Abstract
The objective of this study was to determine whether a commercially available, saponin-adjuvanted, inactivated bovine respiratory syncytial virus (BRSV) vaccine would protect calves from experimental infection with virulent BRSV. This was a randomized controlled trial comprising 14, 8- to 9-week-old calves seronegative for BRSV. Group 1 calves (n = 8) were not vaccinated and group 2 calves (n = 6) were vaccinated on days 0 and 19 with an inactivated BRSV vaccine. All calves were challenged with virulent BRSV on day 46. Clinical signs, arterial PO2, and immune responses were monitored after challenge. Calves were euthanatized on day 54 (8 d after challenge) and lungs were examined for lesions. Vaccination elicited increases in BRSV-specific immunoglobulin (Ig) G and virus neutralizing antibody titers. Challenge with BRSV resulted in severe respiratory tract disease and extensive pulmonary lesions in control calves, but no signs of clinical disease and minimal or no pulmonary lesions in vaccinated calves. Arterial blood oxygen values on day 53 (7 d after challenge) in control calves were significantly lower than those in vaccinated calves, which remained within normal limits. Control calves shed BRSV for several days after challenge, whereas BRSV was not detected on deep nasal swabs from vaccinated calves. In summary, the results indicated that this inactivated BRSV vaccine provided clinical protection from experimental infection with virulent virus 27 d after vaccination and significantly decreased the prevalence and severity of pulmonary lesions. Efficacy was similar to that reported for other commercial inactivated and modified-live BRSV vaccines.
Abstract
Résumé — Efficacité d’un vaccin inactif à adjuvant de saponine contre le virus respiratoire syncytial chez le veau. L’objectif de cette étude était de déterminer si un vaccin inactif à adjuvant de saponine contre le virus respiratoire syncytial bovin (VRSB), disponible dans le commerce, pouvait protéger les veaux contre une infection expérimentale par le VRSB virulent. Cette expérience contrôlée comprenait 14 veaux séronégatifs répartis au hasard et âgés de 8 à 9 semaines. Les veaux du groupe 1 (n = 8) n’ont pas été vaccinés et ceux du groupe 2 (n = 6) ont été vaccinés aux jours 0 et 19 avec un vaccin inactivé contre le VRSB. Tous les veaux ont été infectés avec du VRSB virulent au jour 46. Les signes cliniques, le PO2 artériel et les réponses immunitaires ont été enregistrés après l’infection. Les veaux ont été euthanasiés au jour 54 (8 jours après l’infection) et les poumons ont été examinés dans le but d’y observer des lésions. La vaccination a causé une augmentation du titre des immunoglobulines spécifiques au VRSB (Ig)G et des anticorps neutralisants du virus. L’infection par le VRSB a causé une grave maladie du tractus respiratoire et des lésions pulmonaires extensives chez les veaux témoins, mais aucun signe de maladie clinique et pas de lésions ou des lésions minimes seulement chez les veaux vaccinés. Les valeurs de l’oxygène du sang artériel au jour 53 (7 jours après l’infection) chez les veaux témoins étaient significativement plus basses que celles chez les veaux vaccinés, lesquelles se situaient dans les valeurs normales. Les veaux témoins ont éliminé du VRSB pendant plusieurs jours après l’infection alors que les VRSB n’étaient pas détectés dans les prélèvements provenant des voies nasales profondes des veaux vaccinés. En résumé, les résultats indiquent que ce vaccin inactivé contre le VRSB assure une protection clinique contre une infection expérimentale avec le virus virulent 27 jours après la vaccination et diminue significativement la prévalence et la sévérité des lésions pulmonaires. L’efficacité était semblable à celle rapportée pour d’autres vaccins commerciaux inactivés et vivants modifiés contre le VRSB.
(Traduit par Docteur André Blouin)
Introduction
Bovine respiratory syncytial virus (BRSV) is a para-myxovirus that ubiquitously and endemically infects cattle in many parts of the world (1–3). The virus is genetically and antigenically related to human respiratory syncytial virus (HRSV) and, like its human counterpart, can cause disease in all ages of hosts, but it primarily affects the young, causing recurrent seasonal outbreaks (1–3). Clinical disease in cattle is characterized by pyrexia, coughing, and tachypnea, occasionally progressing rapidly to dyspnea and death in naïve individuals (3,4). Bovine respiratory syncytial virus is also considered to be one of the viral agents that predisposes cattle with bovine respiratory disease (BRD) complex to secondary bacterial infections; however, cattle with fatal BRSV-associated respiratory disease often do not have secondary bacterial infections (5,6). As well, subclinical BRSV infections may cause insidious economic losses due to decreased production (7).
Modified-live virus (MLV) and inactivated single fraction and combination BRSV vaccines have been available for nearly 2 decades; nevertheless, their efficacy and potential disease-enhancing properties remain controversial (3,8). It has been well and consistently documented that MLV and inactivated BRSV vaccines stimulate distinctively different antibody responses in cattle (9–15). Cattle administered MLV vaccines generally develop high concentrations of virus neutralizing (VN) and fusion inhibiting antibodies, compared with low to moderate concentrations of total BRSV-specific immunoglobulin (Ig) G, as measured by use of an enzyme-linked immunosorbent assay (ELISA) (9–11).
