Abstract
Vascular smooth muscle (VSM) cells, endothelial cells (EC), and pericytes that form the walls of vessels in the microcirculation express a diverse array of ion channels that play an important role in the function of these cells and the microcirculation in both health and disease. This brief review focuses on the K+ channels expressed in smooth muscle and endothelial cells in arterioles. Microvascular VSM cells express at least four different classes of K+ channels, including inward-rectifier K+ channels (KIR), ATP-sensitive K+ channels (KATP), voltage-gated K+ channels (KV), and large conductance Ca2+-activated K+ channels (BKCa). VSM KIR participate in dilation induced by elevated extracellular K+ and may also be activated by C-type natriuretic peptide, a putative endothelium-derived hyperpolarizing factor (EDHF). Vasodilators acting through cAMP or cGMP signaling pathways in VSM may open KATP, KV, and BKCa, causing membrane hyperpolarization and vasodilation. VSM BKCa may also be activated by epoxides of arachidonic acid (EETs) identified as EDHF in some systems. Conversely, vasoconstrictors may close KATP, KV, and BKCa through protein kinase C, Rhokinase, or c-Src pathways and contribute to VSM depolarization and vasoconstriction. At the same time KV and BKCa act in a negative feedback manner to limit depolarization and prevent vasospasm. Microvascular EC express at least 5 classes of K+ channels, including small (sKCa) and intermediate (IKCa) conductance Ca2+-activated K+ channels, KIR, KATP, and KV. Both sK and IK are opened by endothelium-dependent vasodilators that increase EC intracellular Ca2+ to cause membrane hyperpolarization that may be conducted through myoendothelial gap junctions to hyperpolarize and relax arteriolar VSM. KIR may serve to amplify sKCa- and IKCa-induced hyperpolarization and allow active transmission of hyperpolarization along EC through gap junctions. EC KIR channels may also be opened by elevated extracellular K+ and participate in K+-induced vasodilation. EC KATP channels may be activated by vasodilators as in VSM. KV channels may provide a negative feedback mechanism to limit depolarization in some endothelial cells.
Keywords: arterioles, endothelium, ion channels, microcirculation, vascular smooth muscle, vasoconstriction, vasodilation
INTRODUCTION
Ion channels play an important role in the regulation of microvascular function. First, ion channels in the plasma membrane, as well as ion channels in the membrane of the smooth endoplasmic reticulum provide the major source of cytoplasmic Ca2+ in smooth muscle (14,54,93), pericytes (78,79,127,130,157,163) and endothelial cells (117) that form the vessels in the microcirculation. In smooth muscle cells (14,54,150), and pericytes (79,157), intracellular Ca2+ determines the state of contraction, or tone, while in endothelial cells, intracellular Ca2+ controls the production and release of endothelial cell autacoids, such as NO (44,59), PGI2 (59), and epoxides of arachidonic acid (21) as well as impacting the barrier function of these cells (120,144). Furthermore, intracellular Ca2+ likely regulates gene expression in all types of microvascular cells (22,126,145,151). Second, ion channels play a central role in setting and modulating membrane potential (69). Membrane potential, in turn controls the activity of voltage-gated Ca2+ channels in vascular smooth muscle cells (69) and pericytes (78,79,127,163), modulating Ca2+ influx, intracellular Ca2+, and microvascular tone.
Studies of macrovascular endothelial cells, primarily in culture, suggest that membrane potential also impacts Ca2+ influx in these cells by affecting the electrochemical gradient for Ca2+ diffusion into endothelial cells through nonselective cation channels (although this may be different in microvascular endothelial cells—see below) (117). Membrane potential also has been suggested to impact other aspects of Ca2+ homeostasis, including Ca2+ release from intracellular stores and Ca2+ sensitivity of smooth muscle contraction (47,89,119,159,160). Thus, ion channels participate in all aspects of microvascular function from control of blood flow to exchange of solutes and water to interactions of endothelial cells with inflammatory cells.
The cells that make up the walls of vessels in the microcirculation (smooth muscle cells, pericytes, and endothelial cells) express a diverse array of ion channels. For example (Figure 1), arteriolar smooth muscle cells have been shown to express at least four different classes of potassium channels (67,69,115), one or more types of voltage-gated Ca2+ channels (63), one or more types of Cl− channels (82), and several nonselective cation channels from the transient receptor potential family (TRP) of channels (5,64). Although not as well studied, pericytes (78,79,121,127,130,157,163) and microvascular endothelial cells (117) also express a similar complexity of ion channels. Furthermore, the function or expression of ion channels in the microcirculation may differ from those in conduit vessels. There is substantial evidence from the pulmonary circulation for differences in expression and function of K+ channels as one moves from conduit vessels toward the microcirculation (11,12,29,107,137). In the coronary circulation, there is a higher density of inward rectifier K+ channel currents in smooth muscle cells from resistance arteries compared to large coronary arteries (123), and the density of currents through L-type Ca2+ channels increases progressively from conduit arteries to large arterioles (16). The Ca2+ threshold for activation (i.e., the Ca2+ setpoint) of large-conductance Ca2+-activated K+ channels is 10-fold higher in smooth muscle cells from skeletal muscle arterioles, compared to smooth muscle isolated from conduit arteries (71). These examples support the hypothesis that ion channels in the cells that make up the walls of vessels in the microcirculation display unique function or expression patterns that remain largely unexplored. For the purposes of this brief review, I focus on potassium channel expression and function in the plasma membrane of smooth muscle and endothelial cells in arterioles in the peripheral microcirculation. Readers are directed to other sources for more comprehensive, in depth reviews of K+ channels and other ion channels in the cardiovascular system (15,63,69,70,115–118,124,138).
Figure 1.
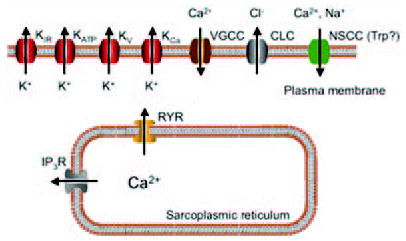
Ion channels expressed in arteriolar smooth muscle cells. Schematic diagram indicating the families of ion channels expressed in smooth muscle cells in the wall of arterioles. Current evidence (see text for details and references) indicate that arteriolar smooth muscle cells express inward rectifier K+ channels, ATP-sensitive K+ channels (KATP), several different types of voltage-gated K+ channels (KV), one or more types of calcium-activated K+ channels (KCa), one or more types of voltage-gated Ca2+ channels (VGCC), two or more types of Cl− channels (CLC), and several different types of nonselective cation channels (NSCC) likely from the transient receptor potential (Trp) family of channels. Intracellular ion channels include Ca2+-activated Ca2+ release channels (ryanodine receptors, RYR) and inositol 3,4,5-trisphosphate receptors (IP3R). It should also be noted that other channels also are likely expressed in intracellular membranes, including those of mitochondria (not shown).
POTASSIUM CHANNELS IN ARTERIOLAR SMOOTH MUSCLE CELLS
Smooth muscle cells in the walls of arterioles in the microcirculation play a central role in the regulation of total peripheral resistance (and hence blood pressure), the distribution of blood flow between and within tissues and organs; and microvascular exchange. Potassium channels importantly contribute to the regulation of smooth muscle tone and, hence, all of these functions (69,70). As in most cells in the body, K+ channels are the dominant ion channels expressed in the plasma membrane of arteriolar smooth muscle cells, and currents through K+ channels contribute substantially to the membrane potential in these cells (Figure 2). Because of the high input resistance of the plasma membrane in smooth muscle cells (on the order of 10 GΩ (67,69,115,116)), it is important to remember that only small changes in steady-state membrane current are required to produce significant changes in membrane potential. Membrane potential, in turn, regulates the activity of voltage-gated Ca2+ channels, controlling Ca2+ influx and intracellular Ca2+. In smooth muscle cells, because of the K+ electrochemical gradient, opening of K+ channels leads to K+ efflux from cells and membrane hyperpolarization. This closes voltage-gated Ca2+ channels, reducing intracellular Ca2+ and leading to vasodilation. Conversely, closure of open K+ channels causes membrane depolarization, opening Ca2+ channels and leading to Ca2+-dependent vasoconstriction. Studies over the past 20 years have identified at least four different classes of K+ channels expressed by arteriolar smooth muscle cells. These include inward rectifier K+ (KIR) channels, ATP-sensitive K+ (KATP) channels, Ca2+-activated K+ (KCa) channels, and voltage-activated K+ (KV) channels, as summarized in Table 1.
Figure 2.
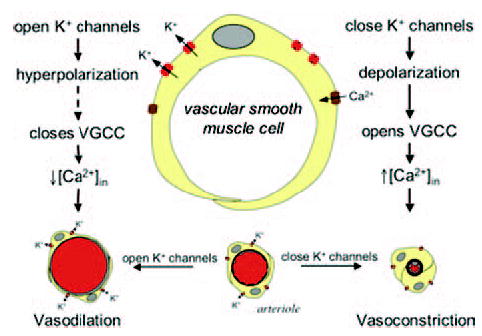
Potassium channels regulate arteriolar smooth muscle tone by affecting membrane potential. Schematic diagram of an arteriolar muscle cell and cross sections of arterioles with open or closed K+ channels. VGCC, voltage-gated Ca2+ channel.
Table 1.
