Abstract
This study was performed to determine airborne manganese levels during welding practice and to establish the relationship between long-term, low-level exposure to manganese and altered serum concentrations of manganese, iron, and proteins associated with iron metabolism in career welders. Ninety-seven welders (average age of 36 years) who have engaged in electric arc weld in a vehicle manufacturer were recruited as the exposed group. Welders worked 7–8 h per day with employment duration of 1–33 years. Control subjects consisted of 91 employees (average age of 35 years) in the same factory but not in the welding profession. Ambient manganese levels in welders’ breathing zone were the highest inside the vehicle (1.5 ± 0.7 mg/m3), and the lowest in the center of the workshop (0.2 ± 0.05 mg/m3). Since the filter size was 0.8 μm, it is possible that these values may be likely an underestimation of the true manganese levels. Serum levels of manganese and iron in welders were about three-fold (p < 0.01) and 1.2-fold (p < 0.01), respectively, higher than those of controls. Serum concentrations of ferritin and transferrin were increased among welders, while serum transferrin receptor levels were significantly decreased in comparison to controls. Linear regression analyses revealed a lack of association between serum levels of manganese and iron. However, serum concentrations of iron and ferritin were positively associated with years of welder experience (p < 0.05). Moreover, serum transferrin receptor levels were inversely associated with serum manganese concentrations (p < 0.05). These findings suggest that exposure to welding fume among welders disturbs serum homeostasis of manganese, iron, and the proteins associated with iron metabolism. Serum manganese may serve as a reasonable biomarker for assessment of recent exposure to airborne manganese.
Keywords: Manganese, Iron, Ferritin, Transferrin, Transferrin receptor, Welder, Welding fume, Biomarker, Airborne manganese
INTRODUCTION
Overexposure to manganese (Mn) leads to a permanent neurodegenerative damage, resulting in syndromes similar to idiopathic Parkinson’s disease (IPD) (Barbeau et al., 1976; Inoue and Makita, 1996; Mena et al., 1967). Despite the similarities in extra-pyramidal symptoms between manganese neurotoxicity and IPD, numerous reports suggest that the sites of manganese-induced neurological lesions are fundamentally different from those observed in IPD. For instance, the primary targeted brain regions in manganese intoxication are the globus pallidus and striatum of the basal ganglia, whereas the neurodegeneration in IPD takes place mainly in the substantia nigra (Calne et al., 1994; Inoue and Makita, 1996; Olanow et al., 1996; Walter et al., 2003; Yamada et al., 1986). Limited human studies, however, suggest that occupationally prolonged exposures to manganese may increase the risk in acquiring and accelerating PD (Crossgrove and Zheng, 2004; Gorell et al., 1997). A more recent investigation on 15 cases of career welders who were diagnosed with PD indicates that these welders had a younger age of onset of PD in comparison to sequentially ascertained PD controls; the clinical symptoms and the response to levodopa treatment among the welder patients were also indistinguishable from those of IPD patients (Racette et al., 2001). A community-based study further suggests that manganese neurotoxicity may be a continuum of dysfunction, with early, subtle changes at lower exposure levels (Mergler et al., 1999).
Cases of manganese intoxication among welders who chronically inhale airborne manganese emitted during the welding process have been documented in literature (Chandra et al., 1981; Mergler et al., 1994; Roels et al., 1987; Sjogren et al., 1996; Smargiassi et al., 2000). The shielded metal arc weld, which is the most commonly used form of arc weld, can generate the fume containing manganese as high as 28.4% weight of hazardous air pollutant (US-EPA, 1994). While manganese is known to be released as concentrated particulates in welding fumes, there have been few reports in the literature that document the actual airborne manganese levels during the welding practice. Even less is known about the time–dose relationship between manganese exposure and its concentrations in the body fluids, e.g., serum and urine, of welders. Currently, no reliable biological indicator (or biomarker) has been established to evaluate manganese exposure. One earlier study suggests that welders exposed to manganese fumes did not result in blood concentrations of manganese higher than those of controls, although the numbers of welders involving in that study (n = 12) was rather limited (Sjogren et al., 1996).
Several lines of evidence suggest that manganese-induced neurotoxicities appear to be associated with altered iron (Fe) metabolism at both systemic and cellular levels (Chua and Morgan, 1996; Li et al., 2004; Malecki et al., 1999; Zheng and Zhao, 2001; Zheng et al., 1999). In vivo manganese exposure in animals appears to facilitate the influx of iron from the blood to the cerebral spinal fluid (CSF) (Zheng et al., 1999). Moreover, in vitro treatment of cultured cells with manganese promotes cellular iron overload (Malecki et al., 1999; Zheng and Zhao, 2001). In humans, a dysfunction in iron metabolism has been seen in IPD patients. High levels of total iron, decreased ferritin, iron-associated oxidative stress, and abnormal mitochondrial complex-I have been repeatedly reported in the postmortem substantia nigra of IPD patients (Dexter et al., 1991; Griffiths and Crossman, 1993; Loeffler et al., 1995; Sofic et al., 1991). An epidemiologic study has established that serum parameters associated with iron metabolism, such as ferritin (Ft), transferring (Tf), total iron-binding capacity, and % iron saturation, are significantly altered in IPD patients compared to normal subjects (Logroscino et al., 1997). It has been postulated that accumulation of iron in selected brain areas may facilitate the generation of iron-mediated reactive oxygen species; the ensuing oxidative stress then lead to the neuronal cell death (Connor, 1997; Youdim et al., 1993). Since manganese exposure alters iron metabolism, we hypothesized that changes in serum concentrations of iron-related proteins may be used as the potential indicator(s) for manganese exposure.
