Abstract
The effects of various growth conditions on spontaneous φLC3 prophage induction in Lactococcus lactis subsp. cremoris IMN-C1814 was analyzed with a half fraction of a 44 factorial experimental design. The four factors included in the study were nutrient availability, acidity, osmolarity, and temperature, each applied at four levels. These environmental factors are related to the fermentation processes in the dairy industry, in which bacteriophage attacks on sensitive starter strains are a constant threat to successful fermentation processes. The frequency of spontaneous φLC3 induction was determined by quantitative analyses of restored DNA attachment sites (attB) on the bacterial chromosomes in a population of lysogenic cells. Statistical analysis revealed that all four environmental factors tested affected φLC3 prophage stability and that the environmental factors were involved in interactions (interactions exist when the effect of one factor depends on the level of another factor). The spontaneous φLC3 induction frequency varied from 0.08 to 1.76%. In general, the induction frequency remained at the same rate or decreased when level 1 to 3 of the four environmental factors was applied. At level 4, which generally gave the least favorable growth conditions, the induction frequency was either unchanged, decreased, or increased, depending on the type of stress. It appeared that the spontaneous induction frequency was independent of the growth behavior of the host. It was the environmental growth conditions that were the decisive factor in induction frequency.
Lactic acid bacteria are widely used to ferment milk for production of cheese and other dairy products. Virulent bacteriophages are a constant threat to such fermentation processes, as they are occur naturally in milk (17) and can survive pasteurization (9). In addition, many of the starter strains used are lysogenic, carrying one ore more potentially inducible prophages, suggesting that they represent a source of phages (10). In fact, recent research has revealed that prophage-related sequences in a number of bacterial genomes take up more than 10% of the DNA content (reviewed in references 7 and 8). The three lactococcal phage species most frequently found in dairy environments are the 936, c2, and P335 phage species (1, 19, 36). The 936 and c2 species contain only virulent phages, while the P335 species encompasses both virulent and temperate phages. Temperate phages and prophage-derived sequences within the P335 species have been shown to give rise to new virulent derivatives (5, 11, 33).
For several years it has been discussed whether spontaneously induced P335 phages are a matter of significant concern to the dairy industry. However, there is still little information available on how and when the bacterial host and the environmental conditions may influence the induction of a prophage. Consequently, improved knowledge about conditions that would prevent or permit spontaneous prophage induction from lysogenic lactic acid bacteria is of importance in starter culture technology as well as a question of fundamental interest.
During milk fermentation processes, lactic acid bacteria are exposed to various environmental stress conditions, such as temperature fluctuations, acid pH, high osmotic pressure, and absence of available nutrients. Many of these conditions will often coincide. Like other bacteria, lactic acid bacteria have evolved intricate stress response systems enabling them to adapt to adverse conditions in order to survive. The stress responses of the industrially important species Lactococcus lactis have gained increased interest in recent years, and reports include studies of responses to heat and cold shock, low pH, UV light, salts, starvation, oxidation, DNA damage, and chloride (reviewed in references 38, 42, and 46).
Environmental conditions affect the switch between the lytic and lysogenic life styles of the well-studied temperate Escherichia coli phage λ (13, 16). The molecular basis for the lysis-lysogeny switch is well understood for λ, but the exact mechanisms for sensing environmental conditions and how the signaling routes are coupled to the molecular regulation mechanisms are not well defined. Recently, regulatory mechanisms involved in the lysis-lysogeny decision have been reported for several lactic acid bacterial phages (3, 4, 6, 15, 18, 20, 23, 24, 30, 35, 45), and similarities exist between the regulatory mechanisms of lysogenic lactic acid bacterial phages and λ.
Only a limited number of studies have been performed regarding the effect of environmental factors on the lysis-lysogeny decision of temperate lactic acid bacterial phages. Feirtag and McKay (14) demonstrated that a shift in growth temperature (from 30 to 40°C) led to the induction of temperate bacteriophages into the lytic life cycle, followed by host lysis. For Lactococcus lactis subsp. cremoris SK110, it was shown that this thermoinducible lysis was strongly dependent on the cell growth rate and the pH of the medium, in which high growth rates and neutral pHs gave the highest thermolytic response (31). We have previously reported that the spontaneous induction frequency of the temperate lactococcal bacteriophage φLC3 in five lysogenic L. lactis strains was affected by growth temperature and varied between 0.32 and 9.1% among the lysogenic strains (29).
The temperate phage φLC3, isolated from Lactococcus lactis subsp. cremoris IMN-C3 (27), belongs to the P335 species of small isometric-headed lactococcal phages. The genome sequence of phage φLC3 was recently determined (2). In this work, phage φLC3 was used as a model to study prophage induction in lactic acid bacteria under diverse environmental conditions related to milk fermentation processes. A statistically designed factorial experiment, in which the environmental factors were varied together, was used instead of the more time-consuming one-factor-at-a-time experimental approach (34). Factorial experiments can reveal relationships that are otherwise difficult to observe by analyzing both the main effect of a factor and the interaction effects between the factors. The spontaneous φLC3 prophage induction frequency was monitored with a recently developed method based on quantitative analysis of specific DNA sites involved in φLC3 phage life cycles (28, 29). This study will provide a better understanding of how dairy-related environmental growth conditions affect prophage maintenance in lactic acid bacteria.