Potassium channels in arteriolar smooth muscle and endothelial cells
| Ion channela | VSMb | ENDOc | Additional subunits | Outward currents activated by: | Outward currents inhibited by: | Antagonists |
|---|---|---|---|---|---|---|
| BKCa | Slo1 (19,141) | - or ? | Slo-β (19,141) | Depolarization, ↑[Ca2+]in (69,115), NO(10), CO (149), EETs(162), PKA(115), PKG(115), cSrc (96)? | Hyperpolarization, ↓[Ca2+]in (115), PKC (91,110), cSrc (6)? | Iberiotoxin(45), charybdotoxin(109), penitrem A(83), paxillin (83), 1 mM TEA (115) |
| sKCa | - or ? | SK3 (87) | Calmodulin (117) | ↑[Ca2+]in (117) | ↓[Ca2+]in (117) | Apamin(117), d-tubocurarine(48), TBA(53) |
| IKCa | — | IK1(87) | Calmodulin (117) | ↑[Ca2+]in (117) | ↓[Ca2+]in (117) | TRAM-39(39,60), TRAM-34(39,60), charybdotoxin(117), clotrimazole(39,60), TBA(114) |
| KV | KV 1.5, 1.6(25,26) + ? | KV 1.5, 1.6? (25,26,37,42,62) | KVβ (31,143) | Depolarization (115), PKA (1–3), ↓pH (13) | Hyperpolarization (115), PKC (4), ↑[Ca2+]in (30), Rho kinase (101) | 4-aminopyridine (25,26,115), correolide, Agitooxin-2 (25,26) |
| KATP | KIR 6.1 (111,139) | KIR 6.1 or 6.2? 1997 (92,133) | SUR2b (111) | ↓ATP, ↑ADP, PKA, PKG (115,124), pinacidil, cromakalim, etc. (115) | ↑ATP, ↓ADP (124), ↑[Ca2+]in (via calcineuin) (155), PKC (27,56,115,142), | Glibenclamide, tolbutamide, TPA, Ba2+ (115,124) |
| KIR | KIR 2.1 (161) | KIR 2.1 (117) | — | Hyperpolarization, ↑[K+]out (124), CNP (24), NO (134) | Depolarization, ↓[K+]out (124), ? | Ba2+, quinidine, phencyclidine (115,124) |
Note. PKA, protein kinase A; PKG, cGMP-activated protein kinase; PKC, protein kinase C (see text for other abbreviations). Inhibitor abbreviations: TEA, tetraethyl ammonium; TBA, tetrabutyl ammonium; TPA, tetrapentyl ammonium. —, not present; ?, present, but specific isoform unclear, or mechanism unclear (see text for references or more information).
See text for definitions of channel abbreviations.
Vascular smooth muscle.
Endothelium.
SMOOTH MUSCLE KIR CHANNELS
Inward-rectifier K+ channels derive their name from the fact that at membrane potentials negative to the potassium equilibrium potential, these channels conduct K+ ions into cells, whereas at more positive potentials, outward K+ current flow is limited (124). Recent studies suggest that the KIR channel isoform expressed in smooth muscle is KIR 2.1 (17,161). These channels are blocked by Ba2+ ions at micromolar concentrations and are activated by increases in extracellular K+ (124). In coronary and cerebral microcirculations, smooth muscle KIR channels act as sensors for increases in extracellular K+, leading to membrane hyperpolarization and vasodilation when extracellular K+ is elevated from 5 mM to 8–15 mM (38,84,115,124,125). Current density through KIR channels in coronary smooth muscle increases from conduit arteries into small, resistance arteries, as noted above (123). This difference in K+ channel expression largely explains the observation that conduit arteries have little response to small elevations in extracellular K+, whereas resistance arteries display a robust dilation (123,124). In skeletal muscle microcirculation, KIR channels appear to play a more modulatory role affecting primarily the duration and kinetics of K+-induced smooth muscle hyperpolarization and vasodilation (20). Inward-rectifier K+ channels also may be activated by C-type natriuretic peptide, a putative endothelium-derived hyperpolarizing factor (EDHF) (24). Bradykinin may activate KIR channels in coronary arterioles, and it has been proposed that these channels participate in propagation of hyperpolarizing signals along arterioles (128). In other systems, KIR channels can be modulated by protein kinases (156) or G-proteins (77), suggesting that their vascular counterparts may also be regulated. This hypothesis is supported by recent observations showing that in some blood vessels, NO may activate KIR channels (134). Inward rectifier K+ channels may be downregulated during hypertension (106,138).
SMOOTH MUSCLE KATP CHANNELS
ATP-sensitive K+ channels close with increases in intracellular ATP, hence their name (124). They also are modulated by a myriad of other intracellular signals, including ADP, H+, and Ca2+ (124). These channels in smooth muscle are likely composed of a tetramer of KIR 6.1 subunits that form the ion conductive pore (111,139), and complementary regulatory sulfonylurea receptor (SUR) subunits, SUR 2B (111). KATP channels are blocked by sulfonylureas like glibenclamide and opened by activators such as pinacidil and cromakalim (124). In skeletal muscle and the heart, KATP channels are open at rest and contribute to resting smooth muscle membrane potential and tone (43,66,73,88,129,146,147). These channels also appear to play a role in the mechanism of action of both vasodilators (66,70,73) and vasoconstrictors (70,115,142,155). Studies have shown that KATP channels may be activated by protein kinase A and c-GMP-dependent protein kinase and have been implicated in the mechanism of action of endogenous vasodilators such as adenosine (34,66), PGI2 (66), calcitonin-gene-related-peptide (CGRP) (115), and NO (115). Conversely, activation of protein kinase C (27,56,115,142) and elevation of intracellular Ca2+ (acting through calcineurin) (155) by vasoconstrictors such as norepinephrine (68), vasopressin (148), endothelin (113), and angiotensin II (56,112) close these channels. Smooth muscle ATP-sensitive K+ channels also appear to be downregulated in diseases such as diabetes (33,104,105,111,164) and hypertension (138), potentially contributing to end-organ problems in these diseases.
SMOOTH MUSCLE KCA CHANNELS
The dominant KCa channels expressed by microvascular smooth muscle cells appear to be large (Big) conductance, BKCa channels. These channels have a high single-channel conductance (240 pS, hence their name) and are activated by membrane depolarization and increases in intracellular Ca2+ (69,115). They are composed of Slo1 subunits that form the pore, and Slo β1 subunits that modulate the Ca2+ sensitivity of the channel (19,141). BKCa channels are blocked by millimolar concentrations of tetraethyl ammonium ions (55); scorpion toxins such as charybdotoxin (also blocks other K+ channels)(109) and iberiotoxin (highly selective) (45); and indoles such as penitrem A and paxilline (83). These channels are activated by compounds such as NS 1619 (115).
Like voltage-gated Ca2+ channels (35,36,80), BKCa channels likely exist in signaling complexes with voltage-gated Ca2+ channels, protein kinases (protein kinase A, cGMP-dependent protein kinase, protein kinase C, cSrc, etc.), phosphatases, and other signaling proteins (6,80,98). These macromolecular signaling complexes also appear to be located adjacent to smooth endoplasmic reticulum Ca2+-activated, Ca2+-release channels (ryanodine receptors) such that focal release of Ca2+ from ryanodine receptors triggered by Ca2+ influx through voltage-gated Ca2+ channels (i.e., Ca2+ sparks) can activate BKCa channels in smooth muscle (75).
Studies of microcirculatory beds in vivo have shown that microvascular BKCa channels do not appear to contribute to resting membrane potential under normal conditions (71,72,100,122). This appears to result, at least in part, from the channels having a high threshold for activation by Ca2+, compared to what has been observed in smooth muscle from conduit arteries (71). However, BKCa channels in the microcirculation are opened and play an important negative feedback role during active agonist- and stretch-induced vasoconstriction (70,71,85,154,158). The impact of vasoconstrictor-induced activation of BKCa channels due to membrane depolarization and elevated intracellular Ca2+ may be moderated by the activity of protein kinase C, which appears to inhibit these channels in some systems (91,110). In addition, recent in vitro studies suggest that these channels may be closed by cSrc tyrosine kinases that are activated by vasoconstrictors such as serotonin (6). These observations are complicated by the findings that tyrosine kinases like cSrc (96), Pyk2, and Hck (95) enhance the activity of BKCa channels expressed in HEK 293 cells.
A number of vasodilators, including NO (10), CO (149) and epoxides of arachidonic acid (EETs) (162), appear to activate BKCa channels in some systems either directly or by activation of protein kinases (see (70)). In hypertension, the functional expression of these channels is upregulated (31,138) and they contribute substantially to resting membrane potential and tone in this disease state (31). Recent data suggest that hypertension also may alter the expression of the modulatory β1 subunits, affecting the Ca2+ sensitivity of these channels in this disease (7,9). Expression and function of BKCa channels also may be altered in hypercholesterolemia and atherosclerosis (138).
Some microvascular smooth muscle also may express small conductance KCa (sKCa) channels (50). These channels require calmodulin for Ca2+ sensitivity (unlike BKCa channels that have an intrinsic Ca2+ sensor), have a single-channel conductance of 10 pS, and are blocked by the bee venom peptide, apamin (15). Their physiological function in smooth muscle has not been well studied but recent evidence suggests that in some smooth muscle cells, sKCa channels may be activated by arachidonic acid (49).
SMOOTH MUSCLE KV CHANNELS
Smooth muscle cells in the microcirculation also express a variety of KV channels, including members of the KV 1.5 and 1.6 families (25,26), and likely others (8,81,143). Like BKCa channels, KV channels are composed of a tetramer of KVα subunits that form the ion conductive pore of the channels, along with accessory KVβ subunits (31,143). These channels are activated by membrane depolarization and likely participate in the negative feedback regulation of membrane potential along with BKCa channels. Blockers of microvascular smooth muscle KV channels include 4-aminopyridine (25,26,31,72,115), correolide, and agitotoxin-2 (25,26). In vitro studies have demonstrated that the activity of KV channels contributes to resting membrane potential and smooth muscle tone (25,26,67,72). These channels appear to be activated by the cAMP-protein kinase A signaling pathway (1–3) such that they may be involved in the mechanism of action of vasodilators such as adenosine, PGI2 and CGRP. Decreased intracellular pH activates KV channels in coronary myocytes (13). As with KATP channels, vasoconstrictors tend to close KV channels through signaling pathways involving protein kinase C (4) and Ca2+ (30). Recent studies indicate that Rho kinase also may close these channels (101). All of these effects likely contribute to vasoconstrictor-induced depolarization of arteriolar smooth muscle. There appears to be a functional downregulation of smooth muscle KV channels in hypertension, despite evidence that expression of the channel protein is increased (31). This functional downregulation may be related to increased intracellular Ca2+ observed in myocytes from hypertensive animals (31). Hyperglycemia has been shown to impair KV channel function and expression in small coronary arteries (99). The function of KV channels in the microcirculation in vivo has not been established due to the ubiquitous expression of multiple classes of these channels and due to the lack of selective inhibitors/activators.