The purposes of this study were: (1) to determine airborne manganese levels during the welding practice, (2) to investigate whether long-term, low-level exposure to manganese in welders was associated with altered serum concentrations of manganese, and (3) to study whether exposure to welding fume influenced the homeostasis of iron as well as proteins associated with iron metabolism among welders. Establishment of relationships between these serum determinants and the welding exposure situation may allow to identify the biomarker(s) suitable for monitoring manganese exposure among welders as well as for other manganese exposure scenarios.
SUBJECTS AND METHODS
Factory and Production Processes
A Beijing vehicle factory was chosen for this study for its intensive, day-to-day indoor welding practice in the production of vehicles. The factory is located in the southwest region of Beijing metropolitan area and is not adjacent to any other metal industries. During work days, the welders perform in a workshop and are expected to weld both inside and outside of vehicles. The electric arc weld has been a primary technique in the welding practice.
Study Population
Ninety-seven welders engaged in daily welding practice were randomly selected from this factory as the exposed group. The welders, of whom 24 are woman, worked 7–8 h per day with the average employment history of 15.9 years (range: 1–33 years). A control group of 91 workers were recruited, frequency-matched to the welder group by sex, age, and work shift distribution, from the same factory, who have been employed in the professions other than welding (such as services, bookkeeping, office workers, cafeteria servants, etc.) and not exposed to welding fumes. The mean age was 35.7 years (range: 19–54 years) and 35.4 years (range: 21–52 years), respectively, for welders and controls. Both groups were also matched for socioeconomic status (salary, education, etc.) and background environmental factors (place of residence, etc.). The demographic data of the study population are summarized in Table 1.
Table 1.
Comparison of demographic information between welders and controls
| Welders (n = 97) | Controls (n = 91) | |
|---|---|---|
| Age (years) | 35.7 ±8.4 (19–54) | 35.4 ± 8.2 (21–52) |
| Age structure | <30/30~/35~/40~/45~/≥50 27/15/35/7/8/5 |
<30/30~/35~/40~/45~/≥50 28/11/33/6/11/2 |
| Sex | 73(M)/24(F) | 59(M)/32(F) |
| Body weight (kg) | 69.5 ± 11 (45–100) | 65.8 ± 10.1 (45–90) |
| Height (cm) | 169.7 ± 7.2 (150–182) | 167.9 ± 6.7 (154–182) |
| Year as welder | 15.9 ± 8.0 (1–33) |
Data represent mean ± S.D. Values in parentheses represent the range. There were no statistical significant differences between welders and controls in all categories analyzed.
Subjects in both groups at the time of interview had reported no exposure to other toxic substances, radiation therapy, or substance abuse. Subjects who had taken special medications, which would interfere with iron metabolism such as Vitamin D, aspirin, or herbal medication, were excluded from the study. There were no statistically significant differences in smoking and alcohol consumption between welders and controls.
Collection of Personal Data and Biological Samples
A scheduled interview lasting approximately 60 min was conducted by trained interviewers to obtain detailed information on occupational history, job description, socioeconomic status, lifestyle, and family and personal medical history.
Blood samples were collected in the morning of the day that each participant gave voluntary informed consent. A volume (10 mL) of venous blood was drawn from a cubital vein of the participants after fasting overnight. Samples were maintained at room temperature for 30 min. The sera were separated by low speed centrifugation and stored at −20 °C until analyses. All test tubes used in the study were free of metal contamination, as pre-tested by atomic absorption spectrophotometry (AAS).
Air Sample Collection and Analysis
Four different locations in the workshop were identified as the monitor sites according to the positions where welders usually work. The airborne manganese concentration was determined in the breathing zone of the welders by station air samplers. Air samples were collected by a Model BFC-35 pump equipped with a micro-porous filter, which has a diameter of 40 mm and the pore size of 0.8 um. Air flow was pumped at a flow rate of 5 L/min for 4 min one hour after welding started. At each monitoring site, samples were collected in duplicates every other hour two more times in the same day (total 5 h). The mean values of all three duplicated samples are presented in this report.
