Abstract
Human replication protein A (RPA), the primary single-stranded DNA-binding protein, was previously found to be inhibited after heat shock by complex formation with nucleolin. Here we show that nucleolin-RPA complex formation is stimulated after genotoxic stresses such as treatment with camptothecin or exposure to ionizing radiation. Complex formation in vitro and in vivo requires a 63-residue glycine-arginine-rich (GAR) domain located at the extreme C terminus of nucleolin, with this domain sufficient to inhibit DNA replication in vitro. Fluorescence resonance energy transfer studies demonstrate that the nucleolin-RPA interaction after stress occurs both in the nucleoplasm and in the nucleolus. Expression of the GAR domain or a nucleolin mutant (TM) with a constitutive interaction with RPA is sufficient to inhibit entry into S phase. Increasing cellular RPA levels by overexpression of the RPA2 subunit minimizes the inhibitory effects of nucleolin GAR or TM expression on chromosomal DNA replication. The arrest is independent of p53 activation by ATM or ATR and does not involve heightened expression of p21. Our data reveal a novel cellular mechanism that represses genomic replication in response to genotoxic stress by inhibition of an essential DNA replication factor.
Genomic stability requires that cell cycle progression is tightly regulated and can be blocked at key transitions in response to genotoxic stress (38). In response to such stresses, eukaryotic cells activate pathways that both prevent entry into S phase and inhibit DNA synthesis in cells currently undergoing replication. Whereas certain mechanisms have been identified that, for example, block kinases necessary for S-phase progression (e.g., references 10, 11, and 19), other inhibitory pathways likely exist. Study of replication protein A (RPA), the primary single-stranded DNA binding protein in eukaryotes (31, 57), has shown that this factor is a target for inactivation in response both to genotoxic stress and heat shock (8, 13, 36, 37, 52, 54, 55). However, the mechanisms of inactivation remain poorly understood.
RPA is composed of three distinct subunits of ∼70 (RPA1), 30 (RPA2), and 14 (RPA3) kDa and is an essential factor in many DNA processing reactions. Genetic and biochemical studies demonstrate that RPA has required roles both in the initiation and in the elongation stages of DNA replication (31, 57). Similarly, RPA is necessary for homologous recombination and for DNA repair events that use the recombination machinery (for example, see reference 53 and references therein). It is also indispensable for nucleotide excision repair (1). Along with stabilizing DNA in its single-stranded form, RPA supports the activity of other factors through obligate interactions. For example, simian virus 40 (SV40) DNA replication can be reconstituted with RPA of a metazoan origin but not with Saccharomyces cerevisiae RPA (6, 39). RPA is intimately involved in the cellular checkpoint response as RPA recruits the ATR-ATRIP complex to sites of DNA damage and supports activation of the ATR kinase (59). RPA also recruits the replication factor C-like Rad17 complex to various DNA structures and assists the binding of the Rad9-Rad1-Hus1 complex (60).
As would be expected of a protein with multiple roles in DNA metabolism and in the response to DNA damage, RPA activity is regulated at various levels. The RPA2 subunit of RPA becomes phosphorylated in response to genotoxic stress by phosphatidylinositol 3-kinase-related kinases, including ATM and DNA-PK (see citations within references 5 and 52). Mutational analysis of the RPA2 phosphorylation sites indicates that RPA phosphorylation prevents recruitment of RPA to replication centers while having no effect on localization to sites of DNA damage (52). Downregulation of RPA activity also occurs by apparent phosphorylation-independent mechanisms. The most clearly identified pathway involves the inhibition of RPA activity by association with the nucleolar factor nucleolin (13, 54).
Nucleolin is an abundant protein that is required for the first step of pre-rRNA processing (22). Mutation of the genes encoding nucleolin homologues in budding and fission yeast disrupts balanced production of the small and large ribosomal subunits (24, 34, 35). Nucleolin has many other diverse activities, including regulation of transcription (20, 23, 26, 45, 58), modulation of mRNA stability (9, 48), and acting as a low-affinity receptor for human immunodeficiency virus on the cell surface (7, 41). In response to DNA damage conditions or heat shock, a significant fraction of the nucleolin pool relocalizes from the nucleolus to the nucleoplasm in a process stimulated by physical association with p53 (13, 14, 54). After heat shock, nucleolin-RPA complex formation is greatly stimulated, and formation of this complex is inhibitory to DNA replication in vitro (13, 54). In vivo, the mobilized nucleolin sequesters RPA at sites distinct from replication centers (13). The mobilization of nucleolin in response to heat shock thus represents a novel pathway for regulating DNA replication.
We examined the interaction of nucleolin and RPA in response to DNA damage. We found that, like heat shock, genotoxic stress strongly induces nucleolin-RPA complex formation. The RPA-interacting domain was localized to the 63-amino-acid (aa) glycine-arginine-rich (GAR) domain at the extreme C terminus of nucleolin. Expression of GAR or a nucleolin mutant with constitutive association with RPA causes a block in the cellular transit from G1 into S phase. The nucleolin-mediated inhibition of chromosomal DNA replication could be prevented by overexpression of RPA2 to increase the cellular level of RPA. These data demonstrate a novel intra-S-phase checkpoint response in response to genotoxic stress through target of RPA by mobilized nucleolin.
MATERIALS AND METHODS
Construction of nucleolin and RPA2 expression vectors.
For in vitro studies, human nucleolin and mutant nucleolin derivatives were expressed in Saccharomyces cerevisiae with N-terminal glutathione S-transferase (GST) tags and were purified as described below. The pKG-derived yeast plasmids that express full-length nucleolin (FL; aa 1 to 707), the N-terminal half of nucleolin (NT; aa 1 to 323), and the C-terminal half of nucleolin (CT; aa 323 to 707) were kindly provided by E. Rubin (University of Medicine and Dentistry of New Jersey [UMDNJ]). Other nucleolin variants, including the combined N terminus and first RNA-binding domain (RBD) (NT/RBD1; aa 1 to 390), the combined N terminus and the complete RBD region (NT/RBD1-4; aa 1 to 648), and the C-terminal GAR domain (GAR; aa 645 to 707), were inserted into the pKG vector by using standard PCR-mediated cloning procedures.
For in vivo studies, nucleolin or nucleolin derivatives were expressed with N-terminal green fluorescent protein (GFP), cyan fluorescent protein (CFP), or Myc epitope tags. GFP and CFP fusion proteins were constructed by using PCR cloning into the pEGFP-C1 or pECFP-C1 vectors (Clontech). Similarly, Myc-tagged nucleolin (FL or mutants) was expressed from the pEF6/Myc-HisA plasmid (Invitrogen), as modified by Vassin et al. (52) to prevent expression of the His tag or, for proliferation studies, from a modified pEGFP-C1 vector in which the GFP tag was replaced by the Myc tag. Human RPA2 containing an N-terminal yellow fluorescent protein (YFP) tag was generated by excising the RPA2 coding sequence from pENeGFP RPA34 (kindly provided by M. C. Cardoso) (50) into pEYFP-C1 vector (Clontech). The construction of the Myc-RPA2 expression vector was described previously (52). The pECFP-C1-H-Ras61L and pEYFP-N1-RasBD expression vectors were kindly provided by Trever Bivona of Mark Philips laboratory (New York University [NYU] School of Medicine). All fusion constructs were sequenced and shown to be faithful copies of the corresponding genes.
Purification of proteins.
GST-tagged nucleolin proteins were purified by the protocol of Haluska et al. (25). After transformation of S. cerevisiae JEL1 strain with the appropriate plasmid, cells were grown in synthetic defined (SD) medium under selection in 2% raffinose, and protein expression was induced by 2% galactose. Extracts from these cultures were made by disruption of the yeast cells by using 25- to 50-μm glass beads in uracil RIPA buffer (50 mM Tris-HCl [pH 7.2], 150 mM NaCl, 0.1% sodium dodecyl sulfate [SDS], 1% Triton X-100, 1% sodium deoxycholate) with protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 0.5 μg of leupeptin/ml, 1 μg of pepstatin/ml), 1 mM EDTA, and 1 mM dithiothreitol (DTT). Glutathione-Sepharose beads (Pharmacia) were then added to the clarified yeast extract, followed by incubation to bind the GST-nucleolin proteins. After three washes with a 10× bead volume of RIPA buffer, the GST-tagged proteins were eluted with 10 mM reduced glutathione and 50 mM Tris-HCl (pH 7.5). After overnight dialysis at 4°C against phosphate-buffered saline (PBS) and 20% glycerol, eluates were assayed for purity by SDS-polyacrylamide gel electrophoresis (PAGE) and Coomassie blue staining.
The human RPA heterotrimer was produced in Escherichia coli BL21 transformed with the p11dtRPA vector and purified as described previously (29, 30).
Far-Western analysis.
Far-Western blotting was carried out basically as described by Jayaraman et al. (32). Purified GST-tagged nucleolin (FL and mutants) proteins were subjected to SDS-PAGE and then transferred to a nitrocellulose membrane. After two incubations in denaturation buffer (6 M guanidine-HCl in PBS) for 5 min at 4°C, the membrane was incubated six times in serial dilutions (1:1 [vol/vol]) of denaturation buffer, each dilution being with PBS containing 1 mM DTT. The membrane was blocked with PBS containing 0.1% Tween 20 (PBS-T) and 5% nonfat dry milk (NFDM) for 45 min at room temperature and washed twice with PBS-T and 0.25% NFDM. The membrane was then incubated with purified human RPA (0.2 μg/ml) in PBS-T, 0.25% NFDM, 1 mM DTT, and 2.5 mM phenylmethylsulfonyl fluoride for 2 h at room temperature and subsequently washed four times in PBS-T and 0.25% NFDM. The presence of bound RPA was probed by using a mouse anti-RPA2 monoclonal antibody (SSB34A; NeoMarkers) and horseradish peroxidase-conjugated sheep anti-mouse antibody as the primary and secondary antibodies, respectively, and detected by using enhanced chemiluminescence (Amersham Biosciences).
