Abstract
Application of the cardiolipin (CL)-specific fluorescent dye 10-N-nonyl-acridine orange has recently revealed CL-rich domains in the septal regions and at the poles of the Bacillus subtilis membrane (F. Kawai, M. Shoda, R. Harashima, Y. Sadaie, H. Hara, and K. Matsumoto, J. Bacteriol. 186:1475-1483, 2004). This finding prompted us to examine the localization of another phospholipid, phosphatidylethanolamine (PE), with the cyclic peptide probe, Ro09-0198 (Ro), that binds specifically to PE. Treatment with biotinylated Ro followed by tetramethyl rhodamine-conjugated streptavidin revealed that PE is localized in the septal membranes of vegetative cells and in the membranes of the polar septum and the engulfment membranes of sporulating cells. When the mutant cells of the strains SDB01 (psd1::neo) and SDB02 (pssA10::spc), which both lack PE, were examined under the same conditions, no fluorescence was observed. The localization of the fluorescence thus evidently reflected the localization of PE-rich domains in the septal membranes. Similar PE-rich domains were observed in the septal regions of the cells of many Bacillus species. In Escherichia coli cells, however, no PE-rich domains were found. Green fluorescent protein fusions to the enzymes that catalyze the committed steps in PE synthesis, phosphatidylserine synthase, and in CL synthesis, CL synthase and phosphatidylglycerophosphate synthase, were localized mainly in the septal membranes in B. subtilis cells. The majority of the lipid synthases were also localized in the septal membranes; this includes 1-acyl-glycerol-3-phosphate acyltransferase, CDP-diacylglycerol synthase, phosphatidylserine decarboxylase, diacylglycerol kinase, glucolipid synthase, and lysylphosphatidylglycerol synthase. These results suggest that phospholipids are produced mostly in the septal membranes and that CL and PE are kept from diffusing out to lateral ones.
The bacterial cell membrane is usually viewed as a matrix in which membrane protein and lipid molecules are homogeneously distributed according to the widely known fluid mosaic model based on the diffusional mobility of membrane lipids (53). However, in recent years, extensive work has shown that bacterial membranes have laterally heterogeneous distribution of lipid molecules (recently reviewed by Dowhan et al. [11]), similar to eukaryotic membranes (13, 32, 37, 52). The existence of heterogeneities in the lateral distribution of phospholipids and glycolipids was first revealed by a photo-cross-linking experiment in the membrane of the gram-positive bacterium Micrococcus luteus (60). The use of lipophilic fluorescent probes has visualized the uneven distribution of lipid domains in mycobacterial membranes (4). In Escherichia coli membranes, application of the lipophilic fluorescent styryl dye FM4-64 has suggested laterally uneven distribution of phospholipids (16). A study with pyrene-labeled phospholipid analogues has supported the heterogeneous distribution, suggesting a segregated distribution of phosphatidylethanolamine (PE) and phosphatidylglycerol (PG) domains in bacterial membranes (59). Staining with the cardiolipin (CL)-specific fluorescent dye 10-N-nonyl-acridine orange (NAO) has provided unequivocal visualization of CL-rich domains in E. coli membranes, which were located mostly in the septal regions and at the poles (33, 34). We have used NAO staining to visualize CL-rich domains in Bacillus subtilis Marburg membranes and have shown that the CL-rich domains are localized clearly in the septal regions and at the poles (22).
Membranes of B. subtilis undergo dynamic rearrangements, which include formation of the polar septa and engulfment and forespore membranes during the sporulation process in addition to the rearrangements that occur during cell division in vegetative growth. CL, in the presence of certain divalent cations, and PE have a propensity to form nonbilayer structures, which may introduce discontinuities in the bilayer membrane structure for dynamic membrane functions such as membrane fusion during cell division, formation of adhesion sites between the outer and the inner membranes, integration of proteins into the membrane, and stabilization of membrane proteins (10). Observations of mutant strains lacking either one of the phospholipids and the impossibility of constructing a viable mutant strain lacking both of these phospholipids (6, 7, 29, 38, 48, 51) suggest that the capacity to form nonbilayer structures in E. coli is required for proper cell function. In B. subtilis, a specific role of CL in the membranes during the sporulation process is suggested by the delay in the development of the spore in mutant cells lacking CL (22). Since B. subtilis mutants lacking PE have no phenotype (30), no specific role for PE has been identified yet. This is probably attributable to the complex lipid composition of B. subtilis membranes; the major lipids consist of the glucolipids (mono-, di-, and triglucosyldiacylglycerol), glycerophosphoglucolipid, and the lysine ester of PG (lysyl-PG), in addition to the common bacterial phospholipids, PG, CL, and PE (8, 15, 30). The majority of the genes of the enzymes responsible for lipid synthesis have been suggested in silico (8, 23), and the verification in vivo is now in progress for the purpose of genetic analysis (Table 1).
TABLE 1.
B. subtilis genes and functions of their products in lipid synthesisa
| Gene | Function |
|---|---|
| gpsA | Glycerol-3-phosphate dehydrogenase (glycerol phosphate synthase) |
| yhdO | Similar to 1-acylglycerol-3-phosphate acyltransferase (product of plsC) |
| cdsA | CDP-diacylglycerol synthetase |
| pgsA | Phosphatidylglycerophosphate synthase |
| clsA (ywnE) | Cardiolipin synthase |
| ywjE | Cardiolipin synthase |
| ywiE | Similar to cardiolipin synthase |
| mprF (yfiX) | Lysylphosphatidylglycerol synthase |
| pssA | Phosphatidylserine synthase |
| psd | Phosphatidylserine decarboxylase |
| dgkA | Diacylglycerol kinase |
| ugtP (ypfP) | UDP-glucose diacylglycerol glucosyltransferase |
The successful localization of CL-rich domains in the membranes in B. subtilis cells (22) and the suggested lateral heterogeneity of lipid molecules in bacterial membranes prompted us to examine the localization of PE with the specific cyclic peptide probe Ro09-0198 (Ro) that has previously been used for the localization of PE at the cleavage furrow of dividing CHO-K1 cells during cytokinesis (13). Here we report that B. subtilis cells contain PE-rich domains in the polar septal membranes and in the engulfment and forespore membranes during the sporulation phase as well as in the membranes of medial septa during the vegetative growth phase. Using green fluorescent protein (GFP) fusions, we demonstrate that the enzymes involved in phospholipid synthesis are also localized in the septal membranes. We suggest further that phospholipids are produced mostly on the septal membranes but that CL and PE are kept from diffusing to lateral membranes.
(A preliminary report of this work was presented at the 75th Annual Meeting of the Genetics Society of Japan, Sendai, Japan, September 2003.)
MATERIALS AND METHODS
Media and bacterial growth.
Luria-Bertani (LB) broth contained 1% tryptone (Difco, Detroit, Mich.), 0.5% yeast extract (Difco), and 1% NaCl. PE-deficient mutant strains of E. coli were grown in LB medium supplemented with 50 mM MgCl2 (48). Sporulation medium (DSM), which contained 0.8% nutrient broth (Difco), 0.1% KCl, 0.025% MgSO4 · 7H2O, 1.0 mM Ca(NO3)2, 10 μM MnCl2, and 1.0 μM FeSO4 (50), was used for cultivation of B. subtilis cells. Synthetic media CI and CII were used for competence development (2). When required, the following supplements were added to the media (per liter); 50 mg of ampicillin (Sigma), 20 mg of neomycin (Wako Pure Chem, Tokyo, Japan), 100 mg of spectinomycin (Sigma), or 0.3 mg of erythromycin (Sigma). The growth of bacteria was monitored by measuring turbidity with a Klett-Summerson photoelectric colorimeter (no. 54 filter). For solid media, 1.5% agar (Difco) was included.
Bacterial strains and plasmids.
B. subtilis Marburg and E. coli K-12 strains and plasmids used in this study are listed in Tables 2 and 3, respectively. Strains of gram-positive bacteria were from H. Saito and from the Institute of Applied Microbiology culture collection. For construction of gfp fusion strains, the following plasmid vectors were used. pSG1729 (26) allows fusions of gfp to the 5′ end of a gene of interest, under the control of Pxyl, and pDHCMGFP (a gift of H. Yamamoto and J. Sekiguchi) allows fusions of gfp to the 3′ end of a gene of interest, under the control of PcitM. Each gene was PCR amplified with a pair of primers designed as follows (Table 4). For construction of a GFP fusion to the N terminus of a gene product, a sense primer that creates an in-frame fusion at the 5′ end of each gene to the 3′-end multicloning site of gfp on pSG1729 and an antisense primer at a position downstream of the termination codon, both with a unique restriction endonuclease recognition sequence, were designed. For construction of a GFP fusion to the C terminus of a gene product, an antisense primer that creates an in-frame fusion, by altering the 3′ end sequence of each gene, to the 5′ end multicloning site of gfp on pDHCMGFP and a sense primer at an upstream position of the initiation codon were designed. pSG1154 (26), which allows fusion of gfp to the 3′ end of a gene of interest under the control of Pxyl, was used to construct ClsA+9-GFP, that is ClsA with a GFP fused to its C terminus through a 9-amino-acid-residue linker. pMm2 (56), which allows fusion of gfp to 3′ end of a gene, was also used to construct strains harboring the fused gene that produces PssA-GFP and ClsA-GFP under the control of its own promoter.