The filters were digested with 5 mL of HClO4–HNO3 mixture (1:9, v/v) at 200 °C. The dry residues were dissolved in 10 mL of 1% HCl. The solutions were diluted by 20–50-fold prior to AAS. Air manganese concentrations were measured by a model HITA-CHI Z-5000 flame AAS according to a China National Standard Operation Protocol (GB/T16018-1995) for occupational safety surveillance.
Determination of Serum Manganese and Iron
Concentrations of manganese and iron in sera were determined by a Perkin-Elmer Model 3030 flameless graphite furnace AAS. Aliquots (0.1 mL) of serum samples were diluted (5–20-fold) with an appropriate volume of 0.8% Triton X-100/0.5% EDTA in distilled, deionized water prior to AAS. The standard curves were established using freshly made manganese standards on the day of analysis. The detection limit for this method was 0.2 ng Mn/mL of assay solution with an intra-day variation <5% and inter-day <9% (Zheng et al., 1998, 1999). Total iron concentrations in sera were measured using the method of Yeh and Zee (1974). The serum samples (0.01 mL) were diluted with 0.49 mL of 0.8% Triton/0.5% EDTA prior to AAS. The detection limit for iron was 0.5 ng Fe/mL of assay solution.
Determination of Serum Ferritin, Transferrin (Tf) and Transferrin Receptor (TfR)
Serum ferritin levels were determined by using an ELISA method. The ELISA quantitation kit was purchased from Ramco Laboratories (Houston, TX, Catalog #S-22). The assay procedure followed the instructions by the manufacturer. Briefly, the sera were diluted 10-fold with sample diluent supplied with the assay kit. The diluted samples were pipetted to the wells pre-coated with polyclonal anti-human ferritin antibody. Following addition of horseradish peroxidase (HRP) conjugated secondary antibody, the reaction mixtures were incubated, washed, and the absorbance at 490 nm recorded. The concentrations of serum ferritin were calculated from a standard curve derived from the same procedure using purified human ferritin.
Serum concentrations of TfR were determined by using the similar ELISA test kit purchased from Ramco Laboratories (Catalog #TF-94). The experimental procedure followed the instructions by the manufacturer. The sera were diluted 100-fold with the sample diluent. The absorbance was determined at 450 nm, and the concentrations of TfR were estimated from a standard curve using human TfR as the standard.
Serum levels of Tf were also determined by an ELISA kit purchased from Bethyl Laboratory (Montgomery, TX; Catalog #E80-128). The assay procedure followed the instructions by the manufacturer. Serum samples were diluted 20,000-fold prior to assay. The absorbance was read at 490 nm and converted to calculate the serum concentrations using purified human Tf as the standard.
Statistical Analyses
Records of interviews and other reports were reviewed and abstracted for demographic data. All data are expressed as the mean ± S.D. unless otherwise stated. For statistical analyses, data were first transformed to logarithm. This transformation is valid with regards to the symmetric distribution and the linearity of variables following the logarithm transformation. Associations between serum manganese, iron, ferritin, TfR, and Tf as the function of years of experience as a welder were analyzed by a linear regression. The differences between two means were analyzed by a standard, parametric ANOVA. A statistics software SPSS/PC+ for Windows (V. 10.0) was used in data analysis (Li et al., 2004).
Materials
Chemicals were obtained from the following sources: human standards of ferritin, Tf, and TfR, bovine serum albumin, and Tween-20 from Sigma Chemical Co., St. Louis, MO, and AAS standards of manganese and iron from Alfa Products, Danvers, MA. All reagents were of analytical grade, HPLC grade or the highest available pharmaceutical grade.
RESULTS
Airborne Manganese Levels in Welders’ Breathing Zone
According to the national standard set out by the Chinese Ministry of Public Health (TJ36-79), the maximum allowable concentration (MAC) of manganese in the work place is 0.2 mg/m3. Airborne manganese levels during the welding practice as determined, except for the monitor located at the center of the workshop which is about 2 m above the ground, were all significantly higher than the MAC values (range: 0.47–1.47 mg/m3) (Table 2). Airborne manganese concentrations outside the vehicles during the active welding were about two- to four-fold higher than the MAC values, whereas the airborne levels of manganese inside the vehicles were 7.4-fold greater than the MAC. It was noticed that the air samples in the current study were taken during a summer season when all windows were open, allowing outdoor air flow to cool down the workshop. Thus, the regular airborne levels of manganese during other seasons with less effective ventilation would be expected to be higher than the values observed in this study.
Table 2.
Concentration of airborne manganese in welders’ breathing zone
| Site of monitor | Airborne Mn level (mg/m3) |
|---|---|
| Welding inside the vehicles | 1.47 ± 0.72 |
| Welding outside on the vehicle body | 0.87 ± 0.70 |
| Welding the parts near the vehicles | 0.47 ± 0.06 |
| Center of welding shop | 0.20 ± 0.05a |
| Average of all sampling spots | 0.56 |
Data represent mean ± S.D. of three duplicated samples over a period of 5 h during an active working day.
The distance was about 2 m above the ground and 5–10 m from the surrounding vehicles.