In vitro DNA replication assay.
The SV40-based in vitro DNA replication assay was described previously (52) and utilized a pBluescript SK+ phagemid (Stratagene) containing a 90-bp SV40 origin region segment (positions 5186 to 32) subcloned into the BamHI and XhoI sites (pBS-ori). Reaction mixtures (25 μl) contained the following: 40 mM HEPES (pH 7.5); 40 mM creatine phosphate; 7 mM MgCl2; 0.5 mM DTT; 4 mM ATP; 200 μM concentrations each of CTP, GTP, and UTP; 100 μM concentrations each of dATP, dGTP, and dTTP; 40 μM [α-32P]dCTP (3,000 cpm/pmol; Perkin-Elmer Life Sciences); 1.25 μg of creatine phosphokinase; 150 ng of pBS-ori; 100 μg of AS65 protein fraction prepared from HeLa cells; 200 ng of RPA; 200 to 400 ng of the GST fusion proteins; and 500 ng of SV40 large T antigen. The reaction mixtures were first preincubated on ice for 30 min without the addition of plasmid DNA, deoxynucleoside triphosphates, ATP, and creatine phosphokinase. After the addition of the remaining factors, the complete reaction mixture was further incubated at 37°C for 2 h. The replication activity was determined by precipitating the high-molecular-weight DNA with trichloroacetic acid and quantitating the amount of incorporated radioactivity in the precipitate by liquid scintillation counting.
Immunoprecipitation and immunoblotting.
Plated U2-OS cells were transfected with 1 μg of specified expression plasmids by using Effectene transfection reagent (Qiagen). The transfection efficiencies of each construct were similar when visualized at 24 h posttransfection. When required, cells were either treated with 1 μM CPT or 2.5 mM hydroxyurea or exposed to 10 Gy of ionizing radiation or 30 J of UV light m−2. The immunoprecipitation reaction was carried out by using the IMMUNOcatcher kit (CytoSignal) according to the manufacturer's instructions. Immunoprecipitated proteins were separated by using SDS-10% PAGE and transferred to a nitrocellulose membrane (Schleicher & Schuell). After incubation with the appropriate primary antibody, the membrane incubated with an horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit secondary antibody, and the presence of bound proteins was detected with ECLplus (Amersham Pharmacia Biotech). The following antibodies were used for both detection and immunoprecipitation: RPA2, mouse monoclonal antibody SSB34A (NeoMarkers); RPA1, mouse monoclonal antibody Ab-1; nucleolin, either the MS3 mouse monoclonal or the H-250 rabbit polyclonal antibody (Santa Cruz Biotechnology); GFP, rabbit polyclonal antibody (Molecular Probes); Myc, rabbit polyclonal antibody (Upstate Biotechnology); p53, DO-1 mouse monoclonal antibody (Santa Cruz Biotechnology); (pSer15)p53, rabbit polyclonal antibody (Cell Signaling Technology); and p21, mouse monoclonal Cip1/WAF1 antibody (BD Biosciences/Pharmingen).
Immunofluorescence microscopy.
To prepare for imaging, U2-OS cells grown on fibronectin-coated coverslips (BD Biotechnology) were treated as described previously (15). Cells were fixed for 20 min at room temperature with 4% (wt/vol) formaldehyde in PBS, permeabilized with 0.5% Triton X-100, rinsed with PBS, and then incubated with PBS containing 0.5% Nonidet P-40. Coverslips were incubated with 1:100 dilution of the appropriate primary antibody for 1 h at room temperature. After three rinses with PBS containing 0.5% Tween 20, coverslips were incubated for 1 h at room temperature with 1:100 dilution of Texas Red- or fluorescein isothiocyanate-conjugated secondary antibody (Jackson Immunoresearch Laboratories). Coverslips were then rinsed three times with PBS containing 0.5% Tween 20 and mounted onto glass slides. Fluorescent signals were detected by using either epifluorescence or confocal microscopy.
FRET.
U2-OS cells were grown and cotransfected with the appropriate YFP- and CFP-tagged expression constructs in 35-mm uncoated glass bottom cell culture dishes (MatTek). Live cell images were obtained with a Zeiss LSM510 Meta laser scanning confocal microscope with a Plan-Apochromat ×63 objective lens and a 30-mW Argon laser set at 50% of total output. CFP as the donor channel was excited with a 458-nm laserline, and CFP fluorescence was collected with a band-pass filter of 475 to 525 nm. YFP, the acceptor channel, was excited at 514 nm, and YFP emission was collected with a long-pass filter of 530 nm. The fluorescence resonance energy transfer (FRET) channel consisted of CFP excited at 458 nm and YFP fluorescence collected with a long-pass filter of 530 nm. Photobleaching was performed with the 514-nm laser line set at 100% power with an average bleach time of 5 s. Specific regions of interest (ROIs) were chosen, and positive FRET was determined graphically based on the decrease of YFP signal, and the subsequent increase in the CFP fluorescence postbleaching. Although transfection of any combination of YFP-RPA2 and CFP-nucleolin (or nucleolin derivative) did not have notable deleterious effects on cell viability, only cells with a normal appearance and relatively low expression levels were tested.
BrdU incorporation assay and FACS.
U2-OS cells were plated at 30% confluency in 60-mm dishes. Plates were mock transfected, transfected with 1 μg of the Myc tag (empty) vector, or 1 μg of the appropriate N-terminal Myc-tagged nucleolin expression construct. At 24 h posttransfection, the cells were incubated for 20 min with 10 μM bromodeoxyuridine (BrdU). Cells were then washed twice with ice-cold PBS and collected by centrifugation at 180 × g for 5 min at 4°C. Pelleted cells were carefully resuspended into 300 μl of 4% (wt/vol) formaldehyde in PBS, fixed for 15 min at room temperature, and washed with PBS twice. Cells were then permeabilized for 15 min on ice with PBS containing 0.2% (vol/vol) Triton X-100 and 1% (wt/vol) bovine serum albumin (BSA), washed once with PBS, and then treated with PBS containing 0.25 mg of DNase/ml for 1 h at 37°C. Cells were incubated with 100 μl of PBS containing rat anti-BrdU (Harlan Sera-Lab) and rabbit anti-Myc (Upstate Biotechnology) polyclonal antibodies and 2% (wt/vol) BSA for 40 min at 37°C. Cells were washed twice with PBS and incubated for 40 min at room temperature with 100 μl of PBS containing anti-rabbit phycoerythrin-conjugated and anti-rat fluorescein isothiocyanate-conjugated antibodies (Jackson Laboratories) and 2% BSA. After preincubation of cells with 4 mM sodium citrate, 30 U of RNase A/ml, and 0.1% (vol/vol) Triton X-100 for 10 min at 37°C, the DNA was stained with 7-aminoactinomycin D (Sigma), and the cells were subjected to fluorescence-activated cell sorting (FACS) analysis.
[3H]thymidine uptake assay.
U2-OS cells were plated into 24-well tissue culture plates in complete McCoy's media containing 10% fetal bovine serum (FBS). The cells were transfected with plasmids (100 ng) expressing one of the following proteins: Myc-tag, Myc-nucleolin TM, or Myc-nucleolin GAR. As indicated, cells were also cotransfected with various amounts of a Myc-RPA2 expression vector. After 6 to 8 h, the medium was changed to a low serum (0.1% FBS) condition and further incubated for 18 h. After recovery in complete medium for 8 to 10 h, the cells were incubated with [3H]thymidine (1 μCi/well) for 10 h. Cells were then washed with ice-cold PBS extensively and treated with 5% trichloroacetic acid for 30 min on ice. After further washes with ice-cold PBS, cells were solubilized in 0.5 N NaOH-0.5% (wt/vol) SDS and harvested, and the amount of incorporated radiolabel was determined with a scintillation counter.
RESULTS
Genotoxic stress induces RPA-nucleolin complex formation.
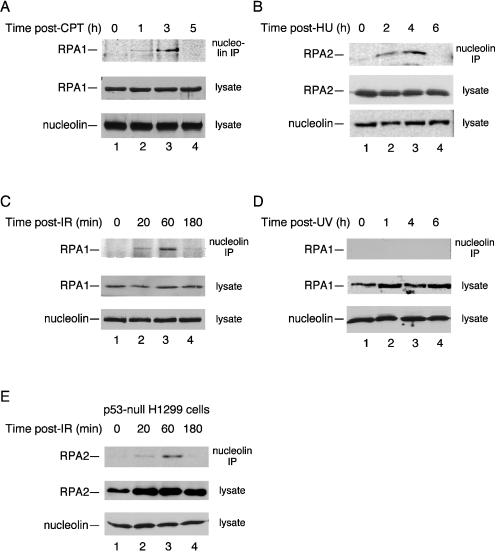
We previously showed that heat shock led to a significant increase in complex formation between endogenous nucleolin and RPA (13). We therefore determined whether this increase was specific for heat shock or a more general effect in response to stress. Human U2-OS osteosarcoma cells were treated with the radiomimetic agent camptothecin (CPT) to cause genotoxic stress (Fig. 1A). Although RPA-nucleolin complex formation was not found in control cells, these complexes were readily detected after CPT treatment with a transient increase in the level of complex formation noted. We estimate that ∼5 to 10% of the RPA pool is coimmunoprecipitated with nucleolin at the peak level of complex formation, although this value would be an underestimate if the complex were transient or unstable under immunoprecipitation conditions. A similar induction of nucleolin-RPA complex formation was observed after treatment with hydroxyurea (to cause replicative stress; Fig. 1B) or exposure to ionizing radiation (10 Gy; Fig. 1C). Nucleolin was not seen to form a complex with RPA after exposure to UV radiation (Fig. 1D) similar to previous observations finding a lack of induced nucleolin-p53 complex and nucleolin relocalization after UV irradiation (14). Induction of nucleolin-RPA complex formation was observed in p53-null H1299 cells after CPT treatment (Fig. 1E). Therefore, although nucleolin relocalization from the nucleolus to the nucleoplasm is p53 dependent (14), this dependence does not extend to nucleolin-RPA complex formation. Note that previous studies from our laboratory indicated that complex formation is not mediated by the presence of DNA and can also be detected by precipitation of RPA rather than nucleolin (13). In general, enhanced nucleolin-RPA complex formation is not restricted to heat shock but is also detected after genotoxic stress.