TABLE 2.
Bacterial strains
| Strain | Relevant characteristic(s) | Source and/or reference |
|---|---|---|
| B. subtilis | ||
| 168 | trpC2 | Laboratory stock |
| SDB01 | 168 psd::neo | 30 |
| SDB02 | 168 pssA10::spc | 30 |
| SDB011 | 168 Pspac-cdsA | T. Yamamoto |
| SDB012 | 168 Pspac-yhdO | T. Yamamoto |
| SDB013 | 168 Pspac-mprF/yfiX | Y. Sadaie |
| SDB014 | 168 mprF/yfiX::tet | K. Misawa |
| SDB110 | 168 Pspac-pgsA | H. Takahashi |
| SDB206 | 168 ywiE2::neo ywjE::spc clsA::pMutin4 | 22 |
| SDB1001 | 168 amyE::(PcitM-gfp cat) | This study |
| SDB1006 | 168 amyE::(Pxyl-gfp-pgsA spc) | This study |
| SDB1010 | 168 amyE::(PcitM-pssA-gfp cat) | This study |
| SDB1012 | 168 amyE::(PcitM-gpsA-gfp cat) | This study |
| SDB1014 | 168 amyE::(Pxyl-gfp-psd spc) | This study |
| SDB1017 | 168 amyE::(Pxyl-gfp-mprF spc) | This study |
| SDB1018 | 168 amyE::(Pxyl-gfp-cdsA spc) | This study |
| SDB1019 | 168 amyE::(Pxyl-gfp-yhdO spc) | This study |
| SDB1020 | 168 amyE::(PcitM-ugtP-gfp cat) | This study |
| SDB1021 | 168 amyE::(Pxyl-gfp-ugtP spc) | This study |
| SDB1022 | 168 amyE::(Pxyl-gfp-dgkA spc) | This study |
| SDB1101 | 168 amyE::(Pxyl-gfp-clsA spt) | This study |
| SDB1109 | 168 amyE::(Pxyl-clsA+9-gfp spc) | This study |
| GS95 | 168 gpsA::spc | G. Schujman, 36 |
| KP261 | PY79 ugtP::neo | K. Price, 46 |
| ASK510 | 168 Pspac-ftsZ | K. Asai |
| SDB1010F | 168 amyE::(PcitM-pssA-gfp cat) Pspac-ftsZ | This study |
| SDB1109F | 168 amyE::(Pxyl-clsA+9-gfp spc) Pspac-ftsZ | This study |
| ts1 | 168 ts1(ftsZ1) | K. Asai, 3 |
| SDB1010T | ts1(ftsZ1) amyE::(PcitM-pssA-gfp cat) | This study |
| SDB1109T | ts1(ftsZ1) amyE::(Pxyl-clsA+7-gfp spc) | This study |
| SDB1209 | 168 clsA-gfp ery | This study |
| SDB1220 | 168 pssA-gfp ery | This study |
| E. coli | ||
| MC4100 | F−araD139 Δ(argF-lac)U169 rpsL150 relA1 flbB5301 deoC1 ptsF25 rbsR | Laboratory stock |
| W3110 | F− λ− IN(rrnD-rrnE)1 rph1 | Laboratory stock |
| GN10 | W3110 ΔpssA10::cat | 48 |
| S107 | W3110 pssA1 | S. Nishijima, 39 |
| UE81 | ΔpssA10::cat ParaBAD-pssA | This study |
| DH5 | supE44 hsdR17 recA1 gyrA96 thi-1 relA1 | Laboratory stock |
TABLE 3.
Plasmids
| Plasmid | Relevant characteristics | Source and/or reference or primer pair used |
|---|---|---|
| pDHCMGFP | bla amyE′3 PcitM-gfp cat amyE′5 | H. Yamamoto |
| pPssA-GFP | pDHCMGFP PcitM-pssA-gfp | pssASph-pssAasNhe |
| pGpsA-GFP | pDHCMGFP PcitM-gpsA-gfp | gpsASph-gpsAasNhe |
| pUgtP-GFP | pDHCMGFP PcitM-ugtP-gfp | ugtPSal-ugtPasNhe |
| pSG1154 | bla amyE′3 spc Pxyl-gfp amyE′5 | P. Lewis, 26 |
| pClsA+9-GFP | pSG1154 Pxyl-clsA+9-gfp | clsAKpnh-clsAasClah |
| pSG1729 | bla amyE′3 spc Pxyl-gfp amyE′5 | P. Lewis, 26 |
| pGFP-PgsA | pSG1729 Pxyl-gfp-pgsA | pgsAXho-pgsAasCla |
| pGFP-ClsA | pSG1729 Pxyl-gfp-clsA | clsAClanm-clsAasEconm |
| pGFP-Psd | pSG1729 Pxyl-gfp-psd | psdHind-psdasEco |
| pGFP-YfiX2 | pSG1729 Pxyl-gfp-mprF | yfiXXho-yfiXasEco2 |
| pGFP-CdsA | pSG1729 Pxyl-gfp-cdsA | cdsAXho-cdsAasCla |
| pGFP-YhdO | pSG1729 Pxyl-gfp-yhdO | yhdOXho-yhdOasCla |
| pGFP-UgtP | pSG1729 Pxyl-gfp-ugtP | ugtPBam-ugtPasClaN |
| pGFP-DgkA | pSG1729 Pxyl-gfp-dgkA | dgkAXho-dgkAasEco |
| pMm2 | ery bla lacZ lacI Pspac gfp | K. Asai, 56 |
| pMPssA-GFP | pMm2 ′pssA-gfp | pssASal-pssAasEco |
| pMClsAj-GFP | pMm2 ′clsA-gfp | clsASalj-clsAasEcoj |
TABLE 4.
Oligonucleotide primers for construction of gfp fusion plasmids
The recognition sequence for the restriction endonuclease is underlined, and mismatched bases with respect to the wild-type B. subtilis sequence are in boldface type. The sites of the initiation codon and termination codon of each gene (the first four letters of the primer name correspond to the gene locus symbol) are marked with a box and thick underline, respectively.
PCR amplification was conducted by using the KOD-Plus DNA polymerase PCR system (Toyobo, Tokyo, Japan) and the ProofStart DNA polymerase PCR system (QIAGEN), and gfp fusion plasmids were constructed by inserting each of the amplified products into the vectors. Other DNA manipulation procedures were performed as described previously (49). In each case, the fused gene under the control of the PcitM or Pxyl promoter was integrated into the amyE locus of the chromosome of wild-type or mutant strains.
The functionality of the GFP fusions of the following essential genes of phospholipid synthase was tested by growth complementation experiments. First, the assignment of the coding sequences of pgsA, yhdO, and cdsA for the genes of phosphatidylglycerophosphate synthase, 1-acyl-glycerol-3-phosphate acyltransferase, and CDP-diacylglycerol synthase, respectively, were confirmed by lipid analysis of the exhausted cells of strains harboring a Pspac-controllable allele (H. Takahashi, T. Yamamoto, H. Hara, and K. Matsumoto, unpublished data). When the GFP-PgsA gene (Pxyl-gfp-pgsA) was integrated into the amyE locus of SDB110 (Pspac-pgsA), which requires isopropyl-β-d-thiogalactopyranoside (IPTG, 0.1 mM) for growth, the resultant strain grew well on an LB plate with 0.1% xylose in the absence of IPTG. GFP-PgsA was thus fully functional. When GFP-YhdO (Pxyl-gfp-yhdO) and GFP-CdsA (Pxyl-gfp-cdsA) genes were integrated into the amyE locus of SDB012 (Pspac-yhdO) and SDB011 (Pspac-csdA) strains that require IPTG (0.1 mM) for growth, respectively, the resultant strains grew well on LB plates with 0.1% xylose in the absence of IPTG. Thus, GFP-YhdO and GFP-CdsA were both fully functional. Glycerol-3-phosphate dehydrogenase (GpsA), which is responsible for the production of glycerol-3-phosphate (36), was included as a control enzyme. When the GpsA-GFP gene (PcitM-gpsA-gfp) was integrated into the amyE locus of GS95 (gpsA::spc) strain, which requires glycerol for growth, the resultant strain grew well on minimal plates containing 3 mM citrate in the absence of glycerol. GFP-GpsA was thus functional.