Serum Levels of Manganese and Iron in Welders and Control Subjects
Serum concentrations of manganese, iron, Ft, Tf, and TfR in welders and controls are summarized in Table 3. By comparing mean values between groups, serum concentrations of manganese in welders of both sexes were about 2.7–3.1-fold higher than those in controls (p < 0.001) (Table 3). When serum manganese levels in welders were plotted as a function of years of experience as a welder, serum manganese concentrations show a tendency to increase as years of welder experience advanced (Fig. 1); however, the correlation did not reach statistically significance.
Table 3.
Serum concentrations of manganese (Mn), iron (Fe), ferritin (Ft), transferrin (Tf), and transferrin receptor (TfR) in welders vs. control subjects
| Welders | Controls | % Change | p-value | ||
|---|---|---|---|---|---|
| Mn (ug/L) | Male | 3.40 ± 1.85 | 1.09 ± 2.58 | +212% | 0.001 |
| Female | 3.41 ± 1.01 | 1.24 ± 2.17 | +175% | 0.001 | |
| Combined | 3.40 ± 1.68 | 1.14 ± 2.43 | +198% | 0.001 | |
| Fe (ug/L) | Male | 1897 ± 483.8 | 1532 ± 744.3 | +24% | 0.001 |
| Female | 1467 ± 569.6 | 1384 ± 659.7 | +6% | 0.625 | |
| Combined | 1790 ± 536.7 | 1480 ± 715.5 | +21% | 0.001 | |
| Ft (ng/mL) | Male | 202.7 ± 111.5 | 188.4 ± 104.4 | +7% | 0.468 |
| Female | 79.9 ± 67.1 | 67.0 ± 66.6 | +19% | 0.484 | |
| Combined | 171.9 ± 115.0 | 145.7 ± 109.3 | +18% | 0.112 | |
| Tf (mg/mL) | Male | 1.67 ± 0.60 | 1.39 ± 0.65 | +20% | 0.016 |
| Female | 1.49 ± 0.44 | 1.48 ± 0.48 | +1% | 0.94 | |
| Combined | 1.63 ± 0.57 | 1.42 ± 0.60 | +14.8% | 0.016 | |
| TfR (ug/mL) | Male | 4.20 ± 0.44 | 4.84 ± 1.31 | −13% | 0.005 |
| Female | 4.03 ± 1.26 | 5.22 ± 1.52 | −23% | 0.004 | |
| Combined | 4.16 ± 1.29 | 4.97 ± 1.39 | −16.3% | 0.0001 |
Data represent mean ± S.D. n = 73 for males and n = 24 for females.
Fig. 1.

Relationship between serum manganese concentration and years of welder experience. Serum manganese concentrations were determined by AAS. Data were analyzed by a simple linear regression (r = 0.132, p = 0.196, n = 97).
The average serum concentrations of iron combined with both sexes were about 1.2-fold higher in welders than in controls (p < 0.001) (Table 3). However, the serum iron levels in female welders did not significantly differ from those of female controls. Linear regression analysis revealed a significant welder’s experience year associated increase in serum iron concentrations (r = 0.217, p = 0.035) (Fig. 2). When the serum iron concentrations were plotted against serum manganese concentrations, no correlation between these two metals was observed (r = 0.36, p = 0.362), suggesting that variations in serum iron appear to be independent of serum manganese level.
Fig. 2.

Relationship between serum iron concentration and years of welder experience. Serum iron concentrations were determined by AAS. Data were analyzed by a simple linear regression (r = 0.217, p = 0.035, n = 97).
Serum Levels of Ferritin, Tf and TfR in Welders and Control Subjects
Levels of serum ferritin, Tf, and TfR are involved in iron homeostasis in the body. These parameters were assessed to investigate the possible detrimental effect of manganese exposure on systemic iron metabolism. In comparison to control subjects, the welders investigated showed approximately 18% and 15% increases in serum ferritin (p = 0.112) and Tf (p = 0.016), respectively, while serum Tf in female welders was not significantly different from those of female controls (p = 0.94). Serum TfR levels among welders of both sexes were significantly decreased by 16% compared to controls (p < 0.001) (Table 3).
By linear regression analyses, serum ferritin levels in welders increased significantly as years of welder experience increased (r = 0.205, p = 0.044) (Fig. 3). The increase of serum Tf concentrations appeared to be positively associated with welding years; the correlation, however, did not reach the statistical significance (r = 0.153, p = 0.135). Similarly, while it was not statistically significant, serum TfR levels indeed showed a tendency to decline as the welding year increased (r = −0.123, p = 0.231).
Fig. 3.
Relationship between serum ferritin concentration and years of welder experience. Serum ferritin concentrations were determined by using a commercially available immunoassay kit. Data were analyzed by a simple linear regression (r = 0.205, p = 0.044, n = 97).