MATERIALS AND METHODS
Experimental design.
A factorial experiment was performed to determine the effects of individual environmental factors and their interactions on spontaneous φLC3 prophage induction frequency in L. lactis subsp. cremoris IMN-C1814. Four factors were studied: nutrition availability, acidity, osmolarity, and growth temperature. The statistical design was generated by using the Minitab statistical software (version 13; Minitab Inc.) and included four levels of each factor, as listed in Table 2, in which the intervals between the levels were kept constant. The experimental design was a half fraction of a 44 design divided into four blocks, each with 32 plots (34). The total experiment included 128 runs.
TABLE 2.
Levels of environmental factors used in the factorial designa
| Factor | Level
|
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Nutrient limitation (M17 broth) | 1× | 0.8× | 0.6× | 0.4× |
| Acidity (pH) | 7.0 | 6.6 | 6.2 | 5.8 |
| Osmolarity (NaCl) | 0.0% | 0.5% | 1.0% | 1.5% |
| Temperature (temp) | 30°C | 31.5°C | 33°C | 34.5°C |
The concentration of glucose in the M17 broth dilutions was kept constant (0.5%). The pH was adjusted initially.
Bacterial strain and culture conditions.
The φLC3 lysogenic strain Lactococcus lactis subsp. cremoris IMN-C1814 (Lac−) (26) was used throughout this study. L. lactis was cultured at 30°C in M17 broth (Oxoid) containing 0.5% (wt/vol) glucose instead of lactose (standard laboratory conditions).
Like other lactic acid bacteria, L. lactis subsp. cremoris strains have complex nutritional requirements, and their optimum growth temperature is 30°C. L. lactis subsp. cremoris is traditionally distinguished from other Lactococcus species by not being able to grow at 40°C or in the presence of 4% NaCl (43). Preliminary bacterial growth analyses were performed to select four levels of each environmental factor to be used in the factorial design. These included dilutions of M17 from 1- to 0.01-fold, NaCl addition in the range from 0 to 3%, initial pHs of between 7.5 and 4.0, and temperatures of between 30 and 40°C. Standard laboratory conditions were used for the nonvaried factors.
The growth media used in the factorial design were prepared as follows. M17 was employed undiluted and with 0.8-, 0.6-, and 0.4-fold concentrations of M17 broth. The concentration of glucose was kept constant (0.5% [wt/vol]). The pHs of the four different M17 media were adjusted to 7.0, 6.6, 6.2, and 5.8 with HCl or NaOH. Each of the resulting growth media was supplemented with 0, 0.5, 1.0, or 1.5% NaCl. In total, 64 different medium combinations were made. Growth was carried out at 30, 31.5, 33, and 34.5°C.
Growth experiments.
Overnight cultures of L. lactis IMN-C1814 were grown in 1-fold M17 broth at 30°C and used to inoculate (2%) media under different combinations of available nutrients (growth broth dilutions), acidity (different initial pHs), osmolarity (NaCl additions), and temperature according to the factorial design. The increase in optical density at 600 nm (OD600) was measured every 30 min for 48 h during growth in a total volume of 200 μl by the use of BioscreenC (Labsystems, Helsinki, Finland). Data acquisition was handled by the Biolink software system (version 5.0; Labsystems, Helsinki, Finland), and the data were further transferred to Minitab (Minitab Inc.) and SAS statistical software (SAS 8.2; SAS Institute Inc., Cary, N.C.) for construction and analysis of the growth curves.
Growth experiments were performed in triplicate vials, and an average value was used in the analysis. The average value was corrected with a standard (i.e., the OD600 in the corresponding medium without inoculated bacterial cells). For the analysis of spontaneous prophage induction, overnight cultures were diluted appropriately in 1.5-ml Eppendorf tubes containing growth medium with the various factor combinations and incubated at the appropriate temperatures. One tube for each condition was taken in the early stationary phase at a time point here called TDNA, and DNA was isolated from this sample.
Determination of the spontaneous induction frequency.
The spontaneous induction frequency of the φLC3 prophage was measured as described previously (29), with a real-time PCR strategy which targets the attachment site for site-specific integration of the φLC3 phage (attB DNA site) located on the bacterial chromosome. The comparative CT method (User Bulletin #2, Applied Biosystems, Foster City, Calif.) was used for calculation of spontaneous prophage induction according to Lunde et al. (29).
Modeling.
Growth behavior was modeled by using a logistic model with the following equation:
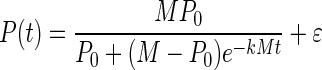 |
(1) |
where P is the cell yield (OD600 measurements), t is time (in hours), e is the base for the natural logarithm, ɛ is random noise, P0 represents the value of P(t) for t = 0, M is the asymptotic maximum value of P(t), and k is an unknown parameter affecting the growth rate. Estimation of M and k was done with the SAS procedure Proc NLIN. From these two parameters, we calculated the maximum growth rate (μmax) and maximum optical density (θmax):
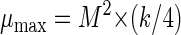 |
(2) |
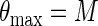 |
(3) |
Statistical analysis.