ARTERIOLAR ENDOTHELIAL CELL K+ CHANNELS
Endothelial cells also express a diverse array of K+ channels (Figure 3). However, relatively little is known about the channels expressed and their function in arteriolar endothelial cells. Data from studies of endothelial cells from different tissues suggest that arteriolar endothelial cells express KATP, KV, KIR, and two types of KCa channels (see below) (117). Potassium channels in endothelial cells appear to play an important role in endothelial cell signaling through regulation of endothelial cell membrane potential (117). Endothelial cells are electrically coupled to one another by gap junctions (117), thus changes in endothelial cell membrane potential have the ability to be summed and transmitted along the endothelium providing a mechanism for long-distance transmission of signals in the microcirculation (41,97). In addition, endothelial cells may be electrically coupled to overlying smooth muscle cells by myoendothelial gap junctions such that changes in endothelial cell membrane potential may be transmitted to smooth muscle cells to modulate arteriolar tone accordingly (40,131,132).
Figure 3.
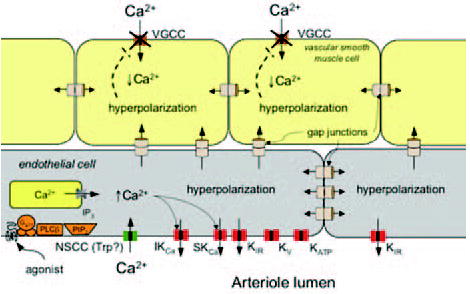
Ion channels expressed in arteriolar endothelial cells. Schematic of a longitudinal section of an arteriole showing cross sections of endothelial cells and smooth muscle cells. Ion channels expressed by microvascular endothelial cells include ATP-sensitive K+ channels (KATP), voltage-gated K+ channels (KV), inward rectifier K+ channels (KIR), small conductance (sKCa) and intermediate conductance (IKCa) Ca+-activated K+ channels, and several types of non-selective cation channels (NSCC), likely from the transient receptor potential (Trp) family of channels. Intracellular ion channels include IP3 receptors (IP3R). Also shown are pathways for activation of endothelial K+ channels leading to endothelial membrane hyperpolarization (see text for references). Agonists such as acetylcholine, substance P, or bradykinin bind to G-protein coupled receptors (Gq/11) and activate phospholipase Cβ (PLCβ) that acts on membrane phospholipids (PIP2) to form IP3. This second messenger binds to IP3 receptors (IP3R) in the membrane of the smooth endoplasmic reticulum, releasing stored Ca2+. Release of Ca2+ from intracellular stores, as well as G-protein-mediated events and activation of other signaling cascades open nonselective cation channels (NSCC), allowing extracellular Ca+2 to diffuse into the cells. Release of Ca2+ and increased Ca2+ influx increase intracellular Ca2+, leading to formation of endothelium derived autacoids such as NO, PGI2 and epoxides of arachidonic acid (EETS) (not shown in figure). Elevated intracellular Ca2+ also activates IKCa and/or sKCa channels, leading to K+ efflux and membrane hyperpolarization. The K+ released through these channels and the membrane hyperpolarization may activate outward currents through KIR channels, supporting the hyperpolarization. Because cells in the walls of arterioles are coupled by gap junctions, the endothelial cell hyperpolarization may be transmitted to other endothelial cells and to smooth muscle cells, leading to smooth muscle hyperpolarization, closure of voltage-gated Ca2+ channels (VGCC), a reduction in intracellular Ca2+, and vasodilation. Transmission of hyperpolarization through gap junctions to adjacent endothelial cells also may activate KIR channels in these cells and allow conduction of hyperpolarizing signals for long distances along the endothelium.
Through their impact on membrane potential, endothelial cell K+ channels also have been proposed to regulate Ca2+ influx into endothelial cells (117). Although there are a few reports that cultured endothelial cells express voltage-gated Ca2+ channels (see (117) for references), most data suggest that calcium influx into endothelial cells occurs through non-selective cation channels, such as those formed by the transient receptor potential (TRP) family of ion channel proteins (117,118). Ion flow through these channels is determined by the electrochemical gradient across the cell membrane, which is determined by the concentration gradient for the ion (Ca2+ in this case), the membrane potential, and the conductance of the ion channel (117). Thus, changes in membrane potential, by altering the electrochemical gradient, can influence the movement of ions through the channel. In cultured endothelial cells (59,117), and perhaps in microvascular endothelial cells in situ (57,58), it has been shown that endothelial cell membrane potential strongly influences Ca2+ influx through nonselective cation channels: depolarization inhibits Ca2+ influx and hyperpolarization increases Ca2+ influx. However, recent data suggest that this may not be the case in endothelial cells in small arteries and arterioles where Ca2+ signaling appears to be independent from changes in membrane potential (see below for more on this topic) (28,102). Table 1 summarizes the K+ channels expressed in endothelial cells and factors that modulate these channels.
ENDOTHELIAL KATP CHANNELS
In addition to their presence in smooth muscle cells, microvascular endothelial cells also may express KATP channels (76,92,133). For the most part, their presence and function has been inferred by effects of the sulphonylurea KATP channel antagonist, gliben-clamide, and by effects of KATP channel agonists such as pinacidil and cromakalim. Endothelial KATP channels have been implicated in arteriolar dilation induced by hyperosmolarity (65), adenosine (90,103), and isofluorane (46). In cultured cells, shear stress has been shown to increase expression of these channels (23). Effects of disease states on endothelial KATP channel expression or function have not be studied.
ENDOTHELIAL KV CHANNELS
Several recent studies suggest that microvascular endothelial cells may express several different classes of KV channels (25,26,37,42,62). However, their physiological function has not been established. It has been suggested that these channels may participate in membrane potential oscillations and negative feedback regulation of membrane potential (37).
ENDOTHELIAL KIR CHANNELS
Endothelial cells in arterioles appear to express strongly rectifying KIR channels, probably KIR 2.1 (117). At first glance, the function of these channels in arterioles is not apparent because the resting membrane potential of arteriolar endothelial cells is on the order of –30 mV (40,152), which is outside of the membrane potential range where these channels significantly contribute to membrane currents with normal extracellular K+ concentrations (124) (Figure 4). However, as in smooth muscle, endothelial cell KIR channels may function as sensors for elevated extracellular K+ and provide a hyperpolarizing signal to alter microvessel function (Figure 4). In fact, in small mesenteric arteries, endothelial KIR channels may contribute to K+-induced dilation as smooth muscle cells in these vessels lack these channels (32). Interestingly, in smooth muscle cells isolated from cremasteric arterioles (20), while KIR channel currents can be recorded, they lack the outward “hump” that is observed in endothelial cells isolated from the same vessels (see Figure 4) and that is required for these currents to contribute to membrane hyperpolarization. Thus, it is possible that endothelial cell KIR channels contribute to K+-induced dilation in cremasteric arterioles as well.
Figure 4.
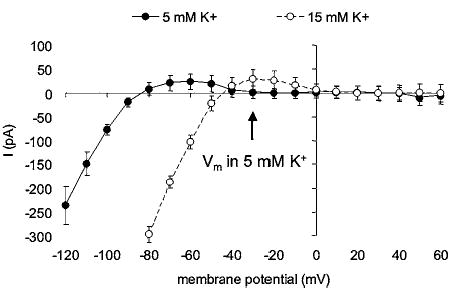
Inward rectifier K+ (KIR) channel currents in arteriolar endothelial cells. Data are means ± SE (n = 5). Ba2+-sensitive (100 μM) difference currents measured in the presence of 5 or 15 mM K+ in the extracellular fluid as indicated. Endothelial cells were isolated enzymatically from hamster cremaster arterioles and membrane currents studied using the amphotericin B perforated patch technique as described previously for arteriolar smooth muscle cells (72). Note that at normal extracellular K+ (5 mM), at the resting membrane potential of these cells (−30 mV), there is little or no outward current. However, with an increase in extracellular K+, the current–voltage relationship shifts to the right such that outward current through the KIR channels is present. This would tend to hyperpolarize the cells toward, in this case, −45 mV. Note also that in physiological K+ (5 mM), membrane hyperpolarization induced by opening of other K+ channels could recruit outward current through KIR channels, amplifying the original hyperpolarization.
In addition to serving as sensors for extracellular K+, KIR channels have the potential to act as amplifiers of hyperpolarization initiated by the opening of other K+ channels, in particular sKCa and IKCa channels (Figures 3 and 4). Hyperpolarization-induced activation of outward currents through KIR channels also may contribute to conduction of hyperpolarizing signals along endothelial cells as proposed for smooth muscle KIR channels (128) (Figure 3).
Studies of cultured macrovascular endothelial cells also suggest that KIR channels may be involved in shear-stress-induced hyperpolarization of endothelial cells (74), and their expression may be modulated by fluid shear (61), although this has not been established in native microvascular endothelial cells. Vasoconstrictors such as angiotensin II, vasopressin, endothelin, and histamine have been reported to inhibit KIR channels in endothelial cells by a G-protein-dependent mechanism (117). However, the functional significance of this phenomenon has yet to be established.