Serum concentrations of ferritin, Tf and TfR were further analyzed by linear regression as the function of serum concentrations of either manganese or iron. There was a significant inverse association between serum TfR and serum manganese levels (r = −0.237, p = 0.019) (Fig. 4). Serum TfR levels, however, did not change as the function of serum iron levels (Fig. 5). No correlation was observed between the serum concentrations of other iron proteins and serum manganese levels (data not shown).
Fig. 4.

Relationship between serum TfR and serum manganese concentrations. Serum TfR concentrations were determined by using a commercially available immunoassay kit. Data were analyzed by a simple linear regression (r = −0.237, p = 0.019, n = 97).
Fig. 5.
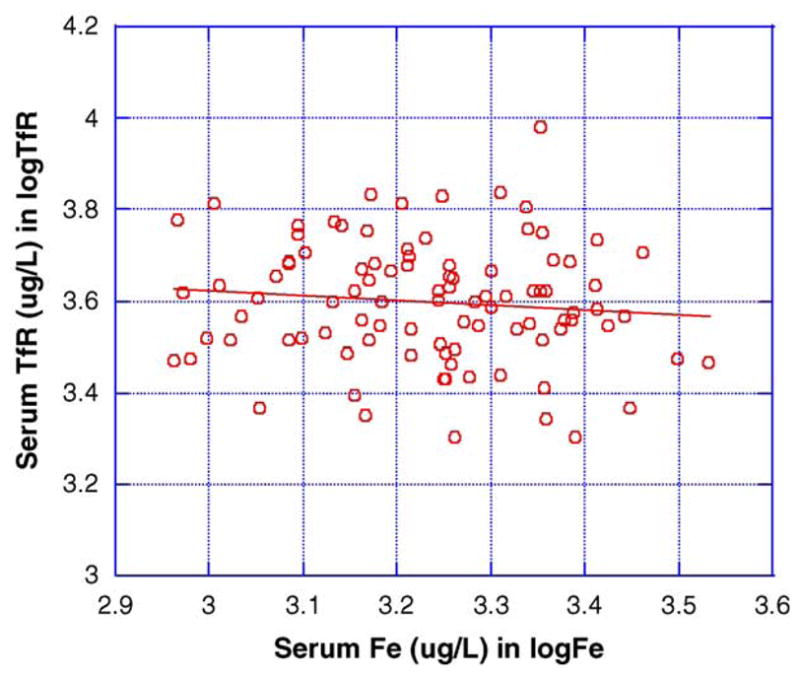
Relationship between serum TfR and serum iron concentrations. Data were analyzed by a simple linear regression (r = −0.106, p = 0.300, n = 97).
DISCUSSION
The welding processes can be divided into several technical categories: manual metal arc, flux cored arc, submerged arc, tungsten inert gas, metal inert/active gas, and plasma arc. All these processes generate fumes, although submerged arc and tungsten inert gas do so at significantly lower levels (US-EPA, 1994). In the factory where the current study was conducted, manual metal electric arc is the primary means employed in daily manufacturing. The welding fume contains a mixture of at least 13 metals including chromium (Cr), cobalt (Co), zinc (Zn), cadmium (Cd), beryllium (Be), mercury (Hg), lead (Pb), iron, and manganese (Mn) (OSHA, 1995). According to US-EPA’s document, the total fume during shielded metal arc emits the manganese as high as 28.4% of weight of hazardous air pollutant (US-EPA, 1994). Our study clearly indicates that manganese is present in ambient air during welding, and its concentration in periods of our assessment far exceeded the maximum allowable concentration (0.2 mg/m3). The high level of manganese inside the vehicle is of particular concern for Mn exposure.
The extent to which the airborne manganese level may cause neurological damage is unknown. Wang et al. (1989) described an outbreak of manganese intoxication due to a failed ventilation system in a ferromanganese smelter. In that study, the air manganese level was found to be greater than 28.8 mg/m3. The highest manganese level in the current study was about 1.5 mg/m3, which is considerably less than the values detected in the smelter. It should be pointed out that during welding, the high temperature generated by the arc (in the order of thousands of degrees Celsius) produces spherical particles having diameters in a range of 0.4–0.8 um (US-EPA, 1994). The filter used in the current study had the diameter of 0.8 um, which may be insufficient in retaining the particles with the diameters less than 0.8 um. Thus, the values we observed could underestimate the true values of manganese concentrations in the ambient air.
Our data suggest that the career welders had significantly higher serum levels of both manganese and iron as compared to control subjects. If one assumes that serum manganese level may reflect the total body burden of manganese, which accumulate over the years of welding practice, one would expect to see a higher serum manganese concentration in welders with a longer employment history. Data presented in this report, however, revealed that serum manganese concentrations were not significantly associated with welder’s professional years. The lack of such an association is not unexpected because a discrepancy between blood half life (t1/2) and tissue t1/2 of manganese has been documented in several studies (Newland et al., 1987; Takeda et al., 1995; Zheng et al., 2000). While the blood t1/2 following i.v. injection of manganese is about 2 h (Zheng et al., 2000), the brain t1/2 of Mn from inorganic manganese exposure ranges between 50 and 70 days (Newland et al., 1987; Takeda et al., 1995). The intracellular distribution and tight tissue binding of manganese likely contribute to the difference between blood and tissue t1/2. Thus, our results suggest that serum manganese levels serve reasonably well as the indicator for recent manganese exposure (e.g., higher in welders than normal controls), but are not suitable for the estimation of the historical accumulation of manganese in the body following long-term, low-level manganese exposure.