The spontaneous induction frequency was used as the response variable in the fractional factorial experiment and analyzed with the appropriate linear model by use of the Proc GLM in SAS. The results were studied by analysis of variance and are presented by P values for tests about equality between the different factor levels and by plots of main and interaction effects. The main effect of a factor is the change in the average response produced by a change in the levels of the factor. Interaction effects exist if the difference in response between the levels of one factor is not the same at all levels of another factor. If there were significant interaction effects, the least significant difference method (34) was used to rank the different factor levels. The standard error of each point in the main plots was estimated from the analysis of variance table. Regression analysis (34) was used to study the relationship between the spontaneous prophage induction frequency and growth behavior, described by the three parameters μmax, θmax and TDNA. These three parameters were used as response variables in the factorial design and analyzed as described above for the spontaneous induction frequency.
RESULTS
Selection of four levels of nutrient availability (M17 broth), osmolarity (NaCl), acidity (pH), and temperature to be used in a statistically designed factorial experiment.
The effect of environmental conditions on the spontaneous φLC3 prophage induction frequency in L. lactis IMN-C1814 was studied with a half fraction of a 44 factorial design. The four environmental factors used in the experimental setup were nutrient availability (growth broth dilutions), acidity (different initial pHs), osmolarity (NaCl addition), and growth temperature above the optimum for L. lactis. Preliminary growth experiments were carried out to select four levels of each environmental factor to be used in the design. These four levels were chosen on the basis of the ability to support growth of the L. lactis IMN-C1814 strain.
Furthermore, the factorial design required intervals of equal size between the levels of factors (34). Therefore, four temperatures at intervals of 1.5°C between 30°C and 34.5°C were chosen, since no growth of L. lactis IMN-C1814 was observed above 35°C in standard M17 medium within 48 h of cultivation. Analyses of which levels of M17 broth concentration, pH, and NaCl addition that supported growth of L. lactis IMN-C1814 were carried out at 30 and 34.5°C as the lower and upper temperature limits in the factorial design. The result of these preliminary growth analyses are shown in Table 1. The four levels of the environmental factors selected to be included in the factorial design are listed in Table 2.
TABLE 1.
Preliminary growth analysis to determine levels of environmental factors to be used in the factorial experiment
| Factor and levels | Growth temp (°C)a
|
|
|---|---|---|
| 30 | 34.5 | |
| M17 brothb (fold) | ||
| 1 | + | + |
| 0.75 | + | + |
| 0.5 | + | + |
| 0.25 | + | + |
| 0.125 | + | − |
| 0.1 | + | − |
| 0.075 | − | − |
| 0.05 | − | − |
| 0.01 | − | − |
| pH | ||
| 7.5 | + | + |
| 7 | + | + |
| 6.5 | + | + |
| 6 | + | + |
| 5.5 | + | − |
| 5 | + | − |
| 4.5 | + | − |
| 4 | − | − |
| NaCl (%) | ||
| 0.75 | + | + |
| 0.1 | + | + |
| 1.5 | + | + |
| 1.8 | − | + |
| 2 | − | − |
| 2.5 | − | − |
| 3 | − | − |
+, growth; −, no significant growth after 48 hours.
The concentration of glucose in the M17 broth dilutions was kept constant (0.5%).
Main effects of nutrient availability, acidity, osmolarity, and growth temperature on spontaneous φLC3 prophage induction.
We have previously shown that spontaneous prophage induction frequency is dependent on the growth phase of the lysogenic bacterial host (29). It was shown that the induction frequency increased in the exponential growth phase and reached a maximum in the early stationary phase, both at growth temperatures of 30 and 15°C. In the present study we have used a factorial experimental design in order to obtain a more complete description of how various factors, both individually and in combination, influence the prophage induction frequency. Consistent with previously reported data (29), a peak in induction frequency in the early stationary phase was observed when several different culture conditions were tested initially (e.g., 0.8-fold M17, 0.6-fold M17, and 0.4-fold M17) (results not shown). Therefore, in order to compare the effects of different environmental conditions on the prophage induction frequency, representative DNA preparations were made from early-stationary-phase cells for all conditions in the factorial design. Growth data (OD600 measurements) were obtained and used to determine the time point (TDNA = time point for cell sampling for DNA analysis) when L. lactis IMN-C1814 entered the stationary phase.
The spontaneous prophage induction frequencies were measured by a recently developed real-time PCR method (29) by using DNA obtained from cells at TDNA. The spontaneous induction frequency, as measured by the level of reestablished attB DNA sites in a bacterial population, varied between 0.082 and 1.76% in the 128 growth conditions analyzed. Analysis of variance showed that all four environmental factors affected the spontaneous induction frequency at a 1% significance level. The results are presented as main effect plots in Fig. 1, in which the main effect of a factor is defined as the change in response produced by a change in the level of the factor (34).