The functionality of the GFP fusions of nonessential genes was examined by measuring production of the corresponding lipids. When the fused PssA-GFP gene (PcitM-pssA-gfp) was integrated into the amyE locus of the strain SDB02 (ΔpssA10::spc), which has a disrupted allele of pssA (30), the resultant strain produced phosphatidylserine, a direct product of the enzyme reaction, and PE after induction with citrate. PssA-GFP was thus fully functional. When the GFP-Psd gene (Pxyl-gfp-psd) was integrated into the amyE locus of SDB01 (psd::neo) (30), the resultant strain produced PE, using phosphatidylserine which was accumulated in SDB01 cells, after induction. GFP-Psd was thus functional. When either the GFP-ClsA gene (Pxyl-gfp-clsA) or the ClsA+9-GFP gene (Pxyl-clsA+9-gfp) was introduced into the amyE locus of strain SDB206, which lacks CL (22), both resultant strains produced CL after the addition of the respective inducers. Both GFP-ClsA and ClsA-GFP were thus functional. The gene yfiX was assigned to lysyl-PG synthase from the lipid analysis of the strain SDB013 harboring Pspac-yfiX (K. Misawa, H. Hara, and K. Matsumoto, unpublished data). The gene was a homologue of mprF from Staphylococcus aureus (45); thus, yfiX is renamed mprF. When the GFP-MprF/YfiX gene (Pxyl-gfp-mprF/yfiX) was integrated into the amyE locus of SDB014 (mprF/yfiX::tet), which lacks lysyl-PG, the resultant strain synthesized lysyl-PG after induction. GFP-MprF/YfiX was thus functional. The gene ugtP/ypfP was assigned to UDP-glucose diacylglycerol glucosyltransferase (21; K. Komori, H. Hara, and K. Matsumoto, unpublished data). When the GFP-UgtP gene (Pxyl-gfp-ugtP) was integrated into the amyE locus of the strain KP261 (ugtP::neo) (46), the resultant strain synthesized glucolipids after xylose induction. Thus, GFP-UgtP was functional. UgtP-GFP, however, was not functional.
Fluorescence microscopy.
For localization of PE, cells were fixed, digested briefly with lysozyme as described previously (44), and then treated with biotinylated Ro (13), followed by incubation with streptavidin conjugated with tetramethyl rhodamine (Molecular Probes Co.) as follows. Cells of wild-type and PE-deficient mutants of B. subtilis and of gram-positive bacterial strains were grown in DSM and harvested (1-ml culture) in the late exponential growth phase and in the sporulation phase at stages T2 and T4. Cells of wild-type and PE-deficient mutants of E. coli were grown in LB medium and LB medium containing 50 mM MgCl2 (48), respectively, and harvested (1-ml culture) in the late exponential growth phase. These cells were fixed in 4.4% (wt/vol) paraformaldehyde--28 mM Na-PO4 (pH 7.4) (100 μl) for 20 min at room temperature, washed three times with PBS (phosphate-buffered saline containing [per liter] 8 g of NaCl, 0.2 g of KCl, 1.44 g of Na2PO4, 0.24 g of KH2PO4 [pH 7.4]), and applied onto poly-l-lysine-coated microscope slides. For E. coli, the cells were incubated in 0.1% Triton X-100-PBS for 15 min before application. The cells on the slides were washed twice with PBS, treated with lysozyme (1 to 2 mg/ml) in 100 μl of GTE (50 mM glucose, 20 mM Tris-HCl [pH 7.5], 10 mM EDTA), for 15 to 45 s (for B. subtilis) or for 1 min (for E. coli) at room temperature, washed three times with PBS, and allowed to dry completely. After rehydration with PBS, the samples on slides were blocked for 20 min with 2% (wt/vol) bovine serum albumin (BSA) in PBS and then incubated in 0.5% (wt/vol) BSA-PBS containing 2 μg of biotinylated Ro/ml (13) for 20 min at room temperature. The slides were washed five times with PBS (for B. subtilis) or 0.05% Tween-PBS (for E. coli), incubated in 0.5% (wt/vol) BSA-PBS containing 5 μg of streptavidin/ml conjugated with tetramethyl rhodamine for 30 min at room temperature, washed five times with PBS (for B. subtilis) or 0.05% Tween-PBS (for E. coli) again, and subjected to microscopic observation. With no fixation, the cells easily lose their rigid form and bulge out even after brief lysozyme treatment (15 s). The cells in the rigid form, though there were not many in the samples, showed essentially the same pattern of Ro localization as that with the fixation. This indicated that the paraformaldehyde fixation did not affect the pattern of localization. For colocalization of CL, NAO (Molecular Probes Co.) at a final concentration of 1 mM was directly added to the washed slides. After incubation at room temperature for 20 min, the cells were washed and subjected to microscopic observation.
Fluorescence images were viewed with an ECLIPS E600 fluorescence microscope (Nikon) and a cooled charge-coupled device camera (ORCA-ER; Hamamatsu Photonics Co., Hamamatsu, Japan). Fluorescence from tetramethyl rhodamine (excitation at 555 nm; emission at 580 nm) and fluorescence from FM4-64 (excitation at 510 nm; emission at 626 nm) were detected with a G-2A filter unit (510- to 560-nm excitation and 590-nm emission). Green fluorescence from GFP and from NAO (excitation at 495 nm; emission at 525 nm) was detected by using a standard GFP(R)-BP filter unit (460- to 500-nm excitation and 510- to 560-nm emission). To minimize the toxicity of high-energy excitation light, the focus was set under phase-contrast conditions and then fluorescence images were captured shortly after the shift to high-energy excitation light. The exposure time for tetramethyl rhodamine was 0.1 s, and that for green fluorescence of GFP and NAO was 5 to 7 s and 0.2 to 0.8 s, respectively. Captured images were processed with Adobe Photoshop, version 6.0. The relative intensities of tetramethyl rhodamine fluorescence were quantified by using NIH Image (Scion, version 4.02).
Deconvolution microscopy was carried out with the ECLIPS TE2000-U fluorescence laser microscope system C1 (Nikon). An argon laser (at 488 nm) was used to detect tetramethyl rhodamine conjugated with streptavidin. Raw data from between 7 and 9 optical z-axis sections (0.1-μm intervals) were collected and deconvoluted with the Metamorph software (Universal Imaging Co.). Captured images were processed as described above.
Lipid analysis.
Mutant and wild-type cells cultivated in DSM broth (50 ml) containing an appropriate inducer were harvested during the late exponential phase, and lipids were extracted by the method of Lacombe and Lubochinsky (24) with minor modifications (22). The method incorporated the following acidic treatment into the method of Ames (1). The harvested cells were suspended in 0.9 M perchloric acid in 1% NaCl and incubated at 0°C for 30 min, followed by the addition (1.88 ml to 0.5 ml of cell suspension) of chloroform-methanol (1:2 [vol/vol]). The mixtures were then subjected to the extraction procedure of Ames (1). Lipid fractions were separated by thin-layer chromatography on silica gel (no. 60; Merck, Darmstadt, Germany) with chloroform-methanol-acetic acid (65:25:10 [vol/vol/vol]). Phospholipids were visualized by uniform spraying with Dittmer-Lester reagent (9). Glucolipids were visualized by spraying with orcinol reagent.
RESULTS
Septal localization of PE-rich domains in B. subtilis membranes.
We have previously found CL-rich domains in the septal regions and at the poles of B. subtilis cells by using the CL-specific fluorescent dye NAO (22). This finding directed our interest toward the possible localization of other phospholipid domains, since it has been suggested that lipid molecules are heterogeneously distributed in several bacterial membranes (11). We thus examined the localization of PE on the membranes with the specific probe Ro (13). After fixation with paraformaldehyde followed by a brief lysozyme digestion, cells were treated with biotinylated Ro and then with streptavidin conjugated to tetramethyl rhodamine. The florescence of tetramethyl rhodamine was observed mainly in the septal region (including those parts of the lateral membranes close to the septum) (Fig. 1A -1). Low-intensity fluorescence was also observed in spots on the polar and lateral membranes. The relative intensity of the fluorescence of the septal region compared to that of the lateral membrane was an average of 6.5 and ranged from 3 to 10 (values obtained from 7 randomly selected cells). This indicates that the relative intensity of the fluorescence of the septum, which contains two cytoplasmic membranes, was obviously more than double that of the lateral membrane. When the mutant cells of SDB01 (psd1::neo) and SDB02 (pssA10::spc), both lacking PE (30), were examined under the same conditions, no fluorescence was observable (Fig. 1A-2 and A -3). Thus, the localization of the fluorescence reflected the localization of PE on the septal regions of cell membranes.