Exposure to welding fumes had a significant impact on serum concentrations of iron and certain proteins associated with iron regulation and metabolism. Not only were serum iron levels significantly higher in welders than in controls, but also increased iron levels among welders were significantly influenced by the years of experience as a welder. Altered serum iron levels among welders could be attributable to co-exposure to airborne iron during welding. Inhalation of iron oxide emitted in welding fumes may lead to a higher serum iron concentration in these welders. Noticeably, the current welder results appear to disagree with those previously reported human and rodent studies (Logroscino et al., 1997; Zheng et al., 1999). Previous studies by Zheng et al. (1999) reveal an increased CSF concentration of iron, but a decreased blood iron level, in rats chronically exposed to manganese. In IPD patients, blood iron levels are also significantly decreased (Logroscino et al., 1997). The airborne iron level was not determined in this study; however, the increased serum iron status in career welders, particularly with regards to a positive association with welder experience years, is conceivably related to co-exposure to airborne iron. Nonetheless, the possibility that the elevated manganese body burden as a result of exposure to welding fumes may alter iron homeostasis by acting on iron regulatory mechanism cannot be excluded.
Under normal physiological conditions, the iron homeostasis is regulated by a group of proteins involving in iron metabolism, such as ferritin, Tf, and TfR. Tf carries iron in the blood and serves as the major vehicle for iron transport in the body. On arriving at the target cells, Tf binds to TfR on the outer surface of cell membranes and delivers iron into the cells by endocytosis. In the cytoplasm, free iron is either utilized in metabolic processes or stored by conjugation in cytosolic ferritin. Excess presence of iron in the body results in an up-regulation of ferritin production and a down-regulation of TfR synthesis, so as to counterbalance the overload of iron molecules (Aschner et al., 1999; Klausner et al., 1993; Ponka, 2000). Earlier studies from this laboratory (Chen et al., 2001; Zheng et al., 1999; Zheng and Zhao, 2001) suggest that manganese may compete with iron for the [Fe–S] cluster in the active center of iron regulatory proteins (IRPs). Such a competition, while suppressing IRP’s enzymatic catalytic function, would increase the protein’s ability to bind to mRNAs encoding TfR and ferritin, which in concert with an enhanced transport of iron at brain barriers may promote the cellular overload of iron in brain, subsequently leading to iron-initiated neuronal oxidative damage.
In the current study, serum ferritin levels among welders were increased nearly 18%; this increase was also associated with the welder’s professional years, suggesting a systemic overload of iron. Serum TfR levels, on the other hand, were significantly decreased among welders, a consequence suggesting a cellular overload of iron (Klausner et al., 1993; Ponka, 2000). These results suggest that exposure to welding fume has led to an altered status of serum ferritin and TfR. While these changes could be a direct result of elevated serum iron among welders, it is interesting to note that a decreased serum TfR level is significantly associated with the increase of serum manganese concentrations, suggesting an involvement of manganese in iron metabolism and regulation. It is also noted that female welders appear to be less sensitive than male welders to changes in serum levels of Fe and Tf.
In summary, the results from this study demonstrate that the welding process does produce high-than-normal manganese levels in ambient air. The welders had serum concentrations of manganese and iron significantly higher than controls. The proteins associated with iron metabolism, e.g., ferritin and TfR, were significantly altered in comparison to the controls. Moreover, we suggest that serum manganese concentrations may serve as a useful biomarker for recent exposure to airborne manganese.
Acknowledgments
This work was initiated while Dr. Zheng was a faculty member at Mailman School of Public Health, Columbia University in New York City. The project was partly supported by a pilot fund by NIH/NIEHS Environmental Health Sciences Center in Northern Manhattan at Columbia University (Grant ES-09089), and also supported by US-National Institute of Environmental Health Sciences Grant ES-08146 and National Natural Science Foundation of China Grant 30000140.