FIG. 1.
Main effect plots of nutrient availability (M17) (A), acidity (pH) (B), osmolarity (NaCl) (C), and temperature (D) on spontaneous prophage induction frequency. Error bars show the standard error of the means.
The average spontaneous induction frequency in all the culture combinations where undiluted M17 was applied (32 in total) was 0.52% (Fig. 1A). No significant difference in induction frequency was observed when the M17 growth broth was diluted 0.8-fold (0.53%, P = 0.87) and 0.6-fold (0.56%, P = 0.45), as shown in the main effect plot in Fig. 1A. When nutrition availability decreased to 0.4-fold M17 (level 4), the spontaneous induction frequency increased to 0.66% (P = 0.01). The main effect plot in Fig. 1B shows that the spontaneous induction frequency decreased with decreasing initial pHs (pH 7.0 = 0.77%, pH 6.6 = 0.69%, pH 6.2 = 0.47%, to pH 5.8 = 0.34%) (P < 0.0001). A decrease in induction frequency was also observed from 0 (0.78% induction) to 0.5% (0.66% induction) and further to 1.0% (0.41% induction) NaCl addition (P < 0.0001), but between 1.0 and 1.5% (0.41% induction) NaCl additions, no difference was observed (P = 0.95) (Fig. 1C). No significant difference in induction frequency was observed at growth temperatures of 30°C (0.55% induction), 31.5°C (0.56% induction), and 33°C (0.47% induction) (P = 0.16) (Fig. 1D), but at the highest temperature (34.5°C), an increase in the induction frequency to 0.69% was significant (P = 0.0094).
The results from the main effect plots indicated a higher spontaneous induction frequency when the lysogenic host were grown at neutral pHs (pH 7.0 and 6.6), at 0.0 or 0.5% NaCl, at high temperature (34.5°C), and in diluted medium (0.4-fold M17). The main effect plots suggested a lower spontaneous induction frequency at acidic pH (pH 6.2 and pH 5.8) and at the highest levels of NaCl addition (1 and 1.5%).
Interaction effects between nutrient availability, acidity, osmolarity, and growth temperature on spontaneous φLC3 prophage induction.
The results above described the main effect of each environmental factor used in this study. However, it was also of interest to examine whether there were any interaction effects between the environmental factors. An interaction is the failure of one factor to produce the same effect on the response at different levels of another factor (34). If an interaction effect exists, the main effect fails to describe the complete relationship, and the effect of one factor will depend on the level of the other factor in question. The results from the statistical analysis indicated significant interaction effects between nutrient availability and osmolarity (M17×NaCl), initial pH and temperature (pH×Temp), and osmolarity and temperature (NaCl×Temp) on the spontaneous induction frequency (Fig. 2). The critical value for significant difference in spontaneous induction frequency was estimated by least significant difference analysis to 0.216%, and values of differences above this number were considered significant (P < 0.05) at a certain level of a factor.
FIG. 2.
Interaction effect plots of nutrient availability and osmolarity (M17×NaCl) (A), acidity and temperature (pH×Temp) (B), and osmolarity and temperature (NaCl×Temp) (C) on spontaneous prophage induction frequency (standard error = 0.08).
No interaction effect between nutrient availability and osmolarity (M17×NaCl) was observed at NaCl levels of 0.0 and 0.5% except when 0.4-fold M17 broth was applied. Then, a decrease in φLC3 induction frequency was observed from 0.0 to 0.5% NaCl (Fig. 2A). Increasing the NaCl addition from 0.5 to 1.0% resulted in a significant decrease in the spontaneous induction frequency in all media except 0.8-fold M17 broth. Further addition of NaCl (1.5%) led to a decrease in induction frequency when 0.8-fold and 0.6-fold M17 broth were applied, while an increase in induction frequency was observed for 1-fold M17. No significant difference in spontaneous induction frequency was observed for 0.4-fold M17 broth in this case. Hence, this interaction effect analysis revealed that exposing the lysogenic cells to 1.5% NaCl caused divergent effects depending on nutrient availability. This effect was masked in the main effect plot (Fig. 1B), where no effect on the spontaneous induction frequency was observed between 1.0 and 1.5% NaCl.
In the interaction effect analysis of acidity and temperature (pH×Temp), no effect was observed on the prophage induction frequency at growth temperatures from 30 to 33°C (Fig. 2B). When the temperature was raised to 34.5°C, interaction effects were observed in which the induction frequency increased at pH 7.0 and 6.6. No differences in induction frequency were observed at pH 6.2 and 5.8 during growth at 34.5°C compared to growth at lower temperatures. This result indicated that the increase in prophage induction seen in the main plot (Fig. 1D) when the growth temperature was changed from 33 to 34.5°C is not true at acidic pH.