FIG. 1.
Visualization of PE-rich domains in B. subtilis cells with Ro. (A) Analysis with mutant cells lacking PE. Wild-type B. subtilis 168 cells (A-1) and the cells of the mutant strains lacking PE, SDB01 (psd1::neo) (A-2) and SDB02 (pssA10::spc) (A-3), were cultivated in DSM at 37°C and harvested in the early stationary phase to visualize PE. The cells were fixed in 4.4% (wt/vol) paraformaldehyde, washed with PBS, applied onto poly-l-lysine-coated microscope slides, washed with PBS, and treated with lysozyme (2 mg/ml). The slides were incubated in 0.5% (wt/vol) BSA-PBS containing 2 μg of biotinylated Ro/ml for 20 min, washed, incubated in BSA-PBS containing 5 μg of tetramethyl rhodamine/ml conjugated with streptavidin for 30 min, then washed, and subjected to microscopic observation. Fluorescence images were taken using a G-2A filter unit (510- to 560-nm excitation and 590-nm emission) as described in Materials and Methods. Corresponding phase-contrast images are also shown. Exposure times for fluorescence and phase-contrast images were 0.35 and 0.025 s, respectively. (B) PE-rich domains in sporulating B. subtilis cells. Wild-type B. subtilis 168 cells were cultivated in DSM at 37°C. Cells were harvested in the late exponential growth phase (B-1), and the sporulation phase at T2 (B-2), T3 (B-3), and T4 (B-4). The cells were fixed and processed to visualize the localization of PE as described above. Corresponding phase-contrast images are also shown. Exposure times for fluorescence and phase-contrast images were 0.35 and 0.025 s, respectively.
FIG. 2.
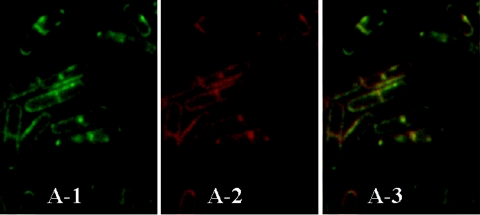
Double staining with NAO and Ro of B. subtilis cells. Wild-type 168 cells in late vegetative growth were harvested and processed with Ro as described in the legend to Fig. 1. After the last wash, NAO at a final concentration of 1 mM was directly added to the washed slides. After incubation for 20 min at room temperature, cells were washed and subjected to microscopic observation. Fluorescence images of NAO (A-1) and tetramethyl rhodamine (A-2) were taken by using a GFP(R)-BP filter unit (460- to 500-nm excitation and 510- to 560-nm emission) and a G-2A filter unit (510- to 560-nm excitation and 590-nm emission), respectively, as described in Materials and Methods. Exposure times for green and red fluorescence were 0.35 and 0.45 s, respectively. (A-3) Colocalization of green (A-1) and red (A-2) fluorescence images.
FIG. 3.
Distribution of PE-dependent fluorescence in E. coli cells. (A) Analysis with mutant cells lacking PE and cells with a reduced PE content. Wild-ype E. coli strain W3110 (A-1), strain GN10 lacking PE (A-2), strain UE81 (A-3), and strain S107 (A-4) cells were cultivated in LB containing MgCl2 to the early stationary phase. UE81 (ΔpssA10::cat ParaBAD-pssA) cells were cultivated in the absence of l-arabinose to reduce PE content to ca. 20% of total phospholipids. S107 (pssA1) cells were cultivated at 42°C to reduce PE content to ca. 30%. These cells were harvested and processed to visualize PE as described in the legend to Fig. 1. Corresponding phase-contrast images are also shown. Exposure times for fluorescence and phase-contrast images were 0.45 and 0.035 s, respectively. (B) Analysis with deconvolution microscopy. Wild-type E. coli strain W3110 (B-1 and B-2) and B. subtilis 168 strain (B-3) cells were treated with Ro and processed as described in Materials and Methods. The processed samples were subjected to deconvolution microscopy with the ECLIPS TE2000-U fluorescence laser microscope system C1 (Nikon). Nine (E. coli) and seven (B. subtilis) optical z-axis sections (0.1-μm intervals) were collected as raw data and deconvoluted with Metamorph software. The resulting images were processed with Adobe Photoshop, version 6.0.
We then examined the localization of PE in sporulating cells. In the sporulation phase, the B. subtilis cell membranes undergo dynamic rearrangements. Two hours after cessation of logarithmic growth (stage T2), the polar septal membrane is produced, the membrane leads to engulfment of the forespore during next stage (T3), and then, at stage T4, the cortex coat starts to accumulate on the forespore membranes. CL is localized in these sporulation-specific membranes of dynamic rearrangements (22). The fluorescence of PE in the cells cultivated in DSM was localized in the polar septal regions at stage T2 and in the engulfment and forespore membranes at later stages of the sporulation phase (Fig. 1B). At a much later stage, forespore membranes showed no fluorescence (Fig. 1B-4), probably because biotinylated Ro and/or streptavidin conjugated with tetramethyl rhodamine could not get through the thick cortex layer of the spore coat to the cytoplasmic membrane.
FIG. 4.
Septal localization of phosphatidylserine synthase, CL synthase, and phosphatidylglycerophosphate synthase in B. subtilis cells. (A) Typical images of localization of GFP fusions. Cells of the B. subtilis strains harboring the gfp fusions in the amyE locus were cultivated in DSM. They were induced with 0.5 mM citrate for PssA-GFP (A-1) or 0.1% xylose for GFP-PgsA (A-2), GFP-ClsA (A-3), and ClsA+9-GFP (A-4). The cells were harvested in the late logarithmic growth phase and subjected to fluorescence microscopy as described in Materials and Methods. Green fluorescence from the GFP fusions was detected by using a standard GFP(R)-BP filter unit. Exposure times were 3 to 5 s. Cells of SDB1220 harboring the PssA-GFP fusion under the natural promoter were cultivated to late log phase (B-1 and B-2), stage T2 (B-3 and B-4), and stage T3 (B-5). Cells of SDB1209 harboring the ClsA-GFP fusion under the natural promoter were cultivated to late log phase (C-1 and C-2). Exposure times were 2 to 3 s.
The localization of PE was then compared with that of CL. Late-logarithmic-growth-phase cells were processed for paraformaldehyde fixation and treated with biotinylated Ro and streptavidin conjugated to tetramethyl rhodamine, followed by NAO treatment. In most cells, the localization in the septal band of red fluorescence of PE overlapped well with that of the green fluorescence of NAO, i.e., CL (Fig. 2). This pattern of distribution of NAO fluorescence is essentially the same as that of the cells without fixation (22), showing that the paraformaldehyde fixation does not change the distribution of phospholipids. Cells stained with NAO but not with Ro were observed occasionally, probably due to ineffective lysozyme treatment.
To investigate whether E. coli cells have similar PE-rich domains or not, the cells of wild-type strains W3110 and MC4100 were fixed, digested briefly with lysozyme, treated with biotinylated Ro, and subjected to microscopic observation. The florescence was distributed uniformly over cell membranes with no apparently intense regions (Fig. 3A-1). When null pssA cells lacking PE (48) were treated in the same way, no fluorescence was observable (Fig. 3A-2), showing that the fluorescence on the cell membranes of the wild-type E. coli is an indication of the distribution of PE. Assuming that the apparently uniform localization may be an artifact of the large amount of PE (as much as 70% of total phospholipids is PE in wild-type cells) (51) and that, in case of scarcity, PE may occur first in regions requiring it most, we examined cells with reduced PE content. Strain S107 (pssA1) reduces its PE content to ca. 30% of total phospholipids after it has been heated to a restrictive temperature (42). UE81 (ΔpssA10::cat ParaBAD-pssA) reduces its PE content to ca. 20% in the absence of l-arabinose (H. Hara, A. Ito, and K. Matsumoto, unpublished data). Both types of cells with reduced PE content, however, showed a uniform distribution of fluorescence (Fig. 3A-3 and A-4), suggesting that PE is uniformly distributed over the whole cell membrane in E. coli. Even after deconvolution, with nine rounds of integration of z-axis sections (0.1-μm intervals), almost uniform distributions of fluorescence were observed with wild-type E. coli W3110 cells (Fig. 3B-1 and B-2), though B. subtilis cells showed quite clear septal localization after deconvolution (Fig. 3B-3).