ENDOTHELIAL KCA CHANNELS
Endothelial cells appear to express at least two classes of calcium-activated K+ channels: small conductance (sKCa, probably sK3 (87)) and intermediate conductance (IKCa, probably IK1 (87)) K+ channels. Distinct from BKCa channels, these channels have a smaller single channel conductance (10 pS for sKCa and 30–80 pS for IKCa (117)), are not voltage-activated, and require the Ca2+ binding protein, calmodulin, to display Ca2+ sensitivity (117). They also have a distinct pharmacology. Both channels are insensitive to iberiotoxin and blockade by millimolar TEA. The bee venom peptide apamin blocks sKCa channels (117), as do tetrabutylammonium ions (53). Charybdotoxin, which blocks, among other things, BKCa channels, also potently blocks IKCa channels (117). IKCa channels also are antagonized by clotrimazole and its derivatives such as TRAM 39 and TRAM 34 (39,60). Depending on the vessel and the species studied, sKCa (Cor de Wit, personal communication), IKCa (102) or both channels mediate agonist-induced hyperpolarization of endothelial cells and play a major role in arteriolar dilation induced by endothelium-dependent vasodilators, such as acetylcholine, substance P, bradykinin, and histamine (21,135,136) (Figure 3). The hyperpolarization induced by Ca2+-triggered opening of these channels may be conducted along the endothelium via endothelial gap junctions (40) and transmitted to smooth muscle cells through myoendothelial gap junctions to cause vasodilation (40,102,131,132) (Figure 3). As noted above, in studies of macrovascular endothelial cells, this hyperpolarization also has been shown to increase the driving force for endothelial cell Ca2+ entry and autacoid production (117). However, recent studies suggest that arteriolar endothelial cell Ca2+ signaling may be independent from changes in endothelial cell membrane potential (28,102,140). This suggests that arteriolar endothelial cells may have novel Ca2+ influx pathways, or that Ca2+ handling in these cells may be different from what has been described previously in cultured cells.
There is a paucity of information on regulation of endothelial IKCa channels by factors other than Ca2+. In cultured cells, shear stress has been demonstrated to increase the expression of IKCa channels (18). The impact of disease states on endothelial KCa channel expression and function in the microcirculation has not been established. However, IKCa channel expression is increased in mesenteric endothelial cells from patients with colonic cancer (86). Conversely, sKCa and IKCa channel expression and function are diminished in regenerated endothelium after balloon injury (87).
QUESTIONS FOR THE FUTURE
Thus, ion channels participate in all aspects of microvascular function. However, given the diversity in expression patterns and multiple mechanisms of regulation of the array of ion channels found in cells that make up microvessels, it is clear that much remains unknown. The use of genomic (52,94) and proteomic approaches, applied specifically to the microcirculation, appear to be efficient approachs to examine the large number and the diversity of expression patterns of ion channels in microvascular smooth muscle cells, pericytes, and endothelial cells. In addition, the use of gene silencing methods, such as antisense oligonucleotides (51) and siRNA (108), in conjunction with conventional pharmacology provides a powerful set of tools to examine the function of specific ion channels in the microcirculation. Indeed, the antisense approach has already proven successful in identification of a component of stretch-activated cation channels in resistance artery smooth muscle cells in vitro (153). These approaches, along with the simultaneous measurement of membrane potential, intracellular Ca2+, and diameter (or other functional assay of vessel function) must also be taken in vivo, so that the true role played by ion channels in the microcirculation in health and disease states can be established.
Acknowledgments
Dr. Jackson is supported by PHS grant HL 32469 from the National Heart, Lung and Blood Institute. Thanks are offered to Susan K. Kovats for her superb technical assistance and to Kenneth D. Cohen for his patch clamp expertise in collecting the data shown in fig. 4.
References
- 1.Aiello EA, Walsh MP, Cole WC. Isoproterenol and forskolin increase and PKI inhibits delayed rectifier K+ current in vascular myocytes isolated from rabbit coronary artery and portal vein. Can J Physiol Pharmacol. 1994;72:47. [Google Scholar]
- 2.Aiello EA, Walsh MP, Cole WC. Phosphorylation by protein kinase A enhances delayed rectifier K+ current in rabbit vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 1995;268:H926–H934. doi: 10.1152/ajpheart.1995.268.2.H926. [DOI] [PubMed] [Google Scholar]
- 3.Aiello EA, Malcolm AT, Walsh MP, Cole WC. Beta-adrenoceptor activation and PKA regulate delayed rectifier K+ channels of vascular smooth muscle cells. Am J Physiol. 1998;275:H448–H459. doi: 10.1152/ajpheart.1998.275.2.H448. [DOI] [PubMed] [Google Scholar]
- 4.Aiello EA, Clement-Chomienne O, Sontag DP, Walsh MP, Cole WC. Protein kinase C inhibits delayed rectifier K+ current in rabbit vascular smooth muscle cells. Am J Physiol. 1996;271:H109–H119. doi: 10.1152/ajpheart.1996.271.1.H109. [DOI] [PubMed] [Google Scholar]
- 5.Albert AP, Large WA. Store-operated Ca2+-permeable non-selective cation channels in smooth muscle cells. Cell Calcium. 2003;33:345–356. doi: 10.1016/s0143-4160(03)00048-4. [DOI] [PubMed] [Google Scholar]
- 6.Alioua A, Mahajan A, Nishimaru K, Zarei MM, Stefani E, Toro L. Coupling of c-Src to large conductance voltage- and Ca2+-activated K+ channels as a new mechanism of agonist-induced vasoconstriction. Proc Natl Acad Sci U S A. 2002;99:14560–14565. doi: 10.1073/pnas.222348099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amberg GC, Santana LF. Downregulation of the BK channel beta1 subunit in genetic hypertension. Circ Res. 2003;93:965–971. doi: 10.1161/01.RES.0000100068.43006.36. [DOI] [PubMed] [Google Scholar]
- 8.Amberg GC, Koh SD, Imaizumi Y, Ohya S, Sanders KM. A-type potassium currents in smooth muscle. Am J Physiol Cell Physiol. 2003;284:C583–C595. doi: 10.1152/ajpcell.00301.2002. [DOI] [PubMed] [Google Scholar]
- 9.Amberg GC, Bonev AD, Rossow CF, Nelson MT, Santana LF. Modulation of the molecular composition of large conductance, Ca(2+) activated K(+) channels in vascular smooth muscle during hypertension. J Clin Invest. 2003;112:717–724. doi: 10.1172/JCI18684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Archer SL, Huang JM, Hampl V, Nelson DP, Shultz PJ, Weir EK. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1994;91:7583–7587. doi: 10.1073/pnas.91.16.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Archer SL, Huang JMC, Reeve HL, Hampl V, Tolarova S, Michelakis E, Weir EK. Differential distributuion of electrophysiologically distinct myocytes in conduit and resistance arteries determines their response to nitric oxide and hypoxia. Circ Res. 1996;78:431–441. doi: 10.1161/01.res.78.3.431. [DOI] [PubMed] [Google Scholar]
- 12.Archer SL, Wu XC, Thebaud B, Nsair A, Bonnet S, Tyrrell B, McMurtry MS, Hashimoto K, Harry G, Michelakis ED. Preferential expression and function of voltage-gated, O2-sensitive K+ channels in resistance pulmonary arteries explains regional heterogeneity in hypoxic pulmonary vasoconstriction: ionic diversity in smooth muscle cells. Circ Res. 2004;95:308–318. doi: 10.1161/01.RES.0000137173.42723.fb. [DOI] [PubMed] [Google Scholar]
- 13.Berger MG, Vandier C, Bonnet P, Jackson WF, Rusch NJ. Intracellular acidosis differentially regulates Kv channels in coronary and pulmonary vascular muscle. Am J Physiol. 1998;275:H1351–H1359. doi: 10.1152/ajpheart.1998.275.4.H1351. [DOI] [PubMed] [Google Scholar]
- 14.Bolton TB, Prestwich SA, Zholos AV, Gordienko DV. Excitation-contraction coupling in gastrointestinal and other smooth muscles. Annu Rev Physiol. 1999;61:85–115. doi: 10.1146/annurev.physiol.61.1.85. [DOI] [PubMed] [Google Scholar]
- 15.Bond CT, Maylie J, Adelman JP. Small-conductance calcium-activated potassium channels. Ann N Y Acad Sci. 1999;868:370–378. doi: 10.1111/j.1749-6632.1999.tb11298.x. [DOI] [PubMed] [Google Scholar]
- 16.Bowles DK, Hu Q, Laughlin MH, Sturek M. Heterogeneity of L-type calcium current density in coronary smooth muscle. Am J Physiol. 1997;273:H2083–H2089. doi: 10.1152/ajpheart.1997.273.4.H2083. [DOI] [PubMed] [Google Scholar]
- 17.Bradley KK, Jaggar JH, Bonev AD, Heppner TJ, Flynn ER, Nelson MT, Horowitz B. Kir2.1 encodes the inward rectifier potassium channel in rat arterial smooth muscle cells. J Physiol. 1999;515:639–651. doi: 10.1111/j.1469-7793.1999.639ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brakemeier S, Kersten A, Eichler I, Grgic I, Zakrzewicz A, Hopp H, Kohler R, Hoyer J. Shear stress-induced up-regulation of the intermediate-conductance Ca(2+)-activated K(+) channel in human endothelium. Cardiovasc Res. 2003;60:488–496. doi: 10.1016/j.cardiores.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Brenner R, Perez GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- 20.Burns WR, Cohen KD, Jackson WF. K+-induced dilation of hamster cremasteric arterioles involves both the Na+/K+-ATPase and inward-rectifier K+ channels. Microcirculation. 2004;11:279–293. doi: 10.1080/10739680490425985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- 22.Cartin L, Lounsbury KM, Nelson MT. Coupling of Ca(2+) to CREB activation and gene expression in intact cerebral arteries from mouse: roles of ryanodine receptors and voltage-dependent Ca(2+) channels. Circ Res. 2000;86:760–767. doi: 10.1161/01.res.86.7.760. [DOI] [PubMed] [Google Scholar]
- 23.Chatterjee S, Al-Mehdi AB, Levitan I, Stevens T, Fisher AB. Shear stress increases expression of a KATP channel in rat and bovine pulmonary vascular endothelial cells. Am J Physiol Cell Physiol. 2003;285:C959–C967. doi: 10.1152/ajpcell.00511.2002. [DOI] [PubMed] [Google Scholar]
- 24.Chauhan SD, Nilsson H, Ahluwalia A, Hobbs AJ. Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc Natl Acad Sci U S A. 2003;100:1426–1431. doi: 10.1073/pnas.0336365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheong A, Dedman AM, Beech DJ. Expression and function of native potassium channel [K(V)alpha1] subunits in terminal arterioles of rabbit. J Physiol. 2001;534:691–700. doi: 10.1111/j.1469-7793.2001.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheong A, Dedman AM, Xu SZ, Beech DJ. K(V)alpha1 channels in murine arterioles: differential cellular expression and regulation of diameter. Am J Physiol Heart Circ Physiol. 2001;281:H1057–H1065. doi: 10.1152/ajpheart.2001.281.3.H1057. [DOI] [PubMed] [Google Scholar]