In Fig. 2C, the NaCl×Temp (osmolarity and temperature) interaction analysis is presented. When growth was carried out at 30, 31.5, and 33°C, addition of 1.0 and 1.5% NaCl gave lower induction frequencies compared to 0.0 and 0.5% NaCl. At 34.5°C, this trend was changed. Here, a large increase in induction frequency was observed at 1.0 and 1.5% NaCl addition, while the induction frequency decreased with no NaCl added. At 0.5% NaCl addition, no significant effect was observed between 33 and 34.5°C. This result clearly indicates that the effect of one environmental factor can be strongly influenced by a second factor.
No significant interaction effects on spontaneous induction frequency were observed when nutrient availability and acidity (GM17×pH), nutrient availability and temperature (GM17×Temp), and acidity and osmolarity (pH×NaCl) were combined (P = 0.91, 0.22, and 0.50, respectively) (plots not shown).
Relationship between growth behavior and spontaneous prophage induction frequency.
The physiological state of a bacterial cell is affected by environmental factors. As expected, the growth curves of L. lactis IMN-C1814 obtained from the 128 factor combinations in the factorial design were highly diverse. These growth curves were modeled by a logistic model (equation 1, Materials and Methods), and the model was used to estimate the maximum growth rates (μmax) and maximum optical densities (θmax) for each of the 128 curves. μmax varied between 0.001 and 0.08 h−1, θmax varied between OD600 = 0.03 and OD600 = 0.5, and TDNA (time point of DNA sampling) varied between 7.5 and 48 h.
Analysis of variance showed that all three growth-describing parameters, μmax, θmax, and TDNA, were affected by acidity, osmolarity, and growth temperature on a 1% significance level, and in addition, θmax was significantly affected by nutrient availability. Main effect and interaction effect plots (plots not shown) revealed that, generally, by growing the φLC3 lysogenic L. lactis strain at higher levels of environmental factors (Table 2), μmax and θmax decreased and TDNA increased, which indicates that the cells became successively stressed when grown at higher levels of factors. This effect is demonstrated in Fig. 3, in which L. lactis IMN-C1814 was grown in undiluted M17 broth (level 1) in growth curve A and in 0.4-fold-diluted M17 broth (level 4) in growth curve B. In these two curves, pH, NaCl concentration, and temperature were kept constant at pH 6.6 (level 2), 0.0% (level 1), and 30°C (level 1), respectively. Increasing the levels of environmental factors (Table 2) did not always result in poorer growth. If the pH, NaCl concentration, and temperature in curves A and B were changed to pH 6.2 (level 3), 1.5% NaCl (level 4), and 33°C (level 3), growth in 0.4-fold-diluted M17 broth (level 4) (curve C) was better than growth in undiluted M17 broth (level 1) (curve D). The spontaneous induction frequency was 1.31, 1.51, 0.362, and 0.083% for curves A, B, C and D, respectively.
FIG. 3.
Growth curves of Lactococcus lactis subsp. cremoris IMN-C1814. The strain was grown in different combinations of four environmental factors according to a factorial design. The curves represent growth behavior in the presence of different combinations of nutrient availability, acidity, osmolarity, and temperature as follows; 1-fold M17, pH 6.6, 0.0% NaCl, and 30°C (A), 0.4-fold M17, pH 6.6, 0.0% NaCl, and 30°C (B), 1-fold M17, pH 6.2, 1.5% NaCl, and 33°C (C) and 0.4-fold M17, pH 6.2, 1.5% NaCl, and 33°C (D).
We examined if there was any relationship between growth behavior (described by μmax, θmax, and TDNA) and spontaneous induction frequency of φLC3 by the use of regression analyses. When θmax, μmax, and TDNA were tested alone, none of them showed any significant relationship with the induction frequency (P = 0.18, 0.87, and 0.94, respectively). However, when μmax and TDNA in combination were tested, a relationship to prophage induction frequency was obtained (P < 0.0001). This result implies that high values of both μmax and TDNA led to high induction rates, while low values of both μmax and TDNA were true for low induction rates.
DISCUSSION
The frequent occurrence of lysogeny among lactococci and the ability of these strains to spontaneously induce their prophages are well established (reviewed in references 21, 25, and 44). However, since the initial discovery of lysogeny in lactic acid bacteria (40), the significance of the lysogenic bacteria as a source of phages that interrupt industrial fermentation processes has been a topic of discussion. In order to address the significance of lysogeny in starter culture technology, we have investigated the effect of nutrient availability, acidity, osmolarity, and growth temperature on the spontaneous φLC3 prophage induction frequency in L. lactis subsp. cremoris IMN-C1814. A fractional factorial design (34) was employed in these analyses, and to our knowledge, the use of a factorial experiment for such a purpose has not been reported previously.
The use of a factorial design when testing the effects of several factors on a certain response has several advantages over the one-factor-at-a-time approach commonly used to carry out experiments. First, a factorial design is more efficient since it facilitates the factors to be varied together. Further, a factorial design is necessary to avoid misleading conclusions when interactions between two factors may be present. Finally, a factorial design allows the effects of a factor to be estimated at several levels of the other factors, yielding conclusions that are valid over a range of experimental conditions (34).