Next, we looked for PE-rich domains in other gram-positive bacteria. Similar treatment revealed PE-rich domains in septal regions of many Bacillus species, including Bacillus amyloliquefaciens 203 (formerly, Bacillus megaterium 203), Bacillus polymyxa IAM1189, and Brevibacillus brevis IAM1031 (data not shown). In the cell membranes of Corynebacterium glutamicum IAM12435, Lactobacillus casei IAM1045 and IAM10062, Lactococcus lactis IAM1198 and IAM12092, and S. aureus IAM1011, which are lacking PE (15, 47), no fluorescence was detected (data not shown). We conclude that PE-rich domains in the septal membranes may be a common feature of gram-positive bacteria having PE.
Septal localization of phosphatidylserine synthase, phosphatidylglycerophosphate synthase, and CL synthase in B. subtilis membranes.
The septal localization of both PE- and CL-rich domains directed our interest to the subcellular localization of the enzymes involved in PE and CL synthesis. The committed step in PE synthesis in B. subtilis is catalyzed by phosphatidylserine synthase (PssA, the gene product of pssA). Its reaction product, phosphatidylserine, is then decarboxylated to form PE (10, 29, 30, 43). CL synthase (ClsA, the product of clsA) and phosphatidylglycerophosphate synthase (PgsA, the product of pgsA) catalyze the committed steps for the synthesis of these anionic phospholipids, respectively (22, 29, 51; unpublished data). For microscopic analysis with GFP fusions, the following strains were constructed. The strains with the genes of PssA and ClsA with a GFP fusion at the C terminus, PssA-GFP and ClsA-GFP, respectively, the expression of which was controlled under their own promoters, were constructed by using pMm2. The strain expressing GFP fused to the C terminus of PssA, PssA-GFP, under the control of PcitM, was constructed by using pDHCMGFP. The strains expressing GFPs fused to the N terminus of PgsA and ClsA, GFP-PgsA and GFP-ClsA, respectively, under the control of Pxyl promoter, were also constructed by using pSG1729. The strain expressing GFP fused to the C terminus of ClsA, with a 9-amino-acid-residue linker, ClsA+9-GFP, was constructed by using pSG1154. In each case, the plasmid DNA having the fused gene under the control of PcitM or Pxyl was inserted into the amyE locus of the wild-type chromosome. These fusion products were functional (see Materials and Methods).
When PssA-GFP was induced, with 0.5 mM citrate, the fluorescence of PssA-GFP was localized to the septal region (Fig. 4A-1). Even with no inducer or at a low concentration (0.1 mM), PssA-GFP was localized on the septal membranes. The pattern of septal membrane localization with 1.5 mM citrate was essentially the same as that for the low concentrations (data not shown). The fusion PssA-GFP produced under its own promoter was obviously localized on the septal membrane, though the intensity of the fluorescence was low (Fig. 4B-1 and B-2). The septal localization is, therefore, an intrinsic characteristic of the enzyme. Possible lateral distribution of PssA-GFP was then examined by inspecting the cells lined up according to their length (as many as 200 of the cells were examined), and we could not find any specific fluorescent foci of the GFP fusion showing the possible predivisional sites on lateral membranes (data not shown). Thus, PssA-GFP was not localized in predivisional sites in predivisional cells in the logarithmic growth phase.
When GFP-PgsA was induced with various concentrations of xylose (from 0.01 to 0.3%), the fluorescence of GFP-PgsA was localized exclusively on the septal membranes (Fig. 4A-2 shows that obtained with 0.1% xylose.). GFP-ClsA induced with 0.1% xylose was also localized on the septal membranes (Fig. 4A-3). With much lower concentrations and with an excess of xylose, localizations of the fluorescence were on the septal membranes (data not shown). ClsA+9-GFP showed similar localization (Fig. 4A-4). The ClsA-GFP fusion produced under its own promoter was clearly localized on the septal membrane, though the intensity of the fluorescence was lower (Fig. 4C-1 and C-2). These results indicated that the septal membrane localization of ClsA and PgsA is an intrinsic characteristic, as in the case of PssA. Thus, we conclude that all of the three enzymes responsible for the committed steps in the synthesis of PE and CL are septally localized in B. subtilis membranes. In these cases, the GFP fluorescence was not found on the cell poles and was confined to the septal regions. This localization was apparently different from that of the NAO fluorescence, which is found in the septal regions and at the poles (Fig. 2) (22).
In sporulating cells, CL-rich and PE-rich domains were observed in the polar septa and on the engulfment and forespore membranes. In these cells, GFP fusion of PssA produced under the natural promoter was only dimly observed in the polar septa and engulfment membranes at stages T2 and T3 (Fig. 4B-3, B-4, and B-5) and was hardly observable at T4. GFP fusion of ClsA was not detectable in these sporulating cells (data not shown). A possible reason for the decline could be that the expression of the enzymes in the sporulation phase is much lower than that during vegetative growth. ClsA may be replaced with YwjE, another CL synthase, in sporulating cells (12, 22).
FIG. 5.
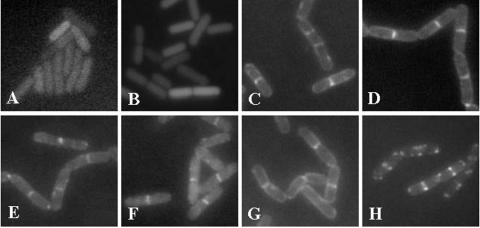
Septal localization of other lipid synthases in B. subtilis cells. Cells of the B. subtilis strains harboring gfp fusions in the amyE locus were cultivated in DSM up to the late logarithmic growth phase. The cells were harvested and subjected to fluorescence microscopy as described in Materials and Methods. The pDHCMGFP vector (A) and the fusions GpsA-GFP (B) and UgtP-GFP (H) were induced with 0.1 mM citrate. Fusions GFP-YhdO (C), GFP-CdsA (D), GFP-Psd (E), GFP-MprF (F), and GFP-DgkA (G) were induced with 0.1% xylose.
Septal localization of other lipid synthases in B. subtilis membranes.
We examined the localization of the following synthases involved in the synthesis of other lipids (Fig. 5). The GFP fusions of enzymes examined include 1-acylglycerol-3-phosphate acyltransferase (YhdO), CDP-diacylglycerol synthase (CdsA), phosphatidylserine decarboxylase (Psd), diacylglycerol kinase (DgkA), UDP-glucose diacylglycerol glucosyltransferase (UgtP), and lysyl-PG synthase (MprF). Glycerol-3-phosphate dehydrogenase (GpsA) was included as a control enzyme. The fluorescence of GpsA-GFP was homogeneously distributed in the cytoplasm (Fig. 5B). GpsA catalyzes the production of glycerol-3-phosphate from dihydroxyacetone-3-phosphate (36), and the predicted sequence of GpsA has no transmembrane domain (examined with the SOSUI program [http://sosui.proteome.bio.tuat.ac.jp]). The cytoplasmic localization of GpsA thus seemed reasonable. Surprisingly, all of the fusions of the phospholipid synthases (GFP-YhdO, GFP-CdsA, GFP-Psd, and GFP-MprF) were localized to the septum in a thick bright fluorescence band (Fig. 5C to F). Since all of the fusions were functional (see Materials and Methods), the septal localization of phospholipid synthases suggests that phospholipids are synthesized mainly in the septal membranes.
Localizations of diacylglycerol kinase (DgkA) and the enzyme that catalyzes the glucolipid synthesis (UgtP) were different from those of the phospholipid synthases. The florescence of DgkA, involved in the phosphorylation of diacylglycerol to reproduce phosphatidic acid, was localized not only in a septal band but also on lateral membranes (Fig. 5G). This tendency of localization on both lateral and septal membranes was observed with various concentrations of the inducer (0.05, 0.1, and 0.5% xylose). The florescence of UgtP-GFP (Fig. 5H) and GFP-UgtP (data not shown) was localized in the septal region, and there were spots at the poles. The localization was, however, not in the typical septal band of phospholipid synthases but in the form of structures with two prominent dots in the regions facing lateral membranes. We interpret the dot-pair structures as two-dimensional projections of three-dimensional open rings that are not filled with fluorescent material. This localization may have something to do with its structure, which is predicted to be that of a cytoplasmic protein with no transmembrane segment (examined with the programs SOSUI and PSORT [http://psort.nibb.ac.jp]).
Septal localization of PE- and CL-rich domains and phospholipid synthases depends on FtsZ.