- 27.Chrissobolis S, Sobey CG. Inhibitory effects of protein kinase C on inwardly rectifying K+- and ATP-sensitive K+ channel-mediated responses of the basilar artery. Stroke. 2002;33:1692–1697. doi: 10.1161/01.str.0000016966.89226.67. [DOI] [PubMed] [Google Scholar]
- 28.Cohen KD, Jackson WF. Membrane hyperpolarization is not required for sustained muscarinic receptor mediated increases in arteriolar endothelial cell Ca2+ FASEB J. 2004;18:A251. doi: 10.1080/10739680590904973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coppock EA, Tamkun MM. Differential expression of K(V) channel alpha- and beta-subunits in the bovine pulmonary arterial circulation. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1350–L1360. doi: 10.1152/ajplung.2001.281.6.L1350. [DOI] [PubMed] [Google Scholar]
- 30.Cox RH, Petrou S. Ca(2+) influx inhibits voltage-dependent and augments Ca(2+)-dependent K(+) currents in arterial myocytes. Am J Physiol. 1999;277:C51–C63. doi: 10.1152/ajpcell.1999.277.1.C51. [DOI] [PubMed] [Google Scholar]
- 31.Cox RH, Rusch NJ. New expression profiles of voltage-gated ion channels in arteries exposed to high blood pressure. Microcirculation. 2002;9:243–257. doi: 10.1038/sj.mn.7800140. [DOI] [PubMed] [Google Scholar]
- 32.Crane GJ, Walker SD, Dora KA, Garland CJ. Evidence for a differential cellular distribution of inward rectifier K channels in the rat isolated mesenteric artery. J Vasc Res. 2003;40:159–168. doi: 10.1159/000070713. [DOI] [PubMed] [Google Scholar]
- 33.Crijns FR, Struijker Boudier HA, Wolffenbuttel BH. Arteriolar reactivity in conscious diabetic rats: influence of aminoguanidine treatment. Diabetes. 1998;47:918–923. doi: 10.2337/diabetes.47.6.918. [DOI] [PubMed] [Google Scholar]
- 34.Dart C, Standen NB. Adenosine-activated potassium current in smooth muscle cells isolated from the pig coronary artery. J Physiol (London) 1993;471:767–786. doi: 10.1113/jphysiol.1993.sp019927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davare MA, Horne MC, Hell JW. Protein phosphatase 2A is associated with class C L-type calcium channels (Cav1.2) and antagonizes channel phosphorylation by cAMP-dependent protein kinase. J Biol Chem. 2000;275:39710–39717. doi: 10.1074/jbc.M005462200. [DOI] [PubMed] [Google Scholar]
- 36.Davare MA, Avdonin V, Hall DD, Peden EM, Burette A, Weinberg RJ, Horne MC, Hoshi T, Hell JW. A beta2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1.2. Science. 2001;293:98–101. doi: 10.1126/science.293.5527.98. [DOI] [PubMed] [Google Scholar]
- 37.Dittrich M, Daut J. Voltage-dependent K(+) current in capillary endothelial cells isolated from guinea pig heart. Am J Physiol. 1999;277:H119–H127. doi: 10.1152/ajpheart.1999.277.1.H119. [DOI] [PubMed] [Google Scholar]
- 38.Edwards FR, Hirst GDS, Silverberg GD. Inward rectification in rat cerebral arterioles: involvement of potassium ions in autoregulation. J Physiol. 1988;404:455–466. doi: 10.1113/jphysiol.1988.sp017299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eichler I, Wibawa J, Grgic I, Knorr A, Brakemeier S, Pries AR, Hoyer J, Kohler R. Selective blockade of endothelial Ca2+-activated small- and intermediate-conductance K+-channels suppresses EDHF-mediated vasodilation. Br J Pharmacol. 2003;138:594–601. doi: 10.1038/sj.bjp.0705075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Emerson GG, Segal SS. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: role in vasomotor control. Circ Res. 2000;87:474–479. doi: 10.1161/01.res.87.6.474. [DOI] [PubMed] [Google Scholar]
- 41.Emerson GG, Segal SS. Endothelial cell pathway for conduction of hyperpolarization and vasodilation along hamster feed artery. Circ Res. 2000;86:94–100. doi: 10.1161/01.res.86.1.94. [DOI] [PubMed] [Google Scholar]
- 42.Fan J, Walsh KB. Mechanical stimulation regulates voltage-gated potassium currents in cardiac microvascular endothelial cells. Circ Res. 1999;84:451–457. doi: 10.1161/01.res.84.4.451. [DOI] [PubMed] [Google Scholar]
- 43.Farouque HM, Worthley SG, Meredith IT, Skyrme-Jones RA, Zhang MJ. Effect of ATP-sensitive potassium channel inhibition on resting coronary vascular responses in humans. Circ Res. 2002;90:231–236. doi: 10.1161/hh0202.103713. [DOI] [PubMed] [Google Scholar]
- 44.Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1–R12. doi: 10.1152/ajpregu.00323.2002. [DOI] [PubMed] [Google Scholar]
- 45.Galvez A, Gimenez-Gallego G, Reuben JP, Roy-Contancin L, Feigenbaum P, Kaczorowski GJ, Garcia ML. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem. 1990;265:11083–11090. [PubMed] [Google Scholar]
- 46.Gamperl AK, Hein TW, Kuo L, Cason BA. Isoflurane-induced dilation of porcine coronary microvessels is endothelium dependent and inhibited by glibenclamide. Anesthesiology. 2002;96:1465–1471. doi: 10.1097/00000542-200206000-00028. [DOI] [PubMed] [Google Scholar]
- 47.Ganitkevich VY, Isenberg G. Membrane potential modulates inositol 1,4,5-trisphosphate-mediated Ca2+ transients in guinea-pig coronary myocytes. J Physiol (London) 1993;470:35–44. doi: 10.1113/jphysiol.1993.sp019845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gater PR, Haylett DG, Jenkinson DH. Neuromuscular blocking agents inhibit receptor-mediated increases in the potassium permeability of intestinal smooth muscle. Br J Pharmacol. 1985;86:861–868. doi: 10.1111/j.1476-5381.1985.tb11108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gauthier KM, Spitzbarth N, Edwards EM, Campbell WB. Apamin-sensitive K+ currents mediate arachidonic acid-induced relaxations of rabbit aorta. Hypertension. 2004;43:413–419. doi: 10.1161/01.HYP.0000110945.84443.d2. [DOI] [PubMed] [Google Scholar]
- 50.Gebremedhin D, Kaldunski M, Jacobs ER, Harder DR, Roman RJ. Coexistence of two types of Ca(2+)-activated K+ channels in rat renal arterioles. Am J Physiol. 1996;270:F69–F81. doi: 10.1152/ajprenal.1996.270.1.F69. [DOI] [PubMed] [Google Scholar]
- 51.Golden T, Dean NM, Honkanen RE. Use of antisense oligonucleotides: advantages, controls, and cardiovascular tissue. Microcirculation. 2002;9:51–64. doi: 10.1038/sj.mn.7800121. [DOI] [PubMed] [Google Scholar]
- 52.Greene AS. Application of physiological genomics to the microcirculation. Microcirculation. 2002;9:3–12. doi: 10.1038/sj.mn.7800117. [DOI] [PubMed] [Google Scholar]
- 53.Groschner K, Graier WF, Kukovetz WR. Activation of a small-conductance Ca2+-dependent K+ channel contributes to bradykinin-induced stimulation of nitric oxide synthesis in pig aortic endothelial cells. Biochim. Biophys Acta Mol Cell Res. 1992;1137:162–170. doi: 10.1016/0167-4889(92)90198-k. [DOI] [PubMed] [Google Scholar]
- 54.Harnett KM, Biancani P. Calcium-dependent and calcium-independent contractions in smooth muscles. Am J Med. 2003;115(Suppl 3A):24S–30S. doi: 10.1016/s0002-9343(03)00232-8. [DOI] [PubMed] [Google Scholar]
- 55.Hart PJ, Overturf KE, Russell SN, Carl A, Hume JR, Sanders KM, Horowitz B. Cloning and expression of a Kv1.2 class delayed rectifier K+ channel from canine colonic smooth muscle. Proc Natl Acad Sci U S A. 1993;90:9659–9663. doi: 10.1073/pnas.90.20.9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hayabuchi Y, Davies NW, Standen NB. Angiotensin II inhibits rat arterial KATP channels by inhibiting steady-state protein kinase A activity and activating protein kinase Cɛ. J Physiol. 2001;530:193–205. doi: 10.1111/j.1469-7793.2001.0193l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He P, Curry FE. Depolarization modulates endothelial cell calcium influx and microvessel permeability. Am J Physiol Heart Circ Physiol. 1991;261:H1246–H1254. doi: 10.1152/ajpheart.1991.261.4.H1246. [DOI] [PubMed] [Google Scholar]
- 58.He P, Curry FE. Endothelial cell hyperpolarization increases [Ca2+]i and venular microvessel permeability. J Appl Physiol. 1994;76:2288–2297. doi: 10.1152/jappl.1994.76.6.2288. [DOI] [PubMed] [Google Scholar]
- 59.Himmel HM, Whorton AR, Strauss HC. Intracellular calcium, currents, and stimulus–response coupling in endothelial cells. Hypertension. 1993;21:112–127. doi: 10.1161/01.hyp.21.1.112. [DOI] [PubMed] [Google Scholar]
- 60.Hinton JM, Langton PD. Inhibition of EDHF by two new combinations of K(+)-channel inhibitors in rat isolated mesenteric arteries. Br J Pharmacol. 2003;138:1031–1035. doi: 10.1038/sj.bjp.0705171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoger JH, Ilyin VI, Forsyth S, Hoger A. Shear stress regulates the endothelial Kir2.1 ion channel. Proc Natl Acad Sci U S A. 2002;99:7780–7785. doi: 10.1073/pnas.102184999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hogg DS, McMurray G, Kozlowski RZ. Endothelial cells freshly isolated from small pulmonary arteries of the rat possess multiple distinct K+ current profiles. Lung. 2002;180:203–214. doi: 10.1007/s004080000094. [DOI] [PubMed] [Google Scholar]
- 63.Hughes AD. Calcium channels in vascular smooth muscle cells. J Vasc Res. 1995;32:353–370. doi: 10.1159/000159111. [DOI] [PubMed] [Google Scholar]
- 64.Inoue R, Mori Y. Molecular candidates for capacitative and non-capacitative Ca2+ entry in smooth muscle. Novartis Found Symp. 2002246:81–90. discussion 221–227. [PubMed] [Google Scholar]
- 65.Ishizaka H, Kuo L. Endothelial ATP-sensitive potassium channels mediate coronary microvascular dilation to hyperosmolarity. Am J Physiol. 1997;273:H104–H112. doi: 10.1152/ajpheart.1997.273.1.H104. [DOI] [PubMed] [Google Scholar]
- 66.Jackson WF. Arteriolar tone is determined by activity of ATP-sensitive potassium channels. Am J Physiol Heart Circ Physiol. 1993;265:H1797–H1803. doi: 10.1152/ajpheart.1993.265.5.H1797. [DOI] [PubMed] [Google Scholar]
- 67.Jackson WF. Potassium channels and regulation of the microcirculation. Microcirculation. 1998;5:85–90. [PubMed] [Google Scholar]
- 68.Jackson WF. Hypoxia does not activate ATP-sensitive K+ channels in arteriolar muscle cells. Microcirculation. 2000;7:137–145. [PMC free article] [PubMed] [Google Scholar]
- 69.Jackson WF. Ion channels and vascular tone. Hypertension. 2000;35:173–178. doi: 10.1161/01.hyp.35.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jackson WF. (2001) Potassium channels in the circulation of skeletal muscle. In: Potassium Channels in the Cardiovascular Biology (SL Archer, NJ Rusch, Eds). New York: Kluwer Academic/Plenum, 505–522.