In fermentation with Lactococcus, milk is typically seeded with about 107 CFU ml−1, which grow to about 5 × 109 CFU ml−1 at the end of fermentation. Typically, cheese whey from a mixed-strain fermentation contains 106 to 108 phage particles ml−1 even in the absence of a noticeable phage attack. Thus, the spontaneous φLC3 induction frequencies of 0.08 to 1.76% obtained in this study appear significant for industrial conditions. Acidity, osmolarity, and growth temperature all showed significant main effects on the spontaneous φLC3 induction frequency (Fig. 1). The statistical analysis revealed that the four environmental factors were involved in significant interactions when applied in the combinations GM17×NaCl, pH×Temp, and NaCl×Temp (Fig. 2).
The overall effect, as observed from the main and interaction effect plots, was that spontaneous induction remained at the same frequency or decreased over the first three levels of all four environmental factors (Fig. 1 and 2). Generally, the cells became successively stressed when they were grown at higher levels of factors (Table 2), as observed from the bacterial growth curves. At the highest level (level 4), the effect on prophage induction was not uniform. In some conditions, the prophage induction frequency was unchanged or decreased, while in other conditions a high prophage induction frequency was seen (Fig. 1 and 2). This indicates that the lysogenic bacterial cells responded to stress conditions by either stabilization or induction of the prophage, depending on the stress conditions. Such a differential response may be a result of the phages' extreme adaptability to their continuously changing environment, in which their main goal is to ensure viral maintenance (13, 16, 32, 37, 39).
The increased stability of the φLC3 prophage observed when the host cells were grown at higher levels of the factors may be due to mechanisms that temperate bacteriophages have evolved to maximize their survival, in which unknown mechanisms sense unfavorable bacterial growth conditions. Moreover, the stimulation of phage induction observed at high stress levels (e.g., 1.0 to 1.5% NaCl in combination with 34.5°C, Fig. 2C) may be a strategy to prevent loss of phage genetic information at growth conditions with lethal effects on the host. High induction frequency (1.3%) was also observed at optimal growth in standard laboratory conditions. This high induction frequency agrees with our previously reported studies on the spontaneous induction frequency of φLC3 in L. lactis IMN-C1814 (29). A rationale for the high induction in optimal growth conditions may be the phage's ability to sense that the cell population is growing well and thus able to propagate a successful lytic infection. The bacterial chromosome exits in a more unfolded and temporary single-stranded form during active DNA replication (22), which may cause instability of the prophage.
It is tempting to suggest that the increased φLC3 induction frequency observed at high stress levels was due to the stress response system in the L. lactis host, where the ubiquitous RecA protein plays a central role (12). In Escherichia coli, the recA gene is a key element in bacteriophage induction by mediating cleavage of the repressor protein (41). The repressor of phage φLC3 (3) is grouped into the phage λ CI-like repressor family, together with repressors of other lactococcal phages such as Tuc2009 (45), BK5-T (6), and r1t (35). However, because of the diverse response to environmental changes, it is likely that other mechanisms are also important in affecting prophage induction frequency.
No significant relationship existed between the spontaneous induction frequency and the growth-describing parameters μmax, θmax, and TDNA except when TDNA and μmax were combined. The results obtained in this study suggest that the prophage induction frequency is mainly an effect of the growth conditions rather than the growth behavior of the lysogenic host.
The results obtained in this study revealed that the spontaneous φLC3 prophage induction frequency was affected by environmental factors related to dairy fermentation processes and that these factors were involved in the interactions. The present study provides a foundation for further studies on prophage stability in starter strains, which should be carried out in environments that mimic the fermentation process even better.
Acknowledgments
This work was supported by the Research Council of Norway (project no. 140438/130).