The septal localization of phospholipid synthases raises the question of whether it depends on FtsZ or not. FtsZ plays a key role in the assembly of the cell division apparatus in cytokinesis. It is at the top of the hierarchy of assembly of division proteins (14). To examine the effect of the depletion of FtsZ on the septal localization of the enzymes, we constructed the fusion strain SDB1010F (PcitM-pssA-gfp Pspac-ftsZ). The cells of SDB1010F were cultivated in DSM containing 3 mM IPTG and 0.5 mM citrate. Depletion of FtsZ by removal of IPTG gave rise to filamentous cells. After 2 h of depletion, the fluorescence of PssA-GFP was dispersed on the membranes, forming randomly dispersed spots (Fig. 6A -2 and A-3). ClsA+9-GFP of SDB1109F (Pxyl-clsA+9-gfp Pspac-ftsZ) showed a similar dispersed localization (data not shown). Cultivation of strains harboring the ftsZ1(Ts) allele at a nonpermissive temperature changes the cells to filaments without septa (3). After incubation of the strain SDB1109T (Pxyl-clsA+9-gfp ftsZ1) at 49°C for 2 h, the fluorescence of ClsA+9-GFP was scattered in the filamentous cells (Fig. 6B-1). PssA-GFP showed a similar dispersed localization in the filamentous cells (data not shown). These results indicate that the septal localization of both PssA and ClsA depends on FtsZ ring formation.
FIG. 6.
FtsZ-dependent localization of PE- and CL-rich domains and phospholipid synthases in B. subtilis cells. (A) Cells of the SDB1010F [amyE::(PcitM-pssA-gfp) Pspac-ftsZ)] strain harboring the fusion gene for PssA-GFP were cultivated in DSM containing 3 mM IPTG and 0.5 mM citrate. Depletion of FtsZ by removal of IPTG gave rise to filamentous cells. The cells were harvested at 0 h (A-1), 2 h (A-2), and 3 h (A-3) after removal of IPTG and subjected to fluorescence microscopy as described in Materials and Methods. The filamentous cells of ASK510 (Pspac-ftsZ) harvested at 4 h after removal of IPTG were subjected to the process for visualization of PE (A-4) as described in Materials and Methods. (B) Cells of the strain SDB1109T [ts1(ftsZ1) amyE::(Pxyl-clsA+9-gfp)] were cultivated in DSM containing 0.1% xylose. The cells were harvested at 2 h after the temperature shift to 49°C, and localization of ClsA+9-GFP (B-1) was observed as described in Materials and Methods. The filamentous cells of the ts1 strain harvested at 1 h (B-2) and at 2 h (B-3) after the temperature shift were subjected to the process for visualization of CL. For visualization of PE (B-4), the filamentous cells harvested at 2 h after the temperature increase were processed as described in Materials and Methods.
In the filamentous cells of these strains (ASK510 and ts1), the fluorescence of PE was dissociated from the septal membranes and dispersed on lateral membranes (Fig. 6A-4 and B-4), forming sporadic long patches in some cases (data not shown). CL, as visualized by the fluorescence of NAO, was localized in many scattered spots over the whole membrane of the filamentous cells of strain ts1 (Fig. 6B-2 and B-3). The distribution of the phospholipids in these filamentous cells was apparently similar to the dispersed distribution of the synthases. The distribution of the phospholipids indicates that the septal localization of PE- and CL-rich domains depends on FtsZ.
DISCUSSION
Treatment of B. subtilis cells with biotinylated Ro, the cyclic peptide probe specific for PE (13), followed by detection with tetramethyl rhodamine-conjugated streptavidin clearly revealed the localization of PE in the septal region of membranes during vegetative growth and in the polar septum and the engulfment and forespore membranes during sporulation. The fluorescence that indicated the localization of Ro was not detectable in mutant cells lacking PE (Fig. 1). We conclude that the localization of the fluorescence reflects localization of PE-rich domains in B. subtilis membranes. In eukaryotic cell membranes, a PE-rich domain has been previously demonstrated at the cleavage furrow of CHO-K1 cells during cytokinesis (13). The present result is the first direct visualization of PE-rich domains in bacterial cell membranes. Similar PE-rich domains in septal regions were found in many other Bacillus species, including B. amyloliquefaciens, B. polymyxa, and B. brevis, though no such domain has been observed in E. coli membranes (Fig. 3). Thus, PE-rich domains in the septal membranes may be a common feature in gram-positive, but not necessarily in gram-negative, bacteria having PE.
B. subtilis cells have CL-rich domains in the septal and polar membranes during vegetative growth and in the polar septum and the engulfment and forespore membranes during sporulation (22); therefore, B. subtilis cells have both PE- and CL-rich domains in their membranes. The localization of PE-rich domains appears to coincide well with that of CL, except that polar CL-rich domains in logarithmic-growth-phase cells were not always associated with PE-rich domains (Fig. 2). Experiments with GFP fusions to phospholipid synthases revealed that the majority of the enzymes are localized in the septal membranes; the enzymes include CL synthase and its upstream enzymes in the biosynthetic pathway and phosphatidylserine synthase, which is responsible for PE synthesis. These results suggest that both PE and CL are produced mostly in the septal membranes.
The septal localization of PE- and CL-rich domains suggests that the septal membranes have reduced contents of other phospholipids, PG and lysyl-PG, and glucolipids, if the septal membranes are not reduced in protein content. Lateral membranes may have lipid domains with less PE and CL and that are enriched with the other phospholipids and glucolipids. How are the PE- and CL-rich domains generated in the septal region of B. subtilis membranes? Since the enzymes, phosphatidylserine synthase and decarboxylase and CL synthase, responsible for the synthesis of these lipids are localized in septal regions, the septal localization of PE and CL is apparently reasonable. However, the majority of the phospholipid synthases are localized in the septal region. This implies that most of the phospholipids are synthesized mainly at the septal region in the B. subtilis membranes. The rate of lateral diffusion of phospholipid molecules in bilayer preparations from E. coli has been determined to be ca. 0.5 × 10−8 cm2/s (20). A similar diffusion coefficient (1.1 × 10−8 cm2/s at 37°C) was observed in the plasma membrane of soybean protoplasts (32). Assuming that the rate of diffusion of lipid molecules is similar in B. subtilis membranes, the time it would take a phospholipid molecule produced at the septal membranes to diffuse into lateral membranes would be less than a minute. Thus, a mechanism(s) that prevents diffusion of PE and CL molecules into lateral membranes or keeps them in the septal membranes will be required.
With our present knowledge, we can hardly envisage the means that may generate septal domains enriched with a particular phospholipid, since B. subtilis membranes have no compartmentalization that could separate lateral and septal regions of the membranes. However, we may imagine the following possibilities. One is that if B. subtilis cells have a PE- or CL-specific lipase, like the CL-specific phosphodiesterase (5) previously reported for E. coli, and if it is localized on lateral membrane, it may have a role in the prevention of diffusion of the phospholipids by decomposition of diffused phospholipids. Another mechanism may include a raft-like structure or a lipid microdomain (37, 52) that contains proteins with an affinity for PE or CL. MurG, a peripheral membrane protein, of E. coli interacts preferentially with CL, and its overexpression results in formation at the poles of vesicles enriched with CL (58). Proteins, such as PssA, DnaA, SecA, and FtsY, with an affinity for acidic phospholipids, including CL and PG, are increasingly reported for E. coli (10, 27, 31, 61). In addition, there are growing numbers of examples of proteins for which specific intracellular or polar localizations are essential for proper function and regulation (25, 28). It may, therefore, be possible that certain septal membrane proteins and proteins responsible for cell division and lipid synthesis have a role in coclustering of such lipids to generate a microdomain, which may be similar to that suggested by Norris et al. (41). MinD, which localizes in the form of a horseshoe on the membranes at the poles of E. coli cells, may be a candidate, since MinD binds with its C-terminal amphiphilic α-helix to liposomes containing anionic phospholipids (19, 35, 54, 55). In B. subtilis, a division site selection protein, DivIVA, which accumulates at the poles independent of FtsZ (17, 18, 57), may also be a candidate. It should be noted that UgtP, which is responsible for glucolipid synthesis, is localized in the form of a two-dot structure that is thickest in the lateral vicinity of the septal face (Fig. 5), different from the phospholipid synthases, which form a sharp band on the septal face. UgtP is probably not an integral membrane protein, since it has no membrane-spanning region, in contrast to phospholipid synthases, which have several membrane-spanning regions. This property of UgtP may have some relation to the difference in its pattern of localization.
The localization of PE usually coincided with that of CL (Fig. 2), except that the polar CL localization observed in logarithmic-growth-phase cells was not always associated with PE. PE-rich domains were found also in the polar septa and on the engulfment and forespore membranes in the sporulating cells. The localization of PE in sporulation-phase cells seems to coincide with that of CL. What is the role of the PE- and CL-rich domains in these membranes? The biological significance of the PE-rich domains has not been clarified for B. subtilis cells, since the mutant cells lacking PE have no obvious growth phenotype (30), though the significance in sporulation of CL has been illustrated with a mutant lacking CL which shows retarded emergence of the polar septal and engulfment membranes that is accompanied by a low frequency of heat-resistant spores (22). Development of the sporulation-specific membranes, polar septal, engulfment, and forespore membranes, may have a polar head structure-specific requirement for CL. Both the PE- and CL-rich domains may contribute to the formation of a nonbilayer structure, which is thought to be required for the formation of division septa and the progression of engulfment, since PE and CL in the presence of certain divalent cations facilitate a dynamic phase shift, causing formation of nonbilayer structures under physiological conditions (10).