- 71.Jackson WF, Blair KL. Characterization and function of Ca++-activated K+ channels in hamster cremasteric arteriolar muscle cells. Am J Physiol Heart Circ Physiol. 1998;274:H27–H34. doi: 10.1152/ajpheart.1998.274.1.H27. [DOI] [PubMed] [Google Scholar]
- 72.Jackson WF, Huebner JM, Rusch NJ. Enzymatic isolation and characterization of single vascular smooth muscle cells from cremasteric arterioles. Microcirculation. 1997;4:35–50. doi: 10.3109/10739689709148316. [DOI] [PubMed] [Google Scholar]
- 73.Jackson WF, König A, Dambacher T, Busse R. Prostacyclin-induced vasodilation in the rabbit heart is mediated by ATP-sensitive potassium channels. Am J Physiol Heart Circ Physiol. 1993;264:H238–H243. doi: 10.1152/ajpheart.1993.264.1.H238. [DOI] [PubMed] [Google Scholar]
- 74.Jacobs ER, Cheliakine C, Gebremedhin D, Birks EK, Davies PF, Harder DR. Shear activated channels in cell-attached patches of cultured bovine aortic endothelial cells. Pflugers Arch. 1995;431:129–131. doi: 10.1007/BF00374386. [DOI] [PubMed] [Google Scholar]
- 75.Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol. 2000;278:C235–C256. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- 76.Janigro D, West GA, Gordon EL, Winn HR. ATP-sensitive K+ channels in rat aorta and brain microvascular endothelial cells. Am J Physiol Cell Physiol. 1993;265:C812–C821. doi: 10.1152/ajpcell.1993.265.3.C812. [DOI] [PubMed] [Google Scholar]
- 77.Kamouchi M, Van Den Bremt K, Eggermont J, Droogmans G, Nilius B. Modulation of inwardly rectifying potassium channels in cultured bovine pulmonary artery endothelial cells. J Physiol. 1997;504:545–556. doi: 10.1111/j.1469-7793.1997.545bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kamouchi M, Kitazono T, Ago T, Wakisaka M, Ooboshi H, Ibayashi S, Iida M. Calcium influx pathways in rat CNS pericytes. Brain Res Mol Brain Res. 2004;126:114–120. doi: 10.1016/j.molbrainres.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 79.Kawamura H, Sugiyama T, Wu DM, Kobayashi M, Yamanishi S, Katsumura K, Puro DG. ATP: a vasoactive signal in the pericyte-containing microvasculature of the rat retina. J Physiol. 2003;551:787–799. doi: 10.1113/jphysiol.2003.047977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Keef KD, Hume JR, Zhong J. Regulation of cardiac and smooth muscle Ca(2+) channels (Ca(V)1.2a,b) by protein kinases. Am J Physiol Cell Physiol. 2001;281:C1743–C1756. doi: 10.1152/ajpcell.2001.281.6.C1743. [DOI] [PubMed] [Google Scholar]
- 81.Kerr PM, Clement-Chomienne O, Thorneloe KS, Chen TT, Ishii K, Sontag DP, Walsh MP, Cole WC. Heteromultimeric Kv1.2-Kv1.5 channels underlie 4-aminopyridine-sensitive delayed rectifier K(+) current of rabbit vascular myocytes. Circ Res. 2001;89:1038–1044. doi: 10.1161/hh2301.100803. [DOI] [PubMed] [Google Scholar]
- 82.Kitamura K, Yamazaki J. Chloride channels and their functional roles in smooth muscle tone in the vasculature. Jpn J Pharmacol. 2001;85:351–357. doi: 10.1254/jjp.85.351. [DOI] [PubMed] [Google Scholar]
- 83.Knaus H-G, McManus OB, Lee SH, Schmalhofer WA, Garcia-Calvo M, Helms LMH, Sanchez M, Giangiacomo K, Reuben JP, Smith AB, III, Kaczorowski GJ, Garcia ML. Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry. 1994;33:5819–5828. doi: 10.1021/bi00185a021. [DOI] [PubMed] [Google Scholar]
- 84.Knot HJ, Zimmermann PA, Nelson MT. Extracellular K(+)-induced hyperpolarizations and dilatations of rat coronary and cerebral arteries involve inward rectifier K(+) channels. J Physiol. 1996;492:419–430. doi: 10.1113/jphysiol.1996.sp021318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Knot HJ, Standen NB, Nelson MT. Ryanodine receptors regulate arterial diameter and wall [Ca2+] in cerebral arteries of rat via Ca2+-dependent K+ channels. J Physiol (London) 1998;508:211–221. doi: 10.1111/j.1469-7793.1998.211br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kohler R, Degenhardt C, Kuhn M, Runkel N, Paul M, Hoyer J. Expression and function of endothelial Ca(2+)-activated K(+) channels in human mesenteric artery: a single-cell reverse transcriptase-polymerase chain reaction and electrophysiological study in situ. Circ Res. 2000;87:496–503. doi: 10.1161/01.res.87.6.496. [DOI] [PubMed] [Google Scholar]
- 87.Kohler R, Brakemeier S, Kuhn M, Behrens C, Real R, Degenhardt C, Orzechowski HD, Pries AR, Paul M, Hoyer J. Impaired hyperpolarization in regenerated endothelium after balloon catheter injury. Circ Res. 2001;89:174–179. doi: 10.1161/hh1401.093460. [DOI] [PubMed] [Google Scholar]
- 88.Kosmas EN, Levy RD, Hussain SN. Acute effects of glyburide on the regulation of peripheral blood flow in normal humans. Eur J Pharmacol. 1995;274:193–199. doi: 10.1016/0014-2999(94)00732-m. [DOI] [PubMed] [Google Scholar]
- 89.Kukuljan M, Rojas E, Catt KJ, Stojilkovic SS. Membrane potential regulates inositol 1,4,5-trisphosphate-controlled cytoplasmic Ca2+ oscillations in pituitary gonadotrophs. J Biol Chem. 1994;269:4860–4865. [PubMed] [Google Scholar]
- 90.Kuo L, Chancellor JD. Adenosine potentiates flow-induced dilation of coronary arterioles by activating KATP channels in endothelium. Am J Physiol. 1995;269:H541–H549. doi: 10.1152/ajpheart.1995.269.2.H541. [DOI] [PubMed] [Google Scholar]
- 91.Lange A, Gebremedhin D, Narayanan J, Harder D. 20-Hydroxyeicosatetraenoic acid-induced vasoconstriction and inhibition of potassium current in cerebral vascular smooth muscle is dependent on activation of protein kinase C. J Biol Chem. 1997;272:27345–27352. doi: 10.1074/jbc.272.43.27345. [DOI] [PubMed] [Google Scholar]
- 92.Langheinrich U, Daut J. Hyperpolarization of isolated capillaries from guinea-pig heart induced by K+ channel openers and glucose deprivation. J Physiol (London) 1997;502:397–408. doi: 10.1111/j.1469-7793.1997.397bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee CH, Poburko D, Kuo KH, Seow CY, van Breemen C. Ca(2+) oscillations, gradients, and homeostasis in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2002;282:H1571–H1583. doi: 10.1152/ajpheart.01035.2001. [DOI] [PubMed] [Google Scholar]
- 94.Li X, Gu W, Mohan S, Baylink DJ. DNA microarrays: their use and misuse. Microcirculation. 2002;9:13–22. doi: 10.1038/sj.mn.7800118. [DOI] [PubMed] [Google Scholar]
- 95.Ling S, Sheng JZ, Braun AP. The calcium-dependent activity of large-conductance, calcium-activated K+ channels is enhanced by Pyk2- and Hck-induced tyrosine phosphorylation. Am J Physiol Cell Physiol. 2004;287:C698–C706. doi: 10.1152/ajpcell.00030.2004. [DOI] [PubMed] [Google Scholar]
- 96.Ling S, Woronuk G, Sy L, Lev S, Braun AP. Enhanced activity of a large conductance, calcium-sensitive K+ channel in the presence of Src tyrosine kinase. J Biol Chem. 2000;275:30683–30689. doi: 10.1074/jbc.M004292200. [DOI] [PubMed] [Google Scholar]
- 97.Little TL, Xia J, Duling BR. Dye tracers define differential endothelial and smooth muscle coupling patterns within the arteriolar wall. Circ Res. 1995;76:498–504. doi: 10.1161/01.res.76.3.498. [DOI] [PubMed] [Google Scholar]
- 98.Liu G, Shi J, Yang L, Cao L, Park SM, Cui J, Marx SO. Assembly of a Ca(2+)-dependent BK channel signaling complex by binding to beta2 adrenergic receptor. EMBO J. 2004;23:2196–2205. doi: 10.1038/sj.emboj.7600228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu Y, Terata K, Rusch NJ, Gutterman DD. High glucose impairs voltage-gated K(+) channel current in rat small coronary arteries. Circ Res. 2001;89:146–152. doi: 10.1161/hh1401.093294. [DOI] [PubMed] [Google Scholar]
- 100.Loeb AL, Gödény I, Longnecker DE. (1997). Functional evidence for inward-rectifier potassium channels in rat cremaster muscle arterioles. Microcirculation 160. [DOI] [PubMed]