This contrasts with the response of cattle to inactivated BRSV vaccines that have generally been shown to stimulate significantly lower concentrations of VN antibodies and high concentrations of virus-specific (non-neutralizing) IgG (9–15), which is further reflected in different ratios of VN antibodies to total virus-specific IgG (11). More-over, differences in T lymphocyte responses have also been reported in cattle, and it has been suggested that natural infection and MLV vaccines stimulate T helper 1 (Th1)-type responses, whereas inactivated BRSV stimulates T helper 2 (Th2)-type responses (12–14).
The dichotomous antibody responses to vaccination in cattle are similar to those reported for human pediatric patients who were given formalin-inactivated HRSV vaccines in the late 1960s and experienced enhanced respiratory disease when they subsequently contracted HRSV (16,17). These findings together with extensive studies examining the differential antibody and T-cell mediated responses in vaccinated and HRSV-infected laboratory rodents led to the conclusion that inactivated BRSV vaccines are not only ineffective but may actually enhance respiratory disease in infected cattle (3,13). Nevertheless, recent studies have documented the efficacy of experimental and commercial inactivated BRSV vaccines in significantly reducing the prevalence and severity of respiratory disease in cattle that were experimentally infected with a virulent field isolate of BRSV (14,15). However, available data from the field and the laboratory indicate that the dose and adjuvant in inactivated vaccines may affect both efficacy and disease-enhancing potential (13–15,18,19), making it difficult to draw generic conclusions about the bovine response to inactivated BRSV vaccines. The objective of this study was to assess responses to a commercially available saponin-adjuvanted inactivated BRSV vaccine in calves.
Materials and methods
Experimental design
Fourteen neonatal calves obtained from local dairies were used in the study. Calves were removed from their dams at birth, fed 1.5 L of pooled bovine colostrum, and reared in isolation from other cattle. After the first 24 h of life, calves were fed 4 L of milk replacer daily and had ad libitum access to water, grass-legume hay, and commercial pelleted calf ration. Calves were randomly assigned to 2 groups: Group 1 consisted of 8 control calves that received no treatment; group 2 consisted of 6 calves that were vaccinated on days 0 and 20 by IM injection of a commercial inactivated vaccine (Triangle 4; Wyeth Animal Health, Guelph, Ontario). This vaccine has a proprietary adjuvant containing saponin. Calves were first vaccinated at approximately 9 wk of age (day 0). All calves were seronegative (serum neutralizing antibody titer < 1:6) for BRSV antibodies at the time of the first vaccination. For challenge, the calves were transferred to isolation facilities at the Western College of Veterinary Medicine (WCVM) where they were kept in pens containing 2 to 3 calves in a shared air space. The challenge inoculum consisted of lung wash obtained from a newborn calf infected with BRSV, as previously described (14). The lung wash was confirmed to be negative for bacterial contamination, Mycoplasma spp, bovine herpesvirus 1, parainfluenza virus 3, bovine corona virus, and bovine viral diarrhea virus by using standard diagnostic methods (culture and fluorescent antibody testing). Lung wash contained 8.75 × 103 plaque-forming units (pfu) per mL of BRSV (14). On day 46, calves were placed in a sealed room and the BRSV was delivered by use of ultrasonic nebulizers (Ultra-Neb 99; Devilbiss, Somerset, Pennsylvania, USA), which were placed near the ceiling in opposite corners of the room. Calves were euthanatized by administration of a barbiturate overdose (Euthanyl Forte; MTC Pharmaceuticals, Cambridge, Ontario) 8 d after challenge (day 54), if they had not already been euthanatized for humane reasons earlier.
Clinical assessment
Calves were observed for clinical signs of respiratory tract disease during the 3 d prior to challenge (study days 44 through 46) and the 8 d after challenge (study days 47 through 54). Clinical assessments were made at the same time each morning by a sole veterinarian, who was not aware of group assignments. Respiratory rate and rectal temperature were recorded, as well as other clinical signs associated with respiratory tract disease, including the presence or absence of cough, dyspnea, or nasal discharge.
Sample collection
Deep nasal swab specimens were collected prior to challenge, on study day 46, and on days 3 to 7 after challenge (study days 49 through 53); they were placed in 1 mL of transport medium consisting of Dulbecco’s modified Eagles medium (DMEM), supplemented with 10% fetal bovine serum (Gibco BRL, Grand Island, New York, USA), 0.5 M MgSO4, 50 mM HEPES, 150 mM NaCl, 200 IU/mL of sodium penicillin, 200 μg/mL of streptomycin sulphate, 200 μg/mL of gentamicin, and 1 μg/mL of amphotericin B, and then frozen at −70°C until analyzed for the presence of virus, as previously described (12). Serum samples were collected from all calves before inclusion in the study and on study days 0, 7, 13, 19, 26, 33, 46, 52, and 54 and analyzed for BRSV-specific IgG and BRSV-specific neutralizing antibody titers. Arterial blood samples were collected from the caudal thoracic aorta (20) on study day 53. Oxygen tension, corrected for rectal temperature, was measured, using a gas analyzer (Model 288; Ciba-Corning, Medfield, Massachusetts, USA).
