-galactosidase fused to the Hly C-terminal signal domain. Mol. Microbiol. 5:2557-2568. [DOI] [PubMed] [Google Scholar]
- 18.Kenny, B., C. Chervaux, and I. B. Holland. 1994. Evidence that residues −15 to −46 of the haemolysin secretion signal are involved in early steps in secretion, leading to recognition of the translocator. Mol. Microbiol. 11:99-109. [DOI] [PubMed] [Google Scholar]
- 19.Koronakis, E., C. Hughes, I. Milisav, and V. Koronakis. 1995. Protein exporter function and in vitro ATPase activity are correlated in ABC-domain mutants of HlyB. Mol. Microbiol. 16:87-96. [DOI] [PubMed] [Google Scholar]
- 20.Kumamoto, C. A., and J. Beckwith. 1983. Mutations in a new gene, secB, cause defective protein localization in Escherichia coli. J. Bacteriol. 154:253-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Missiakas, D., and S. Raina. 1997. Protein folding in the bacterial periplasm. J. Bacteriol. 179:2465-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nossal, N. G., and L. A. Heppel. 1966. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J. Biol. Chem. 241:3055-3062. [PubMed] [Google Scholar]
- 23.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press. Cold Spring Harbor, N.Y.
- 24.Schmitt, L., H. Benabdelhak, M. A. Blight, I. B. Holland, and M. T. Stubbs. 2003. Crystal structure of the nucleotide-binding domain of the ABC-transporter haemolysin B: identification of a variable region within ABC helical domains. J. Mol. Biol. 330:333-342. [DOI] [PubMed] [Google Scholar]
- 25.Schulein, R., I. Gentschev, H. J. Mollenkopf, and W. Goebel. 1992. A topological model for the haemolysin translocator protein HlyD. Mol. Gen. Genet. 234:155-163. [DOI] [PubMed] [Google Scholar]
- 26.Schulein, R., I. Gentschev, S. Schlor, R. Gross, and W. Goebel. 1994. Identification and characterization of two functional domains of the hemolysin translocator protein HlyD. Mol. Gen. Genet. 245:203-211. [DOI] [PubMed] [Google Scholar]
- 27.Spreng, S., and I. Gentschev. 1998. Construction of chromosomally encoded secreted hemolysin fusion proteins by use of mini-TnhlyAs transposon. FEMS Microbiol. Lett. 165:187-192. [DOI] [PubMed] [Google Scholar]
- 28.Stader, J. A., and T. J. Silhavy. 1990. Engineering Escherichia coli to secrete heterologous gene products. Methods Enzymol. 185:166-187. [DOI] [PubMed] [Google Scholar]
- 29.Takagi, H., Y. Morinaga, H. Ikemura, and M. Inouye. 1988. Mutant subtilisin E with enhanced protease activity obtained by site-directed mutagenesis. J. Biol. Chem. 263:19592-19596. [PubMed] [Google Scholar]
- 30.Tzschaschel, B. D., C. A. Guzman, K. N. Timmis, and V. de Lorenzo. 1996. An Escherichia coli hemolysin transport system-based vector for the export of polypeptides: export of Shiga-like toxin IIeB subunit by Salmonella typhimurium aroA. Nat. Biotechnol. 14:765-769. [DOI] [PubMed] [Google Scholar]
- 31.Wandersman, C., and P. Delepelaire. 1990. TolC, an Escherichia coli outer membrane protein required for hemolysin secretion. Proc. Natl. Acad. Sci. USA 87:4776-4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Welch, R. A. 2001. RTX toxin structure and function: a story of numerous anomalies and few analogies in toxin biology. Curr. Top. Microbiol. Immunol. 257:85-111. [DOI] [PubMed] [Google Scholar]