What, then, is the reason for the septal localization of the majority of the lipid synthases? The specific localization implies that most of the phospholipids are synthesized there. At the initial stage of cell division, the small radius of curvature of the developing division site, on the leading edge, requires a lipid with a small head group and large acyl chains, such as PE and CL, in concave regions of the outer monolayers. However, as invagination proceeds to decrease the diameter of the FtsZ ring, the constraints become dominated by the convex nature of the monolayer (40). In the inner monolayers of the bilayer membranes, requirements for the nature of the lipids are opposite. The constraints on the nature of lipids in a particular site in a monolayer in the division site change during the division process, and cells are therefore faced with the problem of ensuring the supply of appropriate lipids at the division site (40). The septally localized phospholipid synthases could meet this need by serving lipids with the appropriate nature at proper times and locations during the division process. This may be the major reason for the septal localization of the majority of the phospholipid synthases.
The last subject to be discussed is the mechanism for the localization of phospholipid synthases. The FtsZ depletion experiments indicated that the localization of these enzymes depended on FtsZ and excluded the possibility of localization at preseptal sites. Thus, the localization probably follows or is associated with the assembly of cell division proteins to execute concerted synthesis of phospholipids with that of peptidoglycan at the leading edge of the invaginating membranes. Two-hybrid analysis of the lipid synthases with cell division proteins and other envelope proteins will visualize possible interaction with them. The lipid synthases should have a specific region or regions that are responsible for the septal localization. Elucidation of the regions responsible for the septal localization by dissection of these enzymes would greatly help our understanding of the mechanism of the septal localization.
Acknowledgments
We thank Peter Lewis, Junichi Sekiguchi, Hironori Yamamoto, Kei Asai, Hiuga Saito, Gustavo Schujman, Diego de Mendoza, Kirsten Price, and Richard Losick for generous gifts of plasmids and bacterial and mutant strains. We thank Yoshinori Hara, Satomi Hirono, Yukari Kudo, Kohei Natori, Hiroyoshi Miyakawa, and Atsuko Taguchi for help in construction of certain GFP fusions and complementation studies. We also thank Yoshito Sadaie and Isao Shibuya for encouragement and helpful discussions. We thank the Genetics Society of Japan for encouraging us by giving A.N. the best-paper award at its 75th annual meeting.
REFERENCES
- 1.Ames, G. F. 1968. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J. Bacteriol. 95:833-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anagnostopoulos, C., and I. P. Crawford. 1961. Transformation studies on the linkage of markers in the tryptophan pathway in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 47:378-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callister, H., T. McGinness, and R. G. Wake. 1983. Timing and other features of the action of the ts1 division initiation gene product of Bacillus subtilis. J. Bacteriol. 154:537-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christensen, H., N. J. Garton, R. W. Horobin, D. E. Minnikin, and M. R. Barer. 1999. Lipid domains of mycobacteria studied with fluorescent molecular probes. Mol. Microbiol. 31:1561-1572. [DOI] [PubMed] [Google Scholar]
- 5.Cole, R., and P. Proulx. 1977. Further studies on the cardiolipin phosphodiesterase of Escherichia coli. Can. J. Biochem. 55:1228-1232. [DOI] [PubMed] [Google Scholar]
- 6.Cronan, J. E., Jr. 2003. Bacterial membrane lipids: where do we stand? Annu. Rev. Microbiol. 57:203-224. [DOI] [PubMed] [Google Scholar]
- 7.DeChavigny, A., P. N. Heacock, and W. Dowhan. 1991. Sequence and inactivation of the pss gene of Escherichia coli. Phosphatidylethanolamine may not be essential for cell viability. J. Biol. Chem. 266:5323-5332. [PubMed] [Google Scholar]
- 8.de Mendoza, D., G. E. Schujman, and P. S. Aguilar. 2002. Biosynthesis and function of membrane lipids, p. 43-55. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives. ASM Press, Washington, D.C.
- 9.Dittmer, J. C., and R. L. Lester. 1964. A simple, specific spray for the detection of phospholipids on thin layer chromatograms. J. Lipid Res. 5:126-127. [PubMed] [Google Scholar]
- 10.Dowhan, W. 1997. Molecular basis for membrane phospholipid diversity: why are there so many lipids? Annu. Rev. Biochem. 66:199-232. [DOI] [PubMed] [Google Scholar]
- 11.Dowhan, W., E. Mileykovskaya, and M. Bogdanov. 2004. Diversity and versatility of lipid-protein interactions revealed by molecular genetic approaches. Biochim. Biophys. Acta 1666:19-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eichenberger, P., S. T. Jensen, E. M. Conlon, C. van Ooij, J. Silvaggi, J.-E. Gonzalez-Pastor, M. Fujita, S. Ben-Yehuda, P. Stragier, J. S. Liu, and R. Losick. 2003. The sigma E regulon and the identification of additional sporulation genes in Bacillus subtilis. J. Mol. Biol. 327:945-972. [DOI] [PubMed] [Google Scholar]
- 13.Emoto, K., T. Kobayashi, A. Yamaji, H. Aizawa, I. Yahara, K. Inoue, and M. Umeda. 1996. Redistribution of phosphatidylethanolamine at the cleavage furrow of dividing cells during cytokinesis. Proc. Natl. Acad. Sci. USA 93:12867-12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Errington, J., R. A. Daniel, and D.-J. Scheffers. 2003. Cytokinesis in bacteria. Microbiol. Mol. Biol. Rev. 67:52-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer, W., M. Nakano, R. A. Laine, and W. Boherer. 1978. On the relationship between glycerophosphoglycolipids and lipoteichoic acids in gram-positive bacteria. I. The occurrence of phosphoglycolipids. Biochim. Biophys. Acta 528:288-297. [DOI] [PubMed] [Google Scholar]
- 16.Fishov, I., and C. L. Woldringh. 1999. Visualization of membrane domains in Escherichia coli. Mol. Microbiol. 32:1166-1172. [DOI] [PubMed] [Google Scholar]
- 17.Hamoen, L. W., and J. Errington. 2003. Polar targeting of DivIVA in Bacillus subtilis is not directly dependent on FtsZ or PBP 2B. J. Bacteriol. 185:693-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harry, E., and P. J. Lewis. 2003. Early targeting of Min proteins to the cell poles in germinated spores of Bacillus subtilis: evidence for division apparatus-independent recruitment of Min proteins to the division site. Mol. Microbiol. 47:37-48. [DOI] [PubMed] [Google Scholar]
- 19.Hu, Z., C. Saez, and J. Lutkenhaus. 2003. Recruitment of MinC, an inhibitor of Z-ring formation, to the membrane in Escherichia coli: role of MinD and MinE. J. Bacteriol. 185:196-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin, A. J., M. Edidin, R. Nossal, and N. L. Gershfeld. 1999. A singular state of membrane lipids at cell growth temperatures. Biochemistry 38:13275-13278. [DOI] [PubMed] [Google Scholar]
- 21.Jorasch, P., F. P. Wolter, U. Zähringer, and E. Heinz. 1998. A UDP glucosyltransferase from Bacillus subtilis successively transfers up to four glucose residues to 1,2-diacylglycerol: expression of ypfP in Escherichia coli and structural analysis of its reaction products. Mol. Microbiol. 29:419-430. [DOI] [PubMed] [Google Scholar]
- 22.Kawai, F., M. Shoda, R. Harashima, Y. Sadaie, H. Hara, and K. Matsumoto. 2004. Cardiolipin domains in Bacillus subtilis Marburg membranes. J. Bacteriol. 186:1475-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi, K., D. S. Ehrlich, et al. 2003. Essential Bacillus subtilis genes. Proc. Natl. Acad. Sci. USA 100:4678-4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lacombe, C., and B. Lubochinsky. 1988. Specific extraction of bacterial cardiolipin from sporulating Bacillus subtilis. Biochim. Biophys. Acta 961:183-187. [DOI] [PubMed] [Google Scholar]
- 25.Lai, E.-M., U. Nair, N. D. Phadke, and J. R. Maddock. 2004. Proteomic screening and identification of differentially distributed membrane proteins in Escherichia coli. Mol. Microbiol. 52:1029-1044. [DOI] [PubMed] [Google Scholar]