FIG. 1.
Nucleolin-RPA complex formation is induced after genotoxic stress. Cell lysates were prepared from p53-positive U2-OS cells (A to D) and p53-null H1299 cells (E) at various times after exposure to various stress treatments as follows: 1 μM CPT for 1 h (A), 2.5 mM hydroxyurea (HU) for 1 h (B), 10 Gy of ionizing radiation (IR) (C and E), and UV irradiation with a single dose of 30 J m−2 (D). After each time point, nucleolin was immunoprecipitated from the lysate with a mouse monoclonal antibody to nucleolin. The precipitate was subjected to SDS-PAGE and immunoblotted for RPA with either an anti-RPA1 or anti-RPA2 antibody (as indicated). As loading controls, aliquots of the lysates were subjected to immunoblotting with anti-nucleolin, anti-RPA1, or anti-RPA2 antibodies.
RPA interacts with the nucleolin GAR domain in vitro.
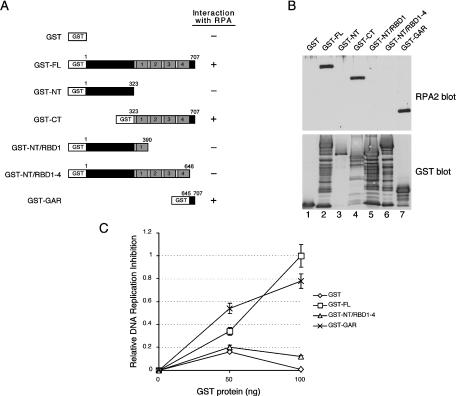
To better characterize the nucleolin-RPA complex, the region on human nucleolin that interacts with RPA was identified by far-Western analysis. Full-length nucleolin or nucleolin truncation mutants were expressed as GST-tagged fusion proteins in yeast and purified. The proteins tested were full-length nucleolin (termed nucleolin FL; aa 1 to 707), the nucleolin N terminus (NT; aa 1 to 323), the C terminus (CT; aa 323 to 707), the N terminus and the first RBD (NT/RBD1; aa 1 to 390), the N terminus and the RBD region (NT/RBD1-4; aa 1 to 648), and the extreme C-terminal GAR domain (GAR; aa 645 to 707) (Fig. 2A). Note that the NT/RBD1-4 construct lacks only the GAR region. After SDS-PAGE and transfer to a nitrocellulose membrane, the immobilized proteins were renatured and incubated with purified RPA to allow for complex formation. The interaction between the fusion proteins and RPA was resolved by Western blotting with an RPA2 antibody (Fig. 2B, upper panel).
FIG. 2.
The nucleolin RPA-binding domain inhibits SV40 DNA replication in vitro. (A) Schematic showing GST-tagged nucleolin and nucleolin mutant proteins as follows: full length (FL), amino terminus (NT), carboxy terminus (CT), amino terminus including the first RBD (NT/RBD1), the GAR deletion mutant (NT/RBD1-4), and only the C-terminal GAR domain (GAR). For each construct, the N-terminal acidic domain is indicated in dark gray; each of the four RBDs have light gray shading and are numbered, and the GAR domain is shown in black. (B) Far-Western analysis of the nucleolin-RPA interaction. Equivalent amounts (500 ng) of nucleolin FL (lane 2), NT (lane 3), CT (lane 4), NT/RBD1 (lane 5), NT/RBD1-4 (lane 6), and GAR (lane 7), with each containing an N-terminal GST tag, were separated by SDS-PAGE. GST alone was also electrophoresed as a control (lane 1). After transfer to a nitrocellulose membrane, the membrane was probed with purified RPA (0.2 μg/ml) (upper panel, lanes 1 to 7). The binding of RPA was visualized by using an RPA2 antibody. To visualize GST-tagged proteins, the membrane was stripped and subjected to immunoblot analysis with a rabbit anti-GST antibody (lower panel, lanes 1 to 7). (C) An SV40 ori-containing plasmid (180 ng) was incubated with AS65 extract (100 μg), T antigen (750 ng), RPA (200 ng), and purified GST-tagged nucleolin proteins (as indicated) for 2 h at 37°C (52). Both FL (□) and GAR (×) GST-tagged nucleolin proteins are proficient in inhibiting SV40 DNA replication in vitro, whereas the NT/RBD1-4 (▵) GST-tagged nucleolin protein and GST alone (⋄) are not. Replication activity was determined by precipitating the reaction mixtures with trichloroacetic acid and determining the amount of 32P in the precipitate by scintillation counting. The data was plotted as the relative DNA replication inhibition compared to that determined by using 100 ng of GST-FL. The maximum degree of inhibition was to 68% that of control levels.
Nucleolin FL formed a complex with RPA (Fig. 2B, upper panel, lane 2), whereas GST alone did not (lane 1). Although no interaction with nucleolin NT was detected (lane 3), RPA bound to the C-terminal half of the protein (lane 4). Longer constructs of nucleolin NT that also contained the first RBD (NT/RBD1; lane 5) or the complete RBD domain (NT/RBD1-4; lane 6) were unable to rescue nucleolin-RPA complex formation. In contrast, RPA effectively bound the 63-aa GAR peptide lacking all other nucleolin domains. Stripping the blot and reprobing the membrane with anti-GST antibodies indicated that similar amounts of each GST fusion protein were loaded on the membrane (Fig. 2B, lower panel). These data demonstrate that the nucleolin GAR domain is necessary and sufficient for RPA binding in vitro. A fraction of each GST construct was invariably present in a degraded form but only the largest FL, CT, or GAR species was observed to bind RPA. The GST constructs are degraded from the C-terminal end because N-terminal deletions would prevent reactivity to the anti-GST antibody (i.e., the GST is located on the N terminus of each construct). We therefore suggest that the extreme C terminus of the GAR domain is required for significant RPA binding.
Effect of the GAR domain on SV40 DNA replication in vitro.
We previously showed that SV40 DNA replication in vitro was inhibited by the addition of nucleolin, purified from human cells, which interfered with RPA action (13). Because our data indicate that the nucleolin GAR domain interacts with RPA, we similarly tested the effect of this peptide on SV40 DNA replication. GST-tagged nucleolin or nucleolin derivatives were purified, and titrated into a T-antigen-dependent SV40 DNA replication reaction (Fig. 2C). In reactions containing nucleolin FL or GAR, DNA synthesis was significantly inhibited as a function of the amount of nucleolin protein added. In contrast, no obvious inhibition was seen by addition of nucleolin NT/RBD1-4 or GST. Thus, nucleolin molecules that are capable of binding RPA also inhibit DNA replication in vitro.
Stress-dependent formation of the nucleolin FL-RPA complex.
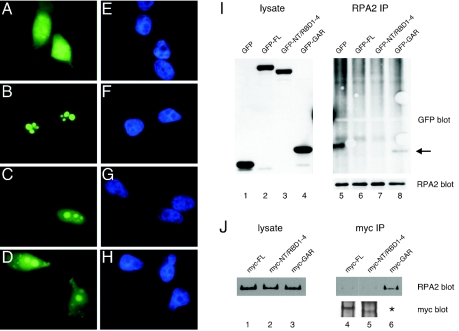
We examined the interaction of nucleolin and the nucleolin mutants with RPA in vivo. Because the cellular localization of nucleolin may be a determinant affecting its interaction with RPA (13), we first examined the localization of the different nucleolin derivatives. GFP-tagged nucleolin FL, NT/RBD1-4, and GAR were expressed in U2-OS cells, and the localization of the fusion proteins captured by indirect immunofluorescence microscopy. Nucleolin FL localized exclusively to nucleolar regions (Fig. 3B), as determined by colocalization with endogenous nucleolin and upstream binding factor (necessary for RNA polymerase I-mediated transcription of rRNA [27]) (data not shown). The NT/RBD1-4 and GAR proteins had primary localization in the nucleolus (Fig. 3C and D, respectively), although the level of nucleolar staining was higher for the NT/RBD1-4 mutant. A significant fraction of each mutant protein pool was located in the nucleoplasm, and both mutants showed a weak but clear cytoplasmic signal. As expected, GFP alone was localized throughout the cell (Fig. 3A). These data are consistent with previous findings that the nucleolin RBD and GAR domains each contribute to nucleolar localization (12, 28, 40, 47).
FIG. 3.