- 101.Luykenaar KD, Brett SE, Wu BN, Wiehler WB, Welsh DG. Pyrimidine nucleotides suppress KDR currents and depolarize rat cerebral arteries by activating Rho kinase. Am J Physiol Heart Circ Physiol. 2004;286:H1088–1100. doi: 10.1152/ajpheart.00903.2003. [DOI] [PubMed] [Google Scholar]
- 102.Marrelli SP, Eckmann MS, Hunte MS. Role of endothelial intermediate conductance KCa channels in cerebral EDHF-mediated dilations. Am J Physiol Heart Circ Physiol. 2003;285:H1590–H1599. doi: 10.1152/ajpheart.00376.2003. [DOI] [PubMed] [Google Scholar]
- 103.Marshall JM, Thomas T, Turner L. A link between adenosine, ATP-sensitive K+ channels, potassium and muscle vasodilatation in the rat in systemic hypoxia. J Physiol (London) 1993;472:1–9. doi: 10.1113/jphysiol.1993.sp019931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mayhan WG. Effect of diabetes mellitus on response of the basilar artery to activation of ATP-sensitive potassium channels. Brain Res. 1994;636:35–39. doi: 10.1016/0006-8993(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 105.Mayhan WG, Faraci FM. Responses of cerebral arterioles in diabetic rats to activation of ATP-sensitive potassium channels. Am J Physiol Heart Circ Physiol. 1993;265:H152–H157. doi: 10.1152/ajpheart.1993.265.1.H152. [DOI] [PubMed] [Google Scholar]
- 106.McCarron JG, Halpern W. Impaired potassium-induced dilation in hypertensive rat cerebral arteries does not reflect altered Na+, K+-ATPase dilation. Circ Res. 1990;67:1035–1039. doi: 10.1161/01.res.67.4.1035. [DOI] [PubMed] [Google Scholar]
- 107.McCulloch KM, Kempsill FE, Buchanan KJ, Gurney AM. Regional distribution of potassium currents in the rabbit pulmonary arterial circulation. Exp Physiol. 2000;85:487–496. [PubMed] [Google Scholar]
- 108.McManus MT, Sharp PA. Gene silencing in mammals by small interfering RNAs. Nat Rev Genet. 2002;3:737–747. doi: 10.1038/nrg908. [DOI] [PubMed] [Google Scholar]
- 109.Miller C, Moczydlowski E, Latorre R, Phillips M. Charybdotoxin, a protein inhibitor of single Ca2+-activated K+ channels from mammalian skeletal muscle. Nature. 1985;313:316–318. doi: 10.1038/313316a0. [DOI] [PubMed] [Google Scholar]
- 110.Minami K, Fukuzawa K, Nakaya Y. Protein kinase C inhibits the Ca(2+)-activated K+ channel of cultured porcine coronary artery smooth muscle cells. Biochem Biophys Res Commun. 1993;190:263–269. doi: 10.1006/bbrc.1993.1040. [DOI] [PubMed] [Google Scholar]
- 111.Miura H, Wachtel RE, Loberiza FR, Jr, Saito T, Miura M, Nicolosi AC, Gutterman DD. Diabetes mellitus impairs vasodilation to hypoxia in human coronary arterioles: reduced activity of ATP-sensitive potassium channels. Circ Res. 2003;92:151–158. doi: 10.1161/01.res.0000052671.53256.49. [DOI] [PubMed] [Google Scholar]
- 112.Miyoshi Y, Nakaya Y. Angiotensin II blocks ATP-sensitive K+ channels in porcine coronary artery smooth muscle cells. Biochem Biophys Res Commun. 1991;181:700–706. doi: 10.1016/0006-291x(91)91247-a. [DOI] [PubMed] [Google Scholar]
- 113.Miyoshi Y, Nakaya Y, Wakatsuki T, Nakaya S, Fujino K, Saito K, Inoue I. Endothelin blocks ATP-sensitive K+ channels and depolarizes smooth muscle cells of porcine coronary artery. Circ Res. 1992;70:612–616. doi: 10.1161/01.res.70.3.612. [DOI] [PubMed] [Google Scholar]
- 114.Nakashima Y, Toki Y, Fukami Y, Hibino M, Okumura K, Ito T. Role of K+ channels in EDHF-dependent relaxation induced by acetylcholine in canine coronary artery. Heart Vessels. 1997;12:287–293. doi: 10.1007/BF02766805. [DOI] [PubMed] [Google Scholar]
- 115.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 116.Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol Cell Physiol. 1990;259:C3–C18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- 117.Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev. 2001;81:1415–1459. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- 118.Nilius B, Droogmans G, Wondergem R. Transient receptor potential channels in endothelium: solving the calcium entry puzzle? Endothelium. 2003;10:5–15. doi: 10.1080/10623320303356. [DOI] [PubMed] [Google Scholar]
- 119.Okada Y, Yanagisawa T, Taira N. BRL 38227 (levcromakalim)-induced hyperpolarization reduces the sensitivity to Ca2+ of contractile elements in canine coronary artery. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:438–444. doi: 10.1007/BF00165396. [DOI] [PubMed] [Google Scholar]
- 120.Pagakis SN, Curry FE. Imaging of Ca2+ transients in endothelial cells of single perfused capillaries: correlation of peak [Ca2+]i with sites of macromolecule leakage. Microcirculation. 1994;1:213–230. doi: 10.3109/10739689409146749. [DOI] [PubMed] [Google Scholar]
- 121.Pallone TL, Cao C, Zhang Z. Inhibition of K+ conductance in descending vasa recta pericytes by AngII. Am J Physiol Renal Physiol. 2004;287:F1213–F1222. doi: 10.1152/ajprenal.00241.2004. [DOI] [PubMed] [Google Scholar]
- 122.Paterno R, Faraci FM, Heistad DD. Role of Ca(2+)-dependent K+ channels in cerebral vasodilatation induced by increases in cyclic GMP and cyclic AMP in the rat. Stroke. 1996;27:1603–1607. doi: 10.1161/01.str.27.9.1603. [DOI] [PubMed] [Google Scholar]
- 123.Quayle JM, Dart C, Standen NB. The properties and distribution of inward rectifier potassium currents in pig coronary arterial smooth muscle. J Physiol (London) 1996;494:715–726. doi: 10.1113/jphysiol.1996.sp021527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev. 1997;77:1165–1232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- 125.Quayle JM, McCarron JG, Brayden JE, Nelson MT. Inward rectifier K+ currents in smooth muscle cells from rat resistance-sized cerebral arteries. Am J Physiol. 1993;265:C1363–C1370. doi: 10.1152/ajpcell.1993.265.5.C1363. [DOI] [PubMed] [Google Scholar]
- 126.Quinlan KL, Naik SM, Cannon G, Armstrong CA, Bunnett NW, Ansel JC, Caughman SW. Substance P activates coincident NF-AT- and NF-kappa B-dependent adhesion molecule gene expression in microvascular endothelial cells through intracellular calcium mobilization. J Immunol. 1999;163:5656–5665. [PubMed] [Google Scholar]
- 127.Rhinehart K, Zhang Z, Pallone TL. Ca(2+) signaling and membrane potential in descending vasa recta pericytes and endothelia. Am J Physiol Renal Physiol. 2002;283:F852–F860. doi: 10.1152/ajprenal.00065.2002. [DOI] [PubMed] [Google Scholar]
- 128.Rivers RJ, Hein TW, Zhang C, Kuo L. Activation of barium-sensitive inward rectifier potassium channels mediates remote dilation of coronary arterioles. Circulation. 2001;104:1749–1753. doi: 10.1161/hc4001.098053. [DOI] [PubMed] [Google Scholar]
- 129.Saito Y, McKay M, Eraslan A, Hester RL. Functional hyperemia in striated muscle is reduced following blockade of ATP-sensitive potassium channels. Am J Physiol Heart Circ Physiol. 1996;270:H1649–H1654. doi: 10.1152/ajpheart.1996.270.5.H1649. [DOI] [PubMed] [Google Scholar]
- 130.Sakagami K, Wu DM, Puro DG. Physiology of rat retinal pericytes: modulation of ion channel activity by serum-derived molecules. J Physiol. 1999;521:637–650. doi: 10.1111/j.1469-7793.1999.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res. 2000;86:341–346. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- 132.Sandow SL, Tare M, Coleman HA, Hill CE, Parkington HC. Involvement of myoendothelial gap junctions in the actions of endothelium-derived hyperpolarizing factor. Circ Res. 2002;90:1108–1113. doi: 10.1161/01.res.0000019756.88731.83. [DOI] [PubMed] [Google Scholar]
- 133.Schnitzler MM, Derst C, Daut J, Preisig-Muller R. ATP-sensitive potassium channels in capillaries isolated from guinea-pig heart. J Physiol. 2000;525:307–317. doi: 10.1111/j.1469-7793.2000.t01-1-00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schubert R, Krien U, Wulfsen I, Schiemann D, Lehmann G, Ulfig N, Veh RW, Schwarz JR, Gago H. Nitric oxide donor sodium nitroprusside dilates rat small arteries by activation of inward rectifier potassium channels. Hypertension. 2004;43:891–896. doi: 10.1161/01.HYP.0000121882.42731.6b. [DOI] [PubMed] [Google Scholar]