REFERENCES
- 1.Bissonnette, F., S. Labrie, H. Deveau, M. Lamoureux, and S. Moineau. 2000. Characterization of mesophilic mixed starter cultures used for the manufacture of aged cheddar cheese. J. Dairy Sci. 83:620-627. [DOI] [PubMed] [Google Scholar]
- 2.Blatny, J. M., L. Godager, M. Lunde, and I. F. Nes. 2004. Complete genome sequence of the Lactococcus lactis temperate phage φLC3: comparative analysis of φLC3 and its relatives in lactococci and streptococci. Virology 318:231-244. [DOI] [PubMed] [Google Scholar]
- 3.Blatny, J. M., P. A. Risoen, D. Lillehaug, M. Lunde, and I. F. Nes. 2001. Analysis of a regulator involved in the genetic switch between lysis and lysogeny of the temperate Lactococcus lactis phage φLC3. Mol. Genet. Genomics 265:189-197. [DOI] [PubMed] [Google Scholar]
- 4.Blatny, J. M., M. Ventura, E. M. Rosenhaven, P. A. Risoen, M. Lunde, H. Brussow, and I. F. Nes. 2003. Transcriptional analysis of the genetic elements involved in the lysogeny/lysis switch in the temperate lactococcal bacteriophage φLC3, and identification of the Cro-like protein ORF76. Mol. Genet. Genomics 269:487-498. [DOI] [PubMed] [Google Scholar]
- 5.Bouchard, J. D., and S. Moineau. 2000. Homologous recombination between a lactococcal bacteriophage and the chromosome of its host strain. Virology 270:65-75. [DOI] [PubMed] [Google Scholar]
- 6.Boyce, J. D., B. E. Davidson, and A. J. Hillier. 1995. Identification of prophage genes expressed in lysogens of the Lactococcus lactis bacteriophage BK5-T. Appl. Environ. Microbiol. 61:4099-4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canchaya, C., C. Proux, G. Fournous, A. Bruttin, and H. Brussow. 2003. Prophage genomics. Microbiol. Mol. Biol. Rev. 67:238-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casjens, S. 2003. Prophages and bacterial genomics: what have we learned so far? Mol. Microbiol. 49:277-300. [DOI] [PubMed] [Google Scholar]
- 9.Chopin, M. C. 1980. Resistance of 17 mesophilic lactic Streptococcus bacteriophages to pasteurization and spray-drying. J. Dairy Res. 47:131-139. [DOI] [PubMed] [Google Scholar]
- 10.Davidson, B. E., I. B. Powell, and A. J. Hillier. 1990. Temperate bacteriophages and lysogeny in lactic acid bacteria. FEMS Microbiol. Rev. 7:79-90. [DOI] [PubMed] [Google Scholar]
- 11.Durmaz, E., and T. R. Klaenhammer. 2000. Genetic analysis of chromosomal regions of Lactococcus lactis acquired by recombinant lytic phages. Appl. Environ. Microbiol. 66:895-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duwat, P., S. D. Ehrlich, and A. Gruss. 1995. The recA gene of Lactococcus lactis: characterization and involvement in oxidative and thermal stress. Mol. Microbiol. 17:1121-1131. [DOI] [PubMed] [Google Scholar]
- 13.Echols, H. 1972. Developmental pathways for the temperate phage: lysis vs lysogeny. Annu. Rev. Genet. 6:157-190. [DOI] [PubMed] [Google Scholar]
- 14.Feirtag, J. M., and L. L. McKay. 1987. Thermoinducible lysis of temperature-sensitive Streptococcus cremoris strains. J. Dairy Sci. 70:1779-1784. [Google Scholar]
- 15.Garcia, P., V. Ladero, J. C. Alonso, and J. E. Suarez. 1999. Cooperative interaction of CI protein regulates lysogeny of Lactobacillus casei by bacteriophage A2. J. Virol. 73:3920-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herskowitz, I., and D. Hagen. 1980. The lysis-lysogeny decision of phage lambda: explicit programming and responsiveness. Annu. Rev. Genet. 14:399-445. [DOI] [PubMed] [Google Scholar]
- 17.Jarvis, A. W. 1987. Sources of lactic streptococcal phages in cheese plants. N. Z. J. Dairy Sci. Technol. 22:93-103. [Google Scholar]
- 18.Johansen, A. H., L. Brondsted, and K. Hammer. 2003. Identification of operator sites of the CI repressor of phage TP901-1: evolutionary link to other phages. Virology 311:144-156. [DOI] [PubMed] [Google Scholar]
- 19.Josephsen, J., N. Andersen, E. Behrndt, E. Brandsborh, G. Christiansen, M. Hansen, E. Nielsen, and F. Vogensen. 1994. An ecological study of lytic bacteriophages of lactococcal bacteriophages of Lactococcus lactis subsp. cremoris isolated in a cheese plant over a 5-year period. Int. Dairy J. 4:123-140. [Google Scholar]
- 20.Kakikawa, M., S. Ohkubo, T. Sakate, M. Sayama, A. Taketo, and K. Kodaira. 2000. Purification and DNA-binding properties of the Cro-type regulatory repressor protein Cng encoded by the Lactobacillus plantarum phage φg1e. Gene 249:161-169. [DOI] [PubMed] [Google Scholar]
- 21.Klaenhammer, T. R. 1984. Interactions of bacteriophages with lactic streptococci. Adv. Appl. Microbiol. 30:1-29. [Google Scholar]
- 22.Kornberg, A., Baker, T. 1992. DNA replication, 2nd ed. W. H. Freeman and Company, New York, N.Y.