Complex formation between the nucleolin GAR domain and RPA in vivo. (A to H) U2-OS cells were transfected with GFP alone (A and E) or the GFP-tagged nucleolin derivatives FL (B and F), NT/RBD1-4 (C and G), or GAR (D and H). At 24 h posttransfection, cells were fixed by treatment with 4% (wt/vol) formaldehyde for 30 min at room temperature and then imaged by epifluorescence microscopy. The staining patterns of the various GFP constructs are shown (A to D), as are images of the same cells stained with DAPI (4′,6′-diamidino-2-phenylindole) (E to H). (I) Immunoprecipitation of endogenous RPA protein in U2-OS cells expressing GFP-tagged nucleolin FL (lane 6), NT/RBD1-4 (lane 7), or GAR (lane 8) or GFP alone (lane 5). The coprecipitation of the expressed GFP-tagged proteins with RPA is shown in the GFP blot. The arrow points to the coprecipitation of GFP-tagged GAR (lane 8). Corresponding lysates were assayed for similar levels of protein expression by blotting for GFP (lanes 1 to 4), whereas equivalent immunoprecipitation of RPA was verified by blotting for RPA2 (right side, lower panel). (J) Reverse immunoprecipitation experiment showing the coprecipitation of endogenous RPA in U2-OS cells expressing Myc-tagged nucleolin FL (lane 4), NT/RBD1-4 (lane 5), and GAR (lane 6). Myc-tagged nucleolin proteins were immunoprecipitated, and coprecipitation of RPA was determined by blotting for RPA2 (upper panel). The immunoprecipitated Myc-tagged proteins are also shown (lower panel). The asterisk indicates that the Myc-tagged GAR could not be detected because of its small size (5 kDa), preventing binding to nitrocellulose membrane during the transfer step. However, similar levels of myc staining were observed for the three constructs when transfected cells were examined by immunofluorescence microscopy (data not shown). The lysates were also blotted for RPA2 as a control (lanes 1 to 3).
The ability of various GFP-tagged nucleolin proteins to associate with endogenous RPA in vivo was tested by coimmunoprecipitation assays. RPA coprecipitated with the GAR domain but did not associate with the NT/RBD1-4 mutant (Fig. 3I, lanes 8 and 7, respectively). Nucleolin FL did not significantly complex with RPA under these nonstress conditions (lane 6) although, because of the higher background in the upper regions of the blot, we cannot rule out a low level of complex formation. To rule out the possibility that the large GFP moiety may sterically block nucleolin complex formation with RPA, reverse immunoprecipitation experiments were repeated with nucleolin tagged with a smaller Myc tag (Fig. 3J). Test of the Myc-tagged nucleolin proteins showed that only nucleolin GAR formed detectable complexes with endogenous RPA (lane 6), whereas nucleolin FL (lane 4) and NT/RBD1-4 (lane 5) did not. Note that detection of Myc-GAR in cell lysates by Western blotting was problematic because of poor association of this 5-kDa species with the nitrocellulose membrane. However, the levels of Myc-tagged nucleolin FL, NT/RBD1-4, and GAR were comparable when examined in parallel experiments by immunofluorescence microscopy, and their cellular localizations were similar to those observed for the analogous GFP fusion proteins (data not shown). In sum, these data indicate that the nucleolin GAR domain is sufficient to support complex formation with RPA in vivo. Concerning the lack of association between nucleolin FL and RPA in vivo, although in apparent contradiction with the results of the Far Western analysis in vitro (above), these results are consistent with those showing a lack of complex formation between endogenous nucleolin and RPA in nonstressed cells (see Fig. 1, zero time points).
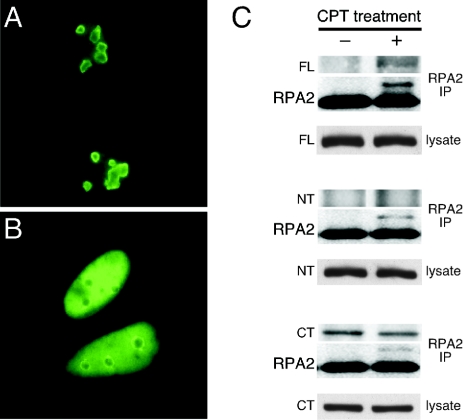
We next examined the effect of CPT treatment on GFP-tagged nucleolin FL localization and on the interaction of RPA with nucleolin FL and the nucleolin derivatives. Although nucleolin FL localized to the nucleolus in the absence of stress (Fig. 4A [see also Fig. 3B above]), incubation with CPT caused a significant fraction of the nucleolin FL pool to move to the nucleoplasm (Fig. 4B), similar to the behavior of endogenous nucleolin (13). In testing the interactions, RPA was observed to associate with nucleolin FL but only after CPT treatment (Fig. 4C, upper panel). In contrast, the NT construct lacking the GAR domain did not form a detectable complex with RPA irrespective of stress (Fig. 4C, middle panel). The CT construct that contains the GAR domain coprecipitated with RPA both in the presence of CPT and in its absence, thus revealing a constitutive interaction (Fig. 4C, lower panel). The localizations of these truncated proteins were not affected by prior CPT treatment (data not shown). These data indicate that although the presence of the GAR is necessary to support detectable complex formation with RPA in vivo, detectable interaction of RPA with the full-length nucleolin also requires stress conditions such as caused by CPT treatment.
FIG. 4.
Complex formation between nucleolin FL and endogenous RPA stimulated by genotoxic stress. (A and B) The cellular localization of GFP-tagged nucleolin FL expressed in U2-OS cells is shown in the absence of CPT treatment (A) and after treatment with 1 μM CPT for 1 h and a 1-h recovery period (B). (C) Immunoprecipitation of endogenous RPA protein with RPA2 antibody in U2-OS cells expressing GFP-tagged nucleolin FL (top set), nucleolin NT (second set), or nucleolin CT (third set). Coprecipitation of the nucleolin proteins was examined in the absence of CPT treatment (−) and 2 h after treatment with 1 μM CPT for 1 h (+) (upper panels). The same blot was reprobed with RPA2 antibody as a control for RPA immunoprecipitation (middle panels). Lysates were assayed for equivalent expression of GFP fusion proteins by probing with an anti-GFP antibody (lower panels).
Nucleolin TM is able to mimic endogenous nucleolin under conditions of stress.
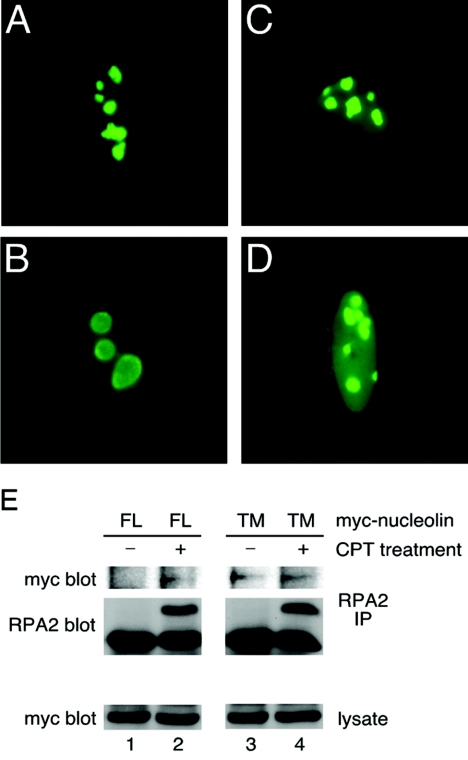
To examine the question of whether nucleolin localization regulates nucleolin-RPA complex formation in vivo, we generated a nucleolin mutant with altered cellular localization. Preliminary studies by our laboratory indicate that the nucleolin phosphorylation pattern at CK2 sites changes in response to stress (K. Kim, M. Daras, and J. A. Borowiec, unpublished data). The three putative CK2 sites at positions S33, S187, and S209 were therefore converted to nonphosphorylatable alanines to generate nucleolin TM (for triple mutant. The localization of GFP-tagged nucleolin TM was examined in untreated U2-OS cells or in cells treated with CPT. Interestingly, nucleolin TM was found to have significant localization in the nucleoplasm in the absence of DNA damage (Fig. 5C) and resembled the localization of nucleolin FL in cells treated with CPT (Fig. 5B). After exposure of the cells to CPT, nucleolin TM was seen to have an even greater fraction of signal arising from the nucleoplasm (Fig. 5D). In testing interactions, the coprecipitation of nucleolin TM with RPA was found to be constitutive and independent of prior CPT treatment (Fig. 5E, lanes 3 and 4), in contrast to nucleolin FL (lanes 1 and 2). Therefore, a nucleolin mutant with a significant degree of nucleoplasmic localization in nonstressed cells also has a constitutive interaction with RPA.
FIG. 5.
The nucleolin TM mutant constitutively interacts with endogenous RPA. The subcellular localization of nucleolin FL (A and B) and nucleolin TM (C and D) in U2-OS cells was determined. At 24 h posttransfection, cells were either mock treated (A and C) or examined 2 h after treatment with 1 μM CPT for 1 h (B and D). Cells were prepared for epifluorescence microscopy as described in Materials and Methods. (E) The coprecipitation of Myc-tagged nucleolin FL and TM with endogenous RPA in U2-OS cells was examined 24 h posttransfection either with or without prior CPT treatment (as described above). Endogenous RPA was precipitated with anti-RPA2 antibody, and the coprecipitation of Myc-tagged nucleolin FL or TM was visualized by Western blotting with an anti-Myc antibody (9E10).
Nucleolin-RPA complex formation examined by FRET.