- 26.Lewis, P., and A. L. Marston. 1999. GFP vectors for controlled expression and dual labeling of protein fusions in Bacillus subtilis. Gene 227:101-109. [DOI] [PubMed] [Google Scholar]
- 27.Li, L., P. Storm, O. P. Karlsson, S. Berg, and Å. Wieslander. 2003. Irreversible binding and activity control of the 1,2-diacylglycerol 3-glucosyltransferase from Acholeplasma laidlawii at an anionic lipid bilayer surface. Biochemistry 42:9677-9686. [DOI] [PubMed] [Google Scholar]
- 28.Lybarger, S. R., and J. R. Maddock. 2001. Polarity in action: asymmetric protein localization in bacteria. J. Bacteriol. 183:3261-3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsumoto, K. 1997. Phosphatidylserine synthase from bacteria. Biochim. Biophys. Acta 1348:214-227. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto, K., M. Okada, Y. Horikoshi, H. Matsuzaki, T. Kishi, M. Itaya, and I. Shibuya. 1998. Cloning, sequencing, and disruption of Bacillus subtilis psd gene coding for phosphatidylserine decarboxylase. J. Bacteriol. 180:100-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsumoto, K. 2001. Dispensable nature of phosphatidylglycerol in Escherichia coli: dual roles of anionic phospholipids. Mol. Microbiol. 39:1427-1433. [DOI] [PubMed] [Google Scholar]
- 32.Metcalf, T. N., J. L. Wang, and M. Schindler. 1986. Lateral diffusion of phospholipids in the plasma membrane of soybean protoplasts: evidence for membrane lipid domains. Proc. Natl. Acad. Sci. USA 83:95-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mileykovskaya, E., and W. Dowhan. 2000. Visualization of phospholipid domains in Escherichia coli by using the cardiolipin-specific fluorescent dye 10-N-nonyl acridine orange. J. Bacteriol. 182:1172-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mileykovskaya, E., W. Dowhan, R. L. Birke, D. Zheng, L. Lutterodt, and T. H. Haines. 2001. Cardiolipin binds nonyl acridine orange by aggregating the dye at exposed hydrophobic domains on bilayer surfaces. FEBS Lett. 507:187-190. [DOI] [PubMed] [Google Scholar]
- 35.Mileykovskaya, E., I. Fishov, X. Fu, B. D. Corbin, W. Margolin, and W. Dowhan. 2003. Effects of phospholipid composition on MinD-membrane interactions in vitro and in vivo. J. Biol. Chem. 278:22193-22198. [DOI] [PubMed] [Google Scholar]
- 36.Morbidoni, H. R., D. de Mendoza, and J. E. Cronan, Jr. 1995. Synthesis of sn-glycerol 3-phosphate, a key precursor of membrane lipids, in Bacillus subtilis. J. Bacteriol. 177:5899-5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Munro, S. 2003. Lipid rafts: elusive or illusive? Cell 115:377-388. [DOI] [PubMed] [Google Scholar]
- 38.Nishijima, S., Y. Asami, N. Uetake, S. Yamagoe, A. Ohta, and I. Shibuya. 1988. Disruption of the Escherichia coli cls gene responsible for cardiolipin synthesis. J. Bacteriol. 170:775-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishijima, S. 1992. Molecular genetic studies on the biosynthesis and biological functions of acidic phospholipids in Escherichia coli. Ph.D. thesis. Saitama University, Saitama, Japan. (In Japanese.)
- 40.Norris, V., G. Misevic, J.-M. Delosme, and A. Ohshima. 2002. Hypothesis: a phospholipid translocase couples lateral and transverse bilayer asymmetries in dividing bacteria. J. Mol. Biol. 318:455-462. [DOI] [PubMed] [Google Scholar]
- 41.Norris, V., C. Woldringh, and E. Mileykovskaya. 2004. A hypothesis to explain division site selection in Escherichia coli by combining nucleoid occlusion and Min. FEBS Lett. 561:3-10. [DOI] [PubMed] [Google Scholar]
- 42.Ohta, A., and I. Shibuya. 1977. Membrane phospholipid synthesis and phenotypic correlation of an Escherichia coli pss mutant. J. Bacteriol. 132:434-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okada, M., H. Matsuzaki, I. Shibuya, and K. Matsumoto. 1994. Cloning, sequencing, and expression in Escherichia coli of the Bacillus subtilis gene for phosphatidylserine synthase. J. Bacteriol. 176:7456-7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pedersen, L. B., E. R. Angert, and P. Setlow. 1999. Septal localization of penicillin-binding protein 1 in Bacillus subtilis. J. Bacteriol. 181:3201-3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peschel, A., R. W. Jack, M. Otto, L. V. Collins, P. Staubitz, G. Nicholson, H. Kalbacher, W. F. Nieuwenhuizen, G. Jung, A. Tarkowski, K. P. M. van Kessel, and J. A. G. van Strijp. 2001. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with L-lysine. J. Exp. Med. 193:1067-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Price, K. D., S. Roels, and R. Losick. 1997. A Bacillus subtilis gene encoding a protein similar to nucleotide sugar transferases influences cell shape and viability. J. Bacteriol. 179:4959-4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ratledge, C., and S. G. Wilkinson. 1988. Microbial lipids, vol. 1. Academic Press, London, England.
- 48.Saha, S. K., S. Nishijima, H. Matsuzaki, I. Shibuya, and K. Matsumoto. 1996. A regulatory mechanism for the balanced synthesis of membrane phospholipid species in Escherichia coli. Biosci. Biotechnol. Biochem. 60:111-116. [DOI] [PubMed] [Google Scholar]
- 49.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 50.Schaeffer, P., H. Ionesco, A. Ryter, and G. Balassa. 1965. La sporulation de Bacillus subtilis: étude génétique et physiologique, p. 553-563. In Mécanismes de régulation des activités cellulaires chez les microorganismes. Centre National de la Recherche Scientifique, Paris, France.
- 51.Shibuya, I. 1992. Metabolic regulations and biological functions of phospholipids in Escherichia coli. Prog. Lipid Res. 31:245-299. [DOI] [PubMed] [Google Scholar]
- 52.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature (London) 387:569-572. [DOI] [PubMed] [Google Scholar]
- 53.Singer, S. J., and G. L. Nicolson. 1972. The fluid mosaic model of the structure of cell membranes. Science 175:720-731. [DOI] [PubMed] [Google Scholar]
- 54.Suefuji, K., R. Valluzzi, and D. RayChaudhuri. 2002. Dynamic assembly of MinD into filament bundles modulated by ATP, phospholipids, and MinE. Proc. Natl. Acad. Sci. USA 99:16776-16781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szeto, T. H., S. L. Rowland, L. I. Rothfield, and G. F. King. 2002. Membrane localization of MinD is mediated by a C-terminal motif that is conserved across eubacteria, archaea, and chloroplasts. Proc. Natl. Acad. Sci. USA 99:15693-15698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takamatsu, H., T. Kodama, A. Imamura, K. Asai, K. Kobayashi, T. Nakayama, N. Ogasawara, and K. Watabe. 2000. The Bacillus subtilis yabG gene is transcribed by sigK RNA polymerase during sporulation, and yabG mutant spores have altered coat protein composition. J. Bacteriol. 182:1883-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomaides, H. B., M. Freeman, M. E. Karoui, and J. Errington. 2001. Division site selection protein DivIVA of Bacillus subtilis has a second distinct function in chromosome segregation during sporulation. Genes Dev. 15:1662-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van den Brink-van der Laan, E., J.-W. P. Boots, R. E. J. Spelbrink, G. M. Kool, E. Breukink, J. A. Killian, and B. de Kruijff. 2003. Membrane interaction of the glycosyltransferase MurG: a special role for cardiolipin. J. Bacteriol. 185:3773-3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vanounou, S., A. H. Parola, and I. Fishov. 2003. Phosphatidylethanolamine and phosphatidylglycerol are segregated into different domains in bacterial membrane. A study with pyrene-labelled phospholipids. Mol. Microbiol. 49:1067-1079. [DOI] [PubMed] [Google Scholar]
- 60.Welby, M., Y. Poquet, and J.-F. Tocanne. 1996. The spatial distribution of phospholipids and glycolipids in the membrane of bacterium Micrococcus luteus varies during the cell cycle. FEBS Lett. 384:107-111. [DOI] [PubMed] [Google Scholar]
- 61.Wikström, M., J. Xie, M. Bogdanov, E. Mileykovskaya, P. N. Heacock, Å. Wieslander, and W. Dowhan. 2004. Monoglucosyldiacylglycerol, a foreign lipid, can substitute for phosphatidylethanolamine in essential membrane-associated functions in Escherichia coli. J. Biol. Chem. 279:10484-10493. [DOI] [PubMed] [Google Scholar]