References
- Aschner M, Vrana KE, Zheng W. Manganese uptake and distribution in the central nervous system (CNS) Neurotoxicology. 1999;20:173–80. [PubMed] [Google Scholar]
- Barbeau A, Inoué N, Cloutier T. Role of manganese in dystonia. Adv Neurol. 1976;14:339–52. [PubMed] [Google Scholar]
- Calne DB, Chu NS, Huang CC, Lu CS, Olanow W. Manganism and idiopathic Parkinsonism: similarities and difference. Neurology. 1994;44:1583–6. doi: 10.1212/wnl.44.9.1583. [DOI] [PubMed] [Google Scholar]
- Chandra SV, Shukla GS, Srivastawa RS, Singh H, Gupta VP. An exploratory study of manganese exposure to welders. Clin Toxicol. 1981;18:407–18. doi: 10.3109/15563658108990264. [DOI] [PubMed] [Google Scholar]
- Chen JY, Tsao GC, Zhao Q, Zheng W. Differential cytotoxicity of Mn(II) and Mn(III): special reference to mitochondrial [Fe–S] containing enzymes. Toxicol Appl Pharmacol. 2001;175:160–8. doi: 10.1006/taap.2001.9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua AC, Morgan EH. Effects of iron deficiency and iron overload on manganese uptake and deposition in the brain and other organs of the rat. Biol Trace Elem Res. 1996;55:39–54. doi: 10.1007/BF02784167. [DOI] [PubMed] [Google Scholar]
- Connor JR. Metals and oxidative damage in neurological disorders. New York: Plenum Press; 1997. pp. 23–39. [Google Scholar]
- Crossgrove JS, Zheng W. Review of manganese toxicity upon overexposure. NMR Biomed. 2004;17(8) doi: 10.1002/nbm.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter DT, Carayon A, Javoy-Agid F, Agid Y, Wells FR, Daniel SE, et al. Alterations in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia. Brain. 1991;114:1953–75. doi: 10.1093/brain/114.4.1953. [DOI] [PubMed] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Brown GG, et al. Occupational exposures to metals as risk factors for Parkinson’s disease. Neurology. 1997;48:650–8. doi: 10.1212/wnl.48.3.650. [DOI] [PubMed] [Google Scholar]
- Griffiths PD, Crossman AR. Distribution of iron in the basal ganglia and neocortex in postmortem tissue in Parkinson’s disease and Alzheimer’s disease. Dementia. 1993;4:61–5. doi: 10.1159/000107298. [DOI] [PubMed] [Google Scholar]
- Inoue N, Makita Y. Neurological aspects in human exposures to manganese. In: Chang LW, editor. Toxicology of metals. Boca Raton: CRC Press; 1996. pp. 415–421. [Google Scholar]
- Klausner RD, Rouault TA, Harford JB. Regulating the fate of mRNA: the control of cellular iron metabolism. Cell. 1993;72:19–28. doi: 10.1016/0092-8674(93)90046-s. [DOI] [PubMed] [Google Scholar]
- Li GJ, Zhang L, Lu L, Wu P, Zheng W. Occupational exposure to welding fume among welders: alterations of manganese, iron, zinc, copper, and lead in body fluids and the oxidative stress status. J Occup Environ Med. 2004;46:241–8. doi: 10.1097/01.jom.0000116900.49159.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffler DA, Connor JR, Juneau PL, Snyder BS, Kanaley L, DeMaggio AJ, et al. Transferrin and iron in normal, Alzheimer’s disease, and Parkinson’s disease brain regions. J Neurochem. 1995;65:710–24. doi: 10.1046/j.1471-4159.1995.65020710.x. [DOI] [PubMed] [Google Scholar]
- Logroscino G, Marder K, Graziano JH, Freyer G, Slavkovich V, LoIacono N, et al. Altered systemic iron metabolism in Parkinson’s disease. Neurology. 1997;49:714–7. doi: 10.1212/wnl.49.3.714. [DOI] [PubMed] [Google Scholar]
- Malecki EA, Devenyi AG, Beard JL, Connor JR. Existing and emerging mechanisms for transport of iron and manganese to the brain. J Neurosci Res. 1999;56:113–22. doi: 10.1002/(SICI)1097-4547(19990415)56:2<113::AID-JNR1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Mena I, Marin O, Fuenzalida S, Cotzias GC. Chronic manganese poisoning: clinical picture and manganese turnover. Neurology. 1967;17:128–36. doi: 10.1212/wnl.17.2.128. [DOI] [PubMed] [Google Scholar]
- Mergler D, Huel G, Bowler R, Iregren A, Belanger S, Baldwin M, et al. Nervous system dysfunction among workers with long-term exposure to manganese. Environ Res. 1994;64:151–80. doi: 10.1006/enrs.1994.1013. [DOI] [PubMed] [Google Scholar]
- Mergler D, Baldwin M, Belanger S, Larribe F, Beuter A, Bowler R, et al. Manganese neurotoxicity, a continuum of dysfunction: results from a community based study. Neurotoxicology. 1999;20:327–42. [PubMed] [Google Scholar]
- Newland MC, Cox C, Hamada R, Oberdorster G, Weiss B. The clearance of manganese chloride in the primates. Fundam Appl Toxicol. 1987;9:314–28. doi: 10.1016/0272-0590(87)90054-6. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Good PF, Shinotoh H, Hewitt KA, Vingerhoets F, Snow BJ, et al. Manganese intoxication in the rhesus monkey: a clinical, imaging, pathologic, and biochemical study. Neurology. 1996;46:492–8. doi: 10.1212/wnl.46.2.492. [DOI] [PubMed] [Google Scholar]
- OSHA. Welding Fumes (Total Particulate) Chemical Sampling Information. 1995 http://www.osha-slc.gov/dts/chemicalsampling/data/CH_276100.html.