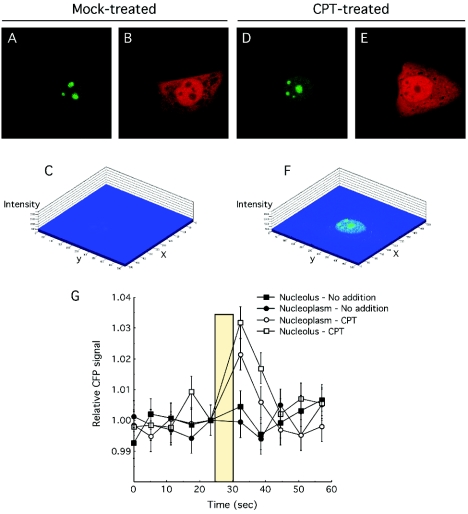
To examine whether a nucleoplasmic localization of nucleolin assists complex formation with RPA, we used FRET to determine the cellular site(s) of interaction. The middle subunit of heterotrimeric RPA (RPA2) was expressed as a YFP fusion, whereas nucleolin and the nucleolin derivatives were coupled to CFP. Previous studies testing GFP-RPA2 indicate that it behaves similarly to the endogenous RPA2 subunit, including the association with replication centers (50, 52). In cells transfected with both YFP-RPA2 and CFP-nucleolin FL, CPT treatment caused nucleolin relocalization (Fig. 6D) as seen above while having little notable affect on YFP-RPA2 (compare Fig. 6B and E). Although no significant FRET signal was detected in the absence of CPT treatment (Fig. 6C), a robust FRET signal was seen when these same doubly transfected cells were analyzed after CPT treatment (Fig. 6F). Because FRET is subject to artifactual detection due to CFP signal bleedthrough into the YFP channel, we performed acceptor photobleaching in which bleach of the YFP fluorescence stimulates the emission from CFP (Fig. 6G) (33). Cells that were either mock treated or treated with CPT were analyzed by using ROIs located in either the nucleolus or the nucleoplasm, and the average CFP signals from these experiments is shown. Consistent with the FRET images, no significant photobleach-dependent stimulation of the CFP signal was observed either in the nucleolus or the nucleoplasm without CPT. When cells were treated with CPT, a robust increase in the CFP signal was detected in the nucleoplasm and the nucleolus after photobleaching. These FRET signals were quantitated and normalized against that found by nucleolin FL and RPA in the nucleoplasm after CPT treatment (Table 1).
FIG. 6.
Nucleolin FL-RPA complex formation occurs both in the nucleolus and in the nucleoplasm after stress. (A to F) U2-OS cells were transfected with CFP-nucleolin and YFP-RPA2 and either mock treated or treated with 1 μM CPT for 1.5 h prior to imaging. Cells were then imaged to capture the CFP-nucleolin signal (A and D), the YFP-RPA2 signal (B and E), or the FRET signal obtained by transfer of the CFP emission energy to YFP (C and F). The FRET images are shown with a pseudo three-dimensional display with the intensity of staining given on the z axis. (G) Acceptor photobleaching analysis of nucleolin-RPA complex formation. The CFP signal from various (ca. 10 to 15) ROIs was determined at 6-s intervals. After the fifth scan, the YFP fluor was photobleached at 514 nm with an average bleach time of 5 s. An increase in the CFP after photobleaching of the YFP signal is indicative of bona fide FRET (33).
TABLE 1.
Effect of stress and cellular localization on FRET intensity
| Constructs transfecteda | Location | Stress status | Relative increase in CFP signal after addition of YFP bleach (%) |
|---|---|---|---|
| Nucleolin FL/RPA2 | Nucleoplasm | None | <15 |
| Nucleolin FL/RPA2 | Nucleolus | None | <15 |
| Nucleolin FL/RPA2 | Nucleoplasm | CPT | 100 |
| Nucleolin FL/RPA2 | Nucleolus | CPT | 68 |
| Nucleolin FL/RPA2 | Nucleoplasm | HS | 164 |
| Nucleolin FL/RPA2 | Nucleolus | HS | <15 |
| Nucleolin GAR/RPA2 | Nucleoplasm | None | 128 |
| Nucleolin GAR/RPA2 | Nucleolus | None | 101 |
| Nucleolin GAR/RPA2 | Nucleoplasm | CPT | 91 |
| Nucleolin GAR/RPA2 | Nucleolus | CPT | 89 |
| Nucleolin TM/RPA2 | Nucleoplasm | None | 134 |
| Nucleolin TM/RPA2 | Nucleolus | None | 170 |
| Nucleolin TM/RPA2 | Nucleoplasm | CPT | 163 |
| Nucleolin TM/RPA2 | Nucleolus | CPT | 150 |
| Nucleolin RBD/RPA2 | Nucleoplasm or nucleolus | None | <15 |
| Nucleolin RBD/RPA2 | Nucleoplasm or nucleolus | CPT | <15 |
| Nucleolin FL only | Nucleoplasm or nucleolus | CPT | <15 |
| H-Ras61L/RPA2 | Nucleoplasm or nucleolus | None | <15 |
| H-Ras61L/Ras-binding domain | Cytoplasm | None | 42 |
Acceptor photobleaching analyses were carried out on U2-OS cells transfected with various expression constructs. As indicated, cells were either mock treated, stressed with 1 μM CPT for 1.5 h, or subjected to a 44°C heat shock (HS) for 15 min prior to analysis. The YFP in each examined ROI was subjected to photobleaching, and the change in intensity of the CFP signal was quantitated. After the averaging of data from >10 ROI for each condition, these data were normalized against the increase in CFP signal detected for CFP-nucleolin FL and YFP-RPA2 in the nucleoplasm after CPT treatment. All nucleolin derivatives and H-Ras61L were expressed with N-terminal CFP tags; RPA2 contained an N-terminal YFP tag, whereas the Ras-binding domain was tagged with YFP on C terminus.
We next performed similar FRET analyses with the nucleolin GAR and NT/RBD1-4 domains. Both nonstressed cells and cells treated with CPT were examined. The average normalized change in the CFP signal after YFP photobleaching is provided (Table 1). From these data, we found that the GAR domain of nucleolin interacts with RPA irrespective of the presence of CPT and equally well in the nucleolus and the nucleoplasm. Similarly, the nucleolin TM mutant showed a very strong FRET signal in both the nucleolus and the nucleoplasm in a CPT-independent fashion. In contrast, the NT-RBD construct was not found to interact with RPA in either the nucleolus or the nucleoplasm and without apparent effect from the CPT. We note that these latter data are subject to the standard concerns of false-negative FRET results due to potential improper orientation of the two fluorescent tags. As a positive control, we show a significant FRET signal from the H-Ras 61L with the Ras-binding domain of Raf1 in the cytoplasm (4). As expected, no FRET signal arises from cells expressing the H-Ras 61L and RPA2 or in cells expressing CFP-nucleolin alone. We also show that heat shock induces a stronger FRET signal compared to CPT treatment, which parallels our previous immunoprecipitation findings that heat shock also greatly stimulated the nucleolin-RPA complex formation (13). It is interesting that heat shock also has a much greater effect on chromosomal DNA replication (an approximately 70 to 85% reduction [see, for example, references 13 and 55) compared to genotoxic stress (an approximately 50% reduction [see, for example, reference 42]). In sum, along with confirming that the nucleolin FL-RPA interaction is stress dependent, these data also indicate that nucleolin-RPA complex formation is stimulated in both the nucleolus and the nucleoplasm after genotoxic stress.
Cell cycle arrest upon overexpression with either nucleolin GAR or TM.
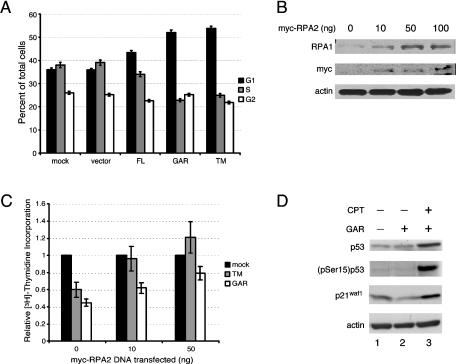
We found previously that heat shock mobilizes nucleolin to move to the nucleoplasm, whereupon it binds RPA at sites distinct from the DNA replication centers (13). These data predict that expression of nucleolin derivatives that bind RPA in nonstressed cells will cause a G1/S arrest. The effect of nucleolin TM and GAR expression on cell cycle progression were therefore investigated by FACS. Both the nontransfected control and the vector control showed a similar distribution, indicating that transfection alone did not inhibit cell cycle transit (Fig. 7A). Expression of nucleolin FL led to only a slight increase in G1-phase cells. However, much more significant effects were observed in cells transfected with nucleolin TM or GAR. The expression of nucleolin GAR resulted in an increase in the G1 population to 52% of cells compared to 36% of vector-transfected cells. We also detected a decrease in S-phase cells from 39% in vector-transfected cells to 23% in GAR-transfected cells. Expression of nucleolin TM had a similar influence on cell cycle progression with G1- and S-phase cells contributing 53 and 25%, respectively, of the total cell pool. In each case, the fraction of G2 cells remained constant. Thus, the expression of nucleolin GAR or TM was sufficient to elicit an arrest in the cell cycle, leading to the accumulation of cells in G1 and a decrease in cells in S phase. We note that the overall degree of replication inhibition in the GAR- or TM-transfected cells (a >40% decrease) is similar to that observed after ionizing irradiation (42).
FIG. 7.