- 135.Sharma NR, Davis MJ. Mechanism of substance P-induced hyperpolarization of porcine coronary artery endothelial cells. Am J Physiol Heart Circ Physiol. 1994;266:H156–H164. doi: 10.1152/ajpheart.1994.266.1.H156. [DOI] [PubMed] [Google Scholar]
- 136.Sharma NR, Davis MJ. Substance P-induced calcium entry in endothelial cells is secondary to depletion of intracellular stores. Am J Physiol Heart Circ Physiol. 1995;268:H962–H973. doi: 10.1152/ajpheart.1995.268.3.H962. [DOI] [PubMed] [Google Scholar]
- 137.Smirnov SV, Beck R, Tammaro P, Ishii T, Aaronson PI. Electrophysiologically distinct smooth muscle cell subtypes in rat conduit and resistance pulmonary arteries. J Physiol. 2002;538:867–878. doi: 10.1113/jphysiol.2001.013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sobey CG. Potassium channel function in vascular disease. Arterioscler Thromb Vasc Biol. 2001;21:28–38. doi: 10.1161/01.atv.21.1.28. [DOI] [PubMed] [Google Scholar]
- 139.Suzuki M, Li RA, Miki T, Uemura H, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Ogura T, Seino S, Marban E, Nakaya H. Functional roles of cardiac and vascular ATP-sensitive potassium channels clarified by Kir6.2-knockout mice. Circ Res. 2001;88:570–577. doi: 10.1161/01.res.88.6.570. [DOI] [PubMed] [Google Scholar]
- 140.Takano H, Dora KA, Spitaler MM, Garland CJ. Spreading dilatation in rat mesenteric arteries associated with calcium-independent endothelial cell hyperpolarization. J Physiol. 2004;556:887–903. doi: 10.1113/jphysiol.2003.060343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tanaka Y, Meera P, Song M, Knaus HG, Toro L. Molecular constituents of maxi KCa channels in human coronary smooth muscle: predominant alpha + beta subunit complexes. J Physiol (London) 1997;502:545–557. doi: 10.1111/j.1469-7793.1997.545bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tateishi J, Faber JE. ATP-sensitive K+ channels mediate alpha 2D-adrenergic receptor contraction of arteriolar smooth muscle and reversal of contraction by hypoxia. Circ Res. 1995;76:53–63. doi: 10.1161/01.res.76.1.53. [DOI] [PubMed] [Google Scholar]
- 143.Thorneloe KS, Chen TT, Kerr PM, Grier EF, Horowitz B, Cole WC, Walsh MP. Molecular composition of 4-aminopyridine-sensitive voltage-gated K(+) channels of vascular smooth muscle. Circ Res. 2001;89:1030–1037. doi: 10.1161/hh2301.100817. [DOI] [PubMed] [Google Scholar]
- 144.Tiruppathi C, Minshall RD, Paria BC, Vogel SM, Malik AB. Role of Ca2+ signaling in the regulation of endothelial permeability. Vascul Pharmacol. 2002;39:173–185. doi: 10.1016/s1537-1891(03)00007-7. [DOI] [PubMed] [Google Scholar]
- 145.Tran PO, Hinman LE, Unger GM, Sammak PJ. A wound-induced [Ca2+]i increase and its transcriptional activation of immediate early genes is important in the regulation of motility. Exp Cell Res. 1999;246:319–326. doi: 10.1006/excr.1998.4239. [DOI] [PubMed] [Google Scholar]
- 146.Vanelli G, Hussain SN. Effects of potassium channel blockers on basal vascular tone and reactive hyperemia of canine diaphragm. Am J Physiol. 1994;266:H43–H51. doi: 10.1152/ajpheart.1994.266.1.H43. [DOI] [PubMed] [Google Scholar]
- 147.Vanelli G, Chang HY, Gatensby AG, Hussain SNA. Contribution of potassium channels to active hyperemia of the canine diaphragm. J App Physiol. 1994;76:1098–1105. doi: 10.1152/jappl.1994.76.3.1098. [DOI] [PubMed] [Google Scholar]
- 148.Wakatsuki T, Nakaya Y, Miyoshi Y, Xiao-Rong Z, Nomura M, Saito K, Inoue I. Effects of vasopressin on ATP-sensitive and Ca2+-activated K+ channels of coronary arterial smooth muscle cells. Jpn J Pharmacol. 1992;58(Suppl 2):339P. [PubMed] [Google Scholar]
- 149.Wang R, Wu L, Wang Z. The direct effect of carbon monoxide on KCa channels in vascular smooth muscle cells. Pflugers Arch. 1997;434:285–291. doi: 10.1007/s004240050398. [DOI] [PubMed] [Google Scholar]
- 150.Webb RC. Smooth muscle contraction and relaxation. Adv Physiol Educ. 2003;27:201–206. doi: 10.1152/advan.00025.2003. [DOI] [PubMed] [Google Scholar]
- 151.Wellman GC, Nelson MT. Signaling between SR and plasmalemma in smooth muscle: sparks and the activation of Ca2+-sensitive ion channels. Cell Calcium. 2003;34:211–229. doi: 10.1016/s0143-4160(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 152.Welsh DG, Jackson WF, Segal SS. Oxygen induces electromechanical coupling in arteriolar smooth muscle cells: a role for L-type Ca2+ channels. Am J Physiol. 1998;274:H2018–H2024. doi: 10.1152/ajpheart.1998.274.6.H2018. [DOI] [PubMed] [Google Scholar]
- 153.Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res. 2002;90:248–250. doi: 10.1161/hh0302.105662. [DOI] [PubMed] [Google Scholar]
- 154.Wesselman JP, Schubert R, VanBavel ED, Nilsson H, Mulvany MJ. KCa-channel blockade prevents sustained pressure-induced depolarization in rat mesenteric small arteries. Am J Physiol Heart Circ Physiol. 1997;272:H2241–H2249. doi: 10.1152/ajpheart.1997.272.5.H2241. [DOI] [PubMed] [Google Scholar]
- 155.Wilson AJ, Jabr RI, Clapp LH. Calcium modulation of vascular smooth muscle ATP-sensitive K(+) channels: role of protein phosphatase-2B. Circ Res. 2000;87:1019–1025. doi: 10.1161/01.res.87.11.1019. [DOI] [PubMed] [Google Scholar]
- 156.Wischmeyer E, Doring F, Karschin A. Acute suppression of inwardly rectifying Kir2.1 channels by direct tyrosine kinase phosphorylation. J Biol Chem. 1998;273:34063–34068. doi: 10.1074/jbc.273.51.34063. [DOI] [PubMed] [Google Scholar]
- 157.Wu DM, Kawamura H, Sakagami K, Kobayashi M, Puro DG. Cholinergic regulation of pericyte-containing retinal microvessels. Am J Physiol Heart Circ Physiol. 2003;284:H2083–H2090. doi: 10.1152/ajpheart.01007.2002. [DOI] [PubMed] [Google Scholar]
- 158.Wu X, Davis MJ. Characterization of stretch-activated cation current in coronary smooth muscle cells. Am J Physiol Heart Circ Physiol. 2001;280:H1751–H1761. doi: 10.1152/ajpheart.2001.280.4.H1751. [DOI] [PubMed] [Google Scholar]
- 159.Yamagishi T, Yanagisawa T, Taira N. K+ channel openers, cromakalim and Ki4032, inhibit agonist-induced Ca2+ release in canine coronary artery. Naunyn Schmiedebergs Arch Pharmacol. 1992;346:691–700. doi: 10.1007/BF00168744. [DOI] [PubMed] [Google Scholar]
- 160.Yanagisawa T, Yamagishi T, Okada Y. Hyperpolarization induced by K+ channel openers inhibits Ca2+ influx and Ca2+ release in coronary artery. Cardiovasc Drugs Ther. 1993;7(Suppl 3):565–574. doi: 10.1007/BF00877622. [DOI] [PubMed] [Google Scholar]
- 161.Zaritsky JJ, Eckman DM, Wellman GC, Nelson MT, Schwarz TL. Targeted disruption of Kir2.1 and Kir2.2 genes reveals the essential role of the inwardly rectifying K(+) current in K(+)-mediated vasodilation. Circ Res. 2000;87:160–166. doi: 10.1161/01.res.87.2.160. [DOI] [PubMed] [Google Scholar]
- 162.Zhang Y, Oltman CL, Lu T, Lee HC, Dellsperger KC, VanRollins M. EET homologs potently dilate coronary microvessels and activate BK(Ca) channels. Am J Physiol Heart Circ Physiol. 2001;280:H2430–H2440. doi: 10.1152/ajpheart.2001.280.6.H2430. [DOI] [PubMed] [Google Scholar]
- 163.Zhang Z, Rhinehart K, Pallone TL. Membrane potential controls calcium entry into descending vasa recta pericytes. Am J Physiol Regul Integr Comp Physiol. 2002;283:R949–R957. doi: 10.1152/ajpregu.00251.2002. [DOI] [PubMed] [Google Scholar]
- 164.Zimmermann PA, Knot HJ, Stevenson AS, Nelson MT. Increased myogenic tone and diminished responsiveness to ATP-sensitive K+ channel openers in cerebral arteries from diabetic rats. Circ Res. 1997;81:996–1004. doi: 10.1161/01.res.81.6.996. [DOI] [PubMed] [Google Scholar]