- 23.Ladero, V., P. Garcia, J. C. Alonso, and J. E. Suarez. 1999. A2 Cro, the lysogenic cycle repressor, specifically binds to the genetic switch region of Lactobacillus casei bacteriophage A2. Virology 262:220-229. [DOI] [PubMed] [Google Scholar]
- 24.Ladero, V., P. Garcia, J. C. Alonso, and J. E. Suarez. 2002. Interaction of the Cro repressor with the lysis/lysogeny switch of the Lactobacillus casei temperate bacteriophage A2. J. Gen. Virol. 83:2891-2895. [DOI] [PubMed] [Google Scholar]
- 25.Lawrence, R. C. 1978. Action of bacteriophages on lactic acid bacteria: consequences and protection. N. Z. J. Dairy Sci. Technol. 13:129-136. [Google Scholar]
- 26.Lillehaug, D., and N. K. Birkeland. 1993. Characterization of genetic elements required for site-specific integration of the temperate lactococcal bacteriophage φLC3 and construction of integration-negative φLC3 mutants. J. Bacteriol. 175:1745-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lillehaug, D., B. Lindqvist, and N. K. Birkeland. 1991. Characterization of φLC3, a Lactococcus lactis subsp. cremoris temperature bacteriophage with cohesive single-stranded DNA ends. Appl. Environ. Microbiol. 57:3206-3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lunde, M., J. M. Blatny, F. Kaper, I. F. Nes, and D. Lillehaug. 2000. The life cycles of the temperate lactococcal bacteriophage φLC3 monitored by a quantitative PCR method. FEMS Microbiol. Lett. 192:119-124. [DOI] [PubMed] [Google Scholar]
- 29.Lunde, M., J. M. Blatny, D. Lillehaug, A. H. Aastveit, and I. F. Nes. 2003. Use of real-time quantitative PCR for the analysis of φLC3 prophage stability in lactococci. Appl. Environ. Microbiol. 69:41-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madsen, P. L., A. H. Johansen, K. Hammer, and L. Brondsted. 1999. The genetic switch regulating activity of early promoters of the temperate lactococcal bacteriophage TP901-1. J. Bacteriol. 181:7430-7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meijer, W., C. Dobbelaar, and J. Hugenholtz. 1998. Thermoinducible lysis in Lactococcus lactis subsp. cremoris SK110: Implications for cheese ripening. Int. Dairy J. 8:275-280. [Google Scholar]
- 32.Mittler, J. E. 1996. Evolution of the genetic switch in temperate bacteriophage. I. Basic theory. J. Theor. Biol. 179:161-172. [DOI] [PubMed] [Google Scholar]
- 33.Moineau, S., S. Pandian, and T. R. Klaenhammer. 1995. Specific chromosomal sequences can contribute to the appearance of a new lytic bacteriophage in Lactococcus. Dev. Biol. Stand. 85:577-580. [PubMed] [Google Scholar]
- 34.Montgomery, D. C. 2001. Design and analysis of experiments, 5th ed. John Wiley & Sons, Inc., New York.
- 35.Nauta, A., D. van Sinderen, H. Karsens, E. Smit, G. Venema, and J. Kok. 1996. Inducible gene expression mediated by a repressor-operator system isolated from Lactococcus lactis bacteriophage r1t. Mol. Microbiol. 19:1331-1341. [DOI] [PubMed] [Google Scholar]
- 36.Prevots, F., M. Mata, and P. Ritzenthaler. 1990. Taxonomic differentiation of 101 lactococcal bacteriophages and characterization of bacteriophages with unusually large genomes. Appl. Environ. Microbiol. 56:2180-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ptashne, M. 1992. A genetic switch: phage lambda and higher organisms, 2nd ed. Blackwell Scientific Publications, Cambridge, Mass.
- 38.Rallu, F., A. Gruss, and E. Maguin. 1996. Lactococcus lactis and stress. Antonie van Leeuwenhoek 70:243-251. [DOI] [PubMed] [Google Scholar]
- 39.Ramirez, E., and A. Villaverde. 1997. Viral spread within ageing bacterial populations. Gene 202:147-149. [DOI] [PubMed] [Google Scholar]
- 40.Reiter, B. 1949. Lysogenic strains of lactic streptococci. Nature 164:667-668. [DOI] [PubMed] [Google Scholar]
- 41.Roberts, J. W., and R. Devoret. 1983. Lysogenic induction, p. 123-144. In R. W. Hendrix, J. W. Roberts, F. W. Stahl, and R. A. Weisberg (ed.), Lambda II. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 42.Sanders, J. W., G. Venema, and J. Kok. 1999. Environmental stress responses in Lactococcus lactis. FEMS Microbiol. Rev. 23:483-501. [Google Scholar]
- 43.Teuber, M., A. Geis, and H. Neve. 1992. The genus Lactococcus, p. 1482-1501. In A. Balows, H. G. Truper, M. Dworkin, W. Harder and K-H. Schleifer (ed.), The procaryotes, 2nd ed., vol. II. Springer-Verlag, New York, N.Y.
- 44.Teuber, M., and J. Lembke. 1983. The bacteriophages of lactic acid bacteria with emphasis on genetic aspects of group N lactic streptococci. Antonie van Leeuwenhoek 49:283-295. [DOI] [PubMed] [Google Scholar]
- 45.van de Guchte, M., C. Daly, G. F. Fitzgerald, and E. K. Arendt. 1994. Identification of the putative repressor-encoding gene cI of the temperate lactococcal bacteriophage Tuc2009. Gene 144:93-95. [DOI] [PubMed] [Google Scholar]
- 46.van de Guchte, M., P. Serror, C. Chervaux, T. Smokvina, S. D. Ehrlich, and E. Maguin. 2002. Stress responses in lactic acid bacteria. Antonie van Leeuwenhoek 82:187-216. [PubMed] [Google Scholar]