RPA overexpression rescues the inhibition of DNA replication caused by nucleolin GAR or TM. (A) Cell cycle distribution of U2-OS cells transfected with nucleolin and nucleolin mutants. FACS analysis was performed 24 h after transfection with N-terminal Myc-tagged nucleolin constructs. The DNA content was quantitated by using the DNA intercalating agent 7-aminoactinomycin D, and cells in S phase were identified by determining BrdU incorporation. (B) Overexpression of myc-tagged RPA2 leads to a corresponding increase in the level of RPA1. Lysates from U2-OS cells transfected with various amounts of myc-tagged RPA2 (10, 50, and 100 ng) were analyzed by Western blotting for the level of RPA1 (first panel). Myc-tagged RPA2 expression (second panel) and β-actin (third panel) are shown as transfection and loading controls, respectively. (C) [3H]thymidine incorporation assay shows that expression of Myc-tagged RPA2 (leading to higher levels of RPA) can rescue the reduction in DNA synthesis caused by expression of nucleolin TM or nucleolin GAR. Each set of U2-OS cells (mock transfected, nucleolin TM transfected, and nucleolin GAR transfected) were cotransfected with 0, 10, or 50 ng of Myc-tagged RPA2. The data are plotted showing the relative amounts of [3H]thymidine incorporation compared to the mock-treated cells at each level of Myc-RPA2 transfected. Expression of Myc-RPA2 alone slightly inhibited cellular DNA synthesis with transfection of 10 ng of the expression construct causing a 16% reduction in [3H]thymidine incorporation. This resulted from inhibitory effects of pEF6/Myc vector transfection rather than expression of Myc-RPA2 per se (data not shown). (D) The nucleolin-mediated checkpoint response does not involve p53 activation. Lysates from U2-OS cells expressing GFP-tagged nucleolin GAR (lanes 2 and 3) or GFP alone (lane 1) were examined after mock treatment (lanes 1 and 2) or 2 h posttreatment with 1 μM CPT for 1 h (lane 3). Lysates were analyzed by Western blotting for total p53 levels (top panel), p53 phosphorylation at serine 15 (second panel), p21waf1, and the loading control, β-actin.
DNA replication inhibition overcome by overexpression of RPA2.
If the inhibition of DNA replication by nucleolin GAR or TM were truly mediated through RPA, then overexpression of heterotrimeric RPA might overcome this inhibition of DNA synthesis. A method of increasing RPA levels arose from our finding that changes in RPA2 levels have coordinate effects on the level of the RPA1 subunit. That is, a decrease in RPA2 levels due to the use of RNAi leads to a corresponding decrease in RPA1 levels (D. Curanovic and J. A. Borowiec, unpublished results), a finding also recently reported by others (16). Similarly, overexpression of Myc-RPA2 caused a parallel increase in the level of RPA1 protein, when examined either by Western blotting (Fig. 7B) or immunofluorescence microscopy (data not shown). Since stable association of the RPA1 and RPA2 subunits requires the smallest RPA3 subunit (which available antibodies only poorly detect), these data indicate that changes in the level of RPA2 regulate the level of RPA in cells. Overexpression of RPA2 thereby provides a method to more directly examine the nucleolin-RPA interplay in inhibiting chromosomal DNA replication.
To test this method, U2-OS cells were transfected with either nucleolin GAR or TM, and the level of DNA replication was measured by determining thymidine incorporation (Fig. 7C). Corroborating the results of the FACS analysis, expression of either nucleolin construct inhibited DNA synthesis by ca. 50%. In parallel reactions, these cells were cotransfected with increasing levels of RPA2. We observed that the degree of replication inhibition caused either by nucleolin GAR or TM expression was progressively reduced by transfection of the Myc-RPA2 expression vector. The stimulatory effect of RPA2 overexpression was somewhat more pronounced in nucleolin TM-transfected cells compared to GAR-transfected cells, for unknown reasons. Transfecting higher levels of RPA2 vector (i.e., 100 ng) caused some toxic effects on cell viability (data not shown). These data strongly indicate that nucleolin can inhibit DNA synthesis by direct interaction with RPA.
Nucleolin GAR expression does not activate p53.
It is possible that the expression of the GAR domain causes a cellular stress response and therefore only inhibits cell cycle progression indirectly. As a test of this possibility, we examined the effect of GAR expression on p53 activation in U2-OS cells (which express wild-type p53). Expression of nucleolin GAR did not increase p53 levels (Fig. 7D, upper panel) or the level of p53 phosphorylated on Ser15, a site modified by the ATM and ATR kinases in response to genotoxic stress (49, 51). The lack of Ser15 phosphorylation demonstrates that p53 and, indirectly, ATM and ATR do not become activated in response to GAR expression (Fig. 7D, second panel). Aliquots of these lysates were probed for the presence of p21waf1, a key stress-induced inhibitor of cyclin-dependent kinases whose expression results in a G1/S arrest. No changes in the level of p21waf1 were detected in response to GAR expression (Fig. 7D, third panel). In contrast, treatment of cells with CPT was found to simultaneously stimulate the levels of p53, (pSer15)p53, and p21waf1. The block in cell cycle progression caused by expression of nucleolin GAR is therefore unrelated to p53 activation, induction of p21, or, likely, activation of ATM or ATR. Instead, our data indicate that nucleolin can itself inhibit DNA replication by binding to RPA and inhibiting RPA activity.
DISCUSSION
In response to genotoxic insult and other stress conditions, eukaryotic cells in S phase use multiple mechanisms to reduce the level of ongoing DNA replication and thereby minimize the detrimental repercussions to the genome. Certain stress response pathways inhibit S-phase kinase complexes Cdk2/cyclin E (10, 19) and Cdc7/Dbf4 (11) whose activities are necessary to allow an origin of replication to fire. Another route apparently mediating the S-phase checkpoint targets the Mre11 recombinational DNA repair complex (43). In contrast, the pathway that we identify involves the inhibition of an essential DNA replication factor, RPA, by stress-dependent complex formation with nucleolin. In this pathway, our data indicate that nucleolin becomes activated in response to stress, leading to heightened complex formation in both the nucleolus and the nucleoplasm. This induced nucleolin-RPA complex can block cellular transit through the G1/S boundary and inhibit DNA replication during S phase. Furthermore, the nucleolin-mediated inhibition can be diminished by heightened expression of RPA.
What is the mechanism by which nucleolin inhibits cell cycle progression? We find that GST-tagged nucleolin FL and the GAR domain each can inhibit SV40 DNA replication in vitro, recapitulating similar inhibitory effects that were observed when endogenous nucleolin purified from human cells was tested (13). Although our studies did not find an inhibitory effect of nucleolin on RPA binding to single-stranded DNA, we do find that nucleolin can inhibit the binding of RPA to duplex molecules containing a central nonpaired region (data not shown). Such data suggest that the nucleolin-RPA complex is selectively inhibitory to the initiation stages of replication. However, we have recently presented data indicating that RPA does not randomly bind to single-stranded DNA at a chromosomal DNA replication fork but is instead actively loaded by a component of the replication machinery (52). Thus, complex formation with nucleolin has the potential to prevent RPA from productive loading onto single-stranded DNA at a replication fork in vivo. Both of these processes could inhibit DNA replication in vivo and cause a reduction in DNA synthesis. Overall, our data indicate that a direct interaction between the GAR domain of nucleolin and RPA is sufficient for replication inhibition in vivo.
Our data lead to the model that genotoxic stress activates nucleolin, such that the GAR domain becomes exposed for complex formation with RPA. In support of this model, consider the following data. First, although the GAR domain is required to bind RPA, nucleolin FL also requires stress conditions to bind RPA. Second, the nucleolin TM molecule that constitutively binds RPA was mutated at three N-terminal positions, whereas the RPA-interacting GAR domain is located on the extreme C terminus of nucleolin. Third, nucleolin relocalization to the nucleoplasm, although an outcome of genotoxic stress and heat shock, is not required for RPA complex formation because our FRET data show interaction in the nucleolus, as well as in the nucleoplasm. Fourth, a requirement for changes in RPA modification does not appear to be required as the GAR domain binds RPA in a stress-independent fashion. Along these same lines, preliminary evidence obtained from test of a hyperphosphorylation mimic of RPA (RPA2D) (52) showed no significant effects on nucleolin complex formation compared to RPA2wt (data not shown). We postulate that changes in nucleolin modification promote conformational changes which remove steric constraints preventing RPA complex formation. Although expression of nucleolin TM or GAR do not cause apparent ATM or ATR activation, it is quite possible that activation of these kinases by genotoxic stress facilitates nucleolin-RPA complex formation, a possibility under investigation.
Our FRET data indicate that nucleolin-RPA complex formation occurs both in the nucleoplasm and in the nucleolus and hence nucleolin relocalization is not required for these two proteins to interact. Even so, nucleolin relocalization probably facilitates interaction with RPA. The nucleolus comprises ca. 10 to 15% of the nuclear volume in human cells (e.g., see reference 18) and a nucleoplasmic localization would provide a larger volume in which complex formation can occur. Although p53 is not required for nucleolin-RPA complex formation, nucleolin relocalization is strongly dependent on p53 (14) (see also below), suggesting that p53 might stimulate the nucleolin-RPA interaction. Testing the ability of p53-positive (U2-OS) and negative (H1299) cells to induce nucleolin-RPA complex formation after stress did not reveal any obvious differences. That said, these cells have genetic differences other than p53 that preclude our drawing firm conclusions on the potential role of p53 in facilitating complex formation at this time.
The mechanism of nucleolin relocalization remains somewhat unclear. Previous study has found that movement of a portion of the nucleolin pool to the nucleoplasm is greatly facilitated by p53 (14). Because genotoxic stress transiently induces nucleolin and p53 complex formation (14), increased nucleoplasmic levels of appropriately modified p53 and nucleolin may lead to more complex formation and hence a net nucleoplasmic flow of nucleolin. The lack of requirement for p53 in supporting nucleolin-RPA complex formation would indicate that an event(s), such as changes to the nucleolin modification state, occurs prior to nucleolin-RPA and nucleolin-p53 complex formation. This event would lead to the apparently independent increase in the association of nucleolin with either p53 or RPA. Along with a p53 requirement in supporting nucleolin mobilization from the nucleolus in response to stress, it has also been recently proposed that p53 activation by stress itself involves nucleolar disruption (ND) (46). In this model, ND interrupts a requisite nucleolar export pathway for p53 destined for degradation. If this model is correct, ND initiates p53 activation, which itself leads to increased ND.