- Ponka P. Iron metabolism: physiology and pathophysiology. J Trace Elem Exp Med. 2000;13:73–83. [Google Scholar]
- Racette BA, McGee-Minnich L, Moerlein SM, Mink JW, Videen TO, Perlmutter JS. Welding-related parkinsonism: clinical features, treatment, and pathophysiology. Neurology. 2001;56:8–13. doi: 10.1212/wnl.56.1.8. [DOI] [PubMed] [Google Scholar]
- Roels H, Lauwerys R, Buchet JP, Genet P, Sarhan MJ, Hanotiau I, et al. Epidemiological survey among workers exposed to manganese: effects on lung, central nervous system, and some biological indices. Am J Ind Med. 1987;11:307–27. doi: 10.1002/ajim.4700110308. [DOI] [PubMed] [Google Scholar]
- Sjogren B, Iregren A, Frech W, Hagman M, Johansson L, Tesarz M, et al. Effects on the nervous system among welders exposed to aluminum and manganese. Occup Environ Med. 1996;53:32–40. doi: 10.1136/oem.53.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smargiassi A, Baldwin M, Savard S, Kennedy G, Mergler D, Zayed J. Assessment of exposure to manganese in welding operations during the assembly of heavy excavation machinery accessories. Appl Occup Environ Hyg. 2000;15:746–50. doi: 10.1080/10473220050129383. [DOI] [PubMed] [Google Scholar]
- Sofic E, Paulus W, Jellinger K, Riederer P, Youdim MB. Selective increase of iron in substantia nigra zona compacta of parkinsonian brains. J Neurochem. 1991;56:978–82. doi: 10.1111/j.1471-4159.1991.tb02017.x. [DOI] [PubMed] [Google Scholar]
- Takeda A, Sawashita J, Okada S. Biological half-lives of zinc and manganese in rat brain. Brain Res. 1995;695:53–8. doi: 10.1016/0006-8993(95)00916-e. [DOI] [PubMed] [Google Scholar]
- US-EPA. Environmental Protection Agency: Office of Air Quality Planning and Standards Emission Inventory Branch. Development of particulate and hazardous emission factors for electric arc welding. 1994:2.1–2.23. 4.1–4.6. EPA Contract No. 68-D2-0159. [Google Scholar]
- Wang JD, Huang CC, Hwang YH, Chiang JR, Lin JM, Chen JS. Manganese induced Parkinsonism: an outbreak due to an unrepaired ventilation control system in a ferromanganese smelter. Br J Ind Med. 1989;46:856–9. doi: 10.1136/oem.46.12.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter U, Niehaus L, Probst T, Benecke R, Meyer BU, Dressler D. Brain parenchyma sonography discriminates Parkinson’s disease and atypical Parkinsonian syndromes. Neurology. 2003;60:74–7. doi: 10.1212/wnl.60.1.74. [DOI] [PubMed] [Google Scholar]
- Yamada M, Ohno S, Okayasu I, Hatakeyama S, Watanabe H, Ushio K, et al. Chronic manganese poisoning: a neuropathological study with determination of manganese distribution in the brain. Acta Neuropathol Berl. 1986;70:273–8. doi: 10.1007/BF00686083. [DOI] [PubMed] [Google Scholar]
- Yeh YY, Zee P. Micromethod for determining total iron-binding capacity by flameless atomic absorption spectrophotometry. Clin Chem. 1974;20:360–4. [PubMed] [Google Scholar]
- Youdim MBH, Ben-Shachar D, Riederer P. The possible role of iron in the etipathology of Parkinson’s disease. Mov Disord. 1993;8:1–12. doi: 10.1002/mds.870080102. [DOI] [PubMed] [Google Scholar]
- Zheng W, Zhao Q. Iron overload following manganese exposure in cultured neuronal, but not neuroglial cells. Brain Res. 2001;897:175–9. doi: 10.1016/s0006-8993(01)02049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Kim H, Zhao Q. Comparative toxicokinetics of manganese chloride and methylcyclo-pentadienyl Mn tricarbonyl in male Sprague–Dawley rats. Toxicol Sci. 2000;54:295–301. doi: 10.1093/toxsci/54.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Ren S, Graziano JH. Manganese inhibits mitochondrial aconitase: a mechanism of manganese neurotoxicity. Brain Res. 1998;799:334–42. doi: 10.1016/s0006-8993(98)00481-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Zhao Q, Slavkovich V, Aschner M, Graziano JH. Alteration of iron homeostasis following chronic exposure to manganese in rats. Brain Res. 1999;833:125–32. doi: 10.1016/s0006-8993(99)01558-9. [DOI] [PMC free article] [PubMed] [Google Scholar]