We identified the nucleolin GAR domain as being necessary for interaction with RPA in vitro and in vivo. The GAR domain is contained within ∼63 residues and includes more than 10 RGG or FGG repeats. Similar RGG/FGG repeat sequences are found in other RNA-binding proteins, including hnRNP A1, hnRNP U, and fibrillarin (3). The RGG region forms a β-spiral structure and binds nonspecifically to single- and double-stranded RNA and DNA (21). The GAR domain of nucleolin interacts with various ribosomal subunits, including L3 (22), and, along with its ability to bind RNA, presumably explains the role of the nucleolin GAR domain in supporting efficient nucleolar localization (12, 28, 40, 47). The nucleolin GAR domain also contains a 12-residue unique lysine-rich element at the extreme C terminus. Our far-Western analysis indicates that nucleolin molecules with small C-terminal deletions do not support RPA binding, suggesting that RPA may bind this unique C-terminal end. Additional studies will be needed to determine the relative contributions of the RGG region and the unique element in supporting complex formation with RPA.
It is becoming clear that the nucleolus is a critical cellular body whose components regulate cell cycle progression. For example, p19ARF (p14ARF in humans) localizes to the nucleolus, where it can bind and sequester the p53 antagonist MDM2 and thereby cause p53 stabilization (56). Similarly, the binding of the human MDM2 RING domain to ATP stimulates nucleolar localization in the absence of p14ARF (44). The yeast Yph1p protein is a BRCT domain-containing nucleolar factor whose depletion causes both G1 and G2 arrest (17). With regard to mitotic progression, it has been found that exit from mitosis is controlled by the Cdc14 protein phosphatase that is sequestered in the nucleolus until anaphase (2). These and other observations, combined with our findings that nucleolus also serves a dual role in ribosome biogenesis and inhibiting S-phase progression in response to genotoxic stress, highlights the importance of the nucleolus in serving to integrate cell growth and cell stress pathways.
Acknowledgments
We thank John Hirsch for assistance with FACS analysis, Eric Rubin (UMDNJ) for providing the GST-nucleolin expression vectors, Cristina Cardoso for the pENeGFP RPA34 plasmid, and Trever Bivona and Mark Philips (NYU School of Medicine) for kindly providing the H-Ras and Ras-binding-domain constructs and other reagents and for their invaluable advice on FRET. We also thank Angus Wilson for insightful comments on our studies and Vitaly Vassin for helpful discussions.
This study was supported by NIH grant AI29963, DOD Breast Cancer Research Program DAMD17-03-1-0299, Philip Morris grant 15-B0001-42171, the NYU Cancer Institute, and the Rita J. and Stanley Kaplan Comprehensive Cancer Center (NCI P30CA16087). Purchase of the confocal microscope was funded by the Shared Instrumentation Grant Program of the NIH (S10 RR017970).
REFERENCES
- 1.Araujo, S. J., and R. D. Wood. 1999. Protein complexes in nucleotide excision repair. Mutat. Res. 435:23-33. [DOI] [PubMed] [Google Scholar]
- 2.Bembenek, J., and H. Yu. 2003. Regulation of CDC14: pathways and checkpoints of mitotic exit. Front. Biosci. 8:d1275-d1287. [DOI] [PubMed] [Google Scholar]
- 3.Biamonti, G., and S. Riva. 1994. New insights into the auxiliary domains of eukaryotic RNA binding proteins. FEBS Lett. 340:1-8. [DOI] [PubMed] [Google Scholar]
- 4.Bivona, T. G., I. Perez De Castro, I. M. Ahearn, T. M. Grana, V. K. Chiu, P. J. Lockyer, P. J. Cullen, A. Pellicer, A. D. Cox, and M. R. Philips. 2003. Phospholipase Cγ activates Ras on the Golgi apparatus by means of RasGRP1. Nature 424:694-698. [DOI] [PubMed] [Google Scholar]
- 5.Block, W. D., Y. Yu, and S. P. Lees-Miller. 2004. Phosphatidyl inositol 3-kinase-like serine/threonine protein kinases (PIKKs) are required for DNA damage-induced phosphorylation of the 32-kDa subunit of replication protein A at threonine 21. Nucleic Acids Res. 32:997-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brill, S. J., and B. Stillman. 1989. Yeast replication factor-A functions in the unwinding of the SV40 origin of replication. Nature 342:92-95. [DOI] [PubMed] [Google Scholar]
- 7.Callebaut, C., J. Blanco, N. Benkirane, B. Krust, E. Jacotot, G. Guichard, N. Seddiki, J. Svab, E. Dam, S. Muller, J. P. Briand, and A. G. Hovanessian. 1998. Identification of V3 loop-binding proteins as potential receptors implicated in the binding of HIV particles to CD4+ cells. J. Biol. Chem. 273:21988-21997. [DOI] [PubMed] [Google Scholar]
- 8.Carty, M. P., M. Zernik-Kobak, S. McGrath, and K. Dixon. 1994. UV light-induced DNA synthesis arrest in HeLa cells is associated with changes in phosphorylation of human single-stranded DNA-binding protein. EMBO J. 13:2114-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, C. Y., R. Gherzi, J. S. Andersen, G. Gaietta, K. Jurchott, H. D. Royer, M. Mann, and M. Karin. 2000. Nucleolin and YB-1 are required for JNK-mediated interleukin-2 mRNA stabilization during T-cell activation. Genes Dev. 14:1236-1248. [PMC free article] [PubMed] [Google Scholar]
- 10.Costanzo, V., K. Robertson, C. Y. Ying, E. Kim, E. Avvedimento, M. Gottesman, D. Grieco, and J. Gautier. 2000. Reconstitution of an ATM-dependent checkpoint that inhibits chromosomal DNA replication following DNA damage. Mol. Cell 6:649-659. [DOI] [PubMed] [Google Scholar]
- 11.Costanzo, V., D. Shechter, P. J. Lupardus, K. A. Cimprich, M. Gottesman, and J. Gautier. 2003. An ATR- and Cdc7-dependent DNA damage checkpoint that inhibits initiation of DNA replication. Mol. Cell 11:203-213. [DOI] [PubMed] [Google Scholar]
- 12.Creancier, L., H. Prats, C. Zanibellato, F. Amalric, and B. Bugler. 1993. Determination of the functional domains involved in nucleolar targeting of nucleolin. Mol. Biol. Cell 4:1239-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daniely, Y., and J. A. Borowiec. 2000. Formation of a complex between nucleolin and replication protein A after cell stress prevents initiation of DNA replication. J. Cell Biol. 149:799-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniely, Y., D. D. Dimitrova, and J. A. Borowiec. 2002. Stress-dependent nucleolin mobilization mediated by p53-nucleolin complex formation. Mol. Cell. Biol. 22:6014-6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dimitrova, D. S., and D. M. Gilbert. 2000. Stability and nuclear distribution of mammalian replication protein A heterotrimeric complex. Exp. Cell Res. 254:321-327. [DOI] [PubMed] [Google Scholar]
- 16.Dodson, G. E., Y. Shi, and R. S. Tibbetts. 2004. DNA replication defects, spontaneous DNA damage, and ATM-dependent checkpoint activation in replication protein A-deficient cells. J. Biol. Chem. 279:34010-34014. [DOI] [PubMed] [Google Scholar]
- 17.Du, Y. C., and B. Stillman. 2002. Yph1p, an ORC-interacting protein: potential links between cell proliferation control, DNA replication, and ribosome biogenesis. Cell 109:835-848. [DOI] [PubMed] [Google Scholar]
- 18.Elias, E., N. Lalun, M. Lorenzato, L. Blache, P. Chelidze, M. F. O'Donohue, D. Ploton, and H. Bobichon. 2003. Cell-cycle-dependent three-dimensional redistribution of nuclear proteins, P 120, pKi-67, and SC 35 splicing factor, in the presence of the topoisomerase I inhibitor camptothecin. Exp. Cell Res. 291:176-188. [DOI] [PubMed] [Google Scholar]
- 19.Falck, J., N. Mailand, R. G. Syljuasen, J. Bartek, and J. Lukas. 2001. The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature 410:842-847. [DOI] [PubMed] [Google Scholar]
- 20.Gabellini, D., M. R. Green, and R. Tupler. 2002. Inappropriate gene activation in FSHD: a repressor complex binds a chromosomal repeat deleted in dystrophic muscle. Cell 110:339-348. [DOI] [PubMed] [Google Scholar]
- 21.Ghisolfi, L., G. Joseph, F. Amalric, and M. Erard. 1992. The glycine-rich domain of nucleolin has an unusual supersecondary structure responsible for its RNA-helix-destabilizing properties. J. Biol. Chem. 267:2955-2959. [PubMed] [Google Scholar]
- 22.Ginisty, H., H. Sicard, B. Roger, and P. Bouvet. 1999. Structure and functions of nucleolin. J. Cell Sci. 112:761-772. [DOI] [PubMed] [Google Scholar]
- 23.Grinstein, E., P. Wernet, P. J. Snijders, F. Rosl, I. Weinert, W. Jia, R. Kraft, C. Schewe, M. Schwabe, S. Hauptmann, M. Dietel, C. J. Meijer, and H. D. Royer. 2002. Nucleolin as activator of human papillomavirus type 18 oncogene transcription in cervical cancer. J. Exp. Med. 196:1067-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gulli, M. P., J. P. Girard, D. Zabetakis, B. Lapeyre, T. Melese, and M. Caizergues-Ferrer. 1995. gar2 is a nucleolar protein from Schizosaccharomyces pombe required for 18S rRNA and 40S ribosomal subunit accumulation. Nucleic Acids Res. 23:1912-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haluska, P., Jr., A. Saleem, T. K. Edwards, and E. H. Rubin. 1998. Interaction between the N terminus of human topoisomerase I and SV40 large T antigen. Nucleic Acids Res. 26:1841-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanakahi, L. A., L. A. Dempsey, M. J. Li, and N. Maizels. 1997. Nucleolin is one component of the B cell-specific transcription factor and switch region binding protein, LR1. Proc. Natl. Acad. Sci. USA 94:3605-3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hannan, K. M., R. D. Hannan, and L. I. Rothblum. 1998. Transcription by RNA polymerase I. Front. Biosci. 3:d376-d398. [DOI] [PubMed] [Google Scholar]
- 28.Heine, M. A., M. L. Rankin, and P. J. DiMario. 1993. The Gly/Arg-rich (GAR) domain of Xenopus nucleolin facilitates in vitro nucleic acid binding and in vivo nucleolar localization. Mol. Biol. Cell 4:1189-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henricksen, L. A., C. B. Umbricht, and M. S. Wold. 1994. Recombinant replication protein A: expression, complex formation, and functional characterization. J. Biol. Chem. 269:11121-11132. [PubMed] [Google Scholar]
- 30.Iftode, C., and J. A. Borowiec. 1998. Unwinding of origin-specific structures by human replication protein A occurs in a two-step process. Nucleic Acids Res. 26:5636-5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iftode, C., Y. Daniely, and J. A. Borowiec. 1999. Replication protein A (RPA): the eukaryotic SSB. Crit. Rev. Biochem. Mol. Biol. 34:141-180. [DOI] [PubMed] [Google Scholar]
- 32.Jayaraman, L., N. C. Moorthy, K. G. Murthy, J. L. Manley, M. Bustin, and C. Prives. 1998. High mobility group protein-1 (HMG-1) is a unique activator of p53. Genes Dev. 12:462-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karpova, T. S., C. T. Baumann, L. He, X. Wu, A. Grammer, P. Lipsky, G. L. Hager, and J. G. McNally. 2003. Fluorescence resonance energy transfer from cyan to yellow fluorescent protein detected by acceptor photobleaching using confocal microscopy and a single laser. J. Microsc. 209:56-70. [DOI] [PubMed] [Google Scholar]
- 34.Kondo, K., and M. Inouye. 1992. Yeast NSR1 protein that has structural similarity to mammalian nucleolin is involved in pre-rRNA processing. J. Biol. Chem. 267:16252-16258. [PubMed] [Google Scholar]
- 35.Lee, W. C., D. Zabetakis, and T. Melese. 1992. NSR1 is required for pre-rRNA processing and for the proper maintenance of steady-state levels of ribosomal subunits. Mol. Cell. Biol. 12:3865-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu, J. S., S. R. Kuo, M. M. McHugh, T. A. Beerman, and T. Melendy. 2000. Adozelesin triggers DNA damage response pathways and arrests SV40 DNA replication through replication protein A inactivation. J. Biol. Chem. 275:1391-1397. [DOI] [PubMed] [Google Scholar]
- 37.Liu, J. S., S. R. Kuo, X. Yin, T. A. Beerman, and T. Melendy. 2001. DNA damage by the enediyne C-1027 results in the inhibition of DNA replication by loss of replication protein A function and activation of DNA-dependent protein kinase. Biochemistry 40:14661-14668. [DOI] [PubMed] [Google Scholar]
- 38.Luch, A. 2002. Cell cycle control and cell division: implications for chemically induced carcinogenesis. Chembiochem 3:506-516. [DOI] [PubMed] [Google Scholar]
- 39.Matsumoto, T., T. Eki, and J. Hurwitz. 1990. Studies on the initiation and elongation reactions in the simian virus 40 DNA replication system. Proc. Natl. Acad. Sci. USA 87:9712-9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Messmer, B., and C. Dreyer. 1993. Requirements for nuclear translocation and nucleolar accumulation of nucleolin of Xenopus laevis. Eur. J. Cell Biol. 61:369-382. [PubMed] [Google Scholar]
- 41.Nisole, S., B. Krust, C. Callebaut, G. Guichard, S. Muller, J. P. Briand, and A. G. Hovanessian. 1999. The anti-HIV pseudopeptide HB-19 forms a complex with the cell-surface-expressed nucleolin independent of heparan sulfate proteoglycans. J. Biol. Chem. 274:27875-27884. [DOI] [PubMed] [Google Scholar]
- 42.Painter, R. B., and B. R. Young. 1980. Radiosensitivity in ataxia-telangiectasia: a new explanation. Proc. Natl. Acad. Sci. USA 77:7315-7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petrini, J. H. 2000. The Mre11 complex and ATM: collaborating to navigate S phase. Curr. Opin. Cell Biol. 12:293-296. [DOI] [PubMed] [Google Scholar]
- 44.Poyurovsky, M. V., X. Jacq, C. Ma, O. Karni-Schmidt, P. J. Parker, M. Chalfie, J. L. Manley, and C. Prives. 2003. Nucleotide binding by the Mdm2 RING domain facilitates Arf-independent Mdm2 nucleolar localization. Mol. Cell 12:875-887. [DOI] [PubMed] [Google Scholar]
- 45.Roger, B., A. Moisand, F. Amalric, and P. Bouvet. 2002. Repression of RNA polymerase I transcription by nucleolin is independent of the RNA sequence that is transcribed. J. Biol. Chem. 277:10209-10219. [DOI] [PubMed] [Google Scholar]
- 46.Rubbi, C. P., and J. Milner. 2003. Disruption of the nucleolus mediates stabilization of p53 in response to DNA damage and other stresses. EMBO J. 22:6068-6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmidt-Zachmann, M. S., and E. A. Nigg. 1993. Protein localization to the nucleolus: a search for targeting domains in nucleolin. J. Cell Sci. 105:799-806. [DOI] [PubMed] [Google Scholar]
- 48.Sengupta, T. K., S. Bandyopadhyay, D. J. Fernandes, and E. K. Spicer. 2003. Identification of nucleolin as an AU-rich element binding protein involved in bcl-2 mRNA stabilization. J. Biol. Chem. 279:10855-10863. [DOI] [PubMed] [Google Scholar]
- 49.Siliciano, J. D., C. E. Canman, Y. Taya, K. Sakaguchi, E. Appella, and M. B. Kastan. 1997. DNA damage induces phosphorylation of the amino terminus of p53. Genes Dev. 11:3471-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sporbert, A., A. Gahl, R. Ankerhold, H. Leonhardt, and M. C. Cardoso. 2002. DNA polymerase clamp shows little turnover at established replication sites but sequential de novo assembly at adjacent origin clusters. Mol. Cell 10:1355-1365. [DOI] [PubMed] [Google Scholar]
- 51.Tibbetts, R. S., K. M. Brumbaugh, J. M. Williams, J. N. Sarkaria, W. A. Cliby, S. Y. Shieh, Y. Taya, C. Prives, and R. T. Abraham. 1999. A role for ATR in the DNA damage-induced phosphorylation of p53. Genes Dev. 13:152-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vassin, V. M., M. S. Wold, and J. A. Borowiec. 2004. Replication protein A (RPA) phosphorylation prevents RPA association with replication centers. Mol. Cell. Biol. 24:1930-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang, X., and J. E. Haber. 2004. Role of Saccharomyces single-stranded DNA-binding protein RPA in the strand invasion step of double-strand break repair. PLoS Biol. 2:104-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang, Y., J. Guan, H. Wang, D. Leeper, and G. Iliakis. 2001. Regulation of DNA replication after heat shock by replication protein A-nucleolin interactions. J. Biol. Chem. 276:20579-20588. [DOI] [PubMed] [Google Scholar]
- 55.Wang, Y., A. R. Perrault, and G. Iliakis. 1998. Replication protein A as a potential regulator of DNA replication in cells exposed to hyperthermia. Radiat. Res. 149:284-293. [PubMed] [Google Scholar]
- 56.Weber, J. D., L. J. Taylor, M. F. Roussel, C. J. Sherr, and D. Bar-Sagi. 1999. Nucleolar Arf sequesters Mdm2 and activates p53. Nat. Cell Biol. 1:20-26. [DOI] [PubMed] [Google Scholar]
- 57.Wold, M. S. 1997. Replication protein A: a heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism. Annu. Rev. Biochem. 66:61-92. [DOI] [PubMed] [Google Scholar]
- 58.Ying, G. G., P. Proost, J. van Damme, M. Bruschi, M. Introna, and J. Golay. 2000. Nucleolin, a novel partner for the Myb transcription factor family that regulates their activity. J. Biol. Chem. 275:4152-4158. [DOI] [PubMed] [Google Scholar]
- 59.Zou, L., and S. J. Elledge. 2003. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 300:1542-1548. [DOI] [PubMed] [Google Scholar]
- 60.Zou, L., D. Liu, and S. J. Elledge. 2003. Replication protein A-mediated recruitment and activation of Rad17 complexes. Proc. Natl. Acad. Sci. USA 100:13827-13832. [DOI] [PMC free article] [PubMed] [Google Scholar]