Antibody assays
Tripling dilutions were used to determine BRSV-specific neutralizing antibody titers (11,12). An ELISA was used to measure BRSV-specific IgG titer, as previously described (10); a 1:50 dilution of serum was used. Convalescent serum from an unvaccinated calf with naturally occurring BRSV was used as the positive standard; fetal bovine serum was used as the negative standard. Optical density (OD) values for test samples were expressed as “units” that were calculated as follows: 100 × (sample OD — negative standard OD)/(positive standard OD — negative standard OD).
Quantitative virus isolation
Virus shedding was quantitatively determined by use of a microisolation plaque assay with bovine embryonic lung fibroblasts (21). This assay has a maximum calculated sensitivity of 10 pfu/mL, as previously described (21).
Postmortem analysis
At necropsy, the respiratory tract of each calf was harvested and the percentage of lung tissue that was pneumonic was calculated (14). Briefly, the dorsal and ventral surfaces of the lungs were photographed. Tracings were made from the projected slides of the dorsal and ventral surfaces, outlining total area and areas that were depressed, dark-red, and obviously pneumonic, as well as emphysematous areas and bullae. For each set of lungs, the total percentage of lung affected comprising both surfaces was determined by use of computer software (Image 1; Universal Imaging Corporation, West Chester, Pennsylvania, USA). Sections of affected lung from unvaccintated control calves were fixed in neutral-buffered 10% formalin and processed for histologic examination and immunohistochemical staining for BRSV antigen, using caprine antibody against BRSV (VMRD, Pullman, Washington, USA), as previously described (22).
Data analysis
Changes in respiratory rate and rectal temperatures after challenge were determined for each calf by subtracting the values obtained after challenge (study days 47 through 54) from baseline. Baseline was calculated for each calf as the mean value for study days 44 to 46. The differences in respiratory rate and rectal temperature were compared between vaccinated and control calves by using generalized estimating equations (GEE) to account for the repeated measures design. Data were analyzed by using a statistical computer software program to look at the effects of vaccination, time, and the potential interaction between vaccination status and time. Model specifications included a normal distribution, id link function, repeated statement with subject equal to calf identification, and an autoregressive (1) correlation structure. Variables remaining in the final multivariable model at P < 0.05, based on the robust empirical standard errors produced by the GEE analysis, were considered statistically significant and were reported. Viral shedding was compared between vaccinated and unvaccinated controls by using GEE as above. Virus neutralization results were compared between groups at study day 46 by using a Wilcoxon rank sum test. Blood oxygen values were compared by using a 2-sample t-test, and lung lesion scores were compared by using a Wilcoxon rank sum test. All analyses were completed with a computerized statistics program (SAS/PC, version 8.2 for Windows [PROC GENMOD]; SAS Institute, Cary, North Carolina, USA). P < 0.05 was considered statistically significant in all cases.
Results
Clinical data
The 6 vaccinated calves had no clinical signs of pneumonia following infection. There were no significant changes from baseline values in rectal temperatures or respiratory rates. No spontaneous coughing, dyspnea, or nasal discharge was observed. In contrast, the 8 unvaccinated control calves had significant (P < 0.001) increases in rectal temperatures (0.74°C; 95% CI, 0.58 to 0.89) and significant (P < 0.001) increases in respiratory rates (22.5 breaths/min; 95% CI, 15.6 to 29.3) (Figures 1 and 2). There were significant (P < 0.001) differences between the groups in arterial blood oxygen tensions, with the 6 vaccinated calves having higher mean blood oxygen tensions that remained in the normal range (80 to 110 mmHg) (Clinical Pathology Laboratory Protocol Manual, Department of Veterinary Microbiology, Western College of Veterinary Medicine, 1998) than those of the 8 control calves that were markedly decreased (37.1 mmHg, 95% CI, 26.6 to 47.6 mmHg) (Figure 3A).
Figure 1.

Mean (± standard deviation) rectal temperatures after challenge on day 0 with virulent bovine respiratory syncytial virus (BRSV) in unvaccinated control calves (♦) and calves vaccinated with an inactivated BRSV vaccine (□).
Figure 2.

Mean (± standard deviation) respiration rates after challenge on day 0 with virulent bovine respiratory syncytial virus (BRSV) in unvaccinated control calves (♦) and calves vaccinated with an inactivated BRSV vaccine (□).
Figure 3.

Scatter plot of arterial oxygen values on day 7 after challenge (A) and percentage pneumonic lung at necropsy (B). Symbols represent values from individual vaccinated (□) and control (♦) calves. Bars represent group mean values.
Viral shedding
No shedding of BRSV was detected in any of the 14 calves on study day 46 (before challenge). The 6 vaccinated calves did not shed detectable BRSV after challenge. In contrast, the 8 unvaccinated control calves shed variable amounts of virus from days 3 to 7 after infection (Figure 4). On average, between days 3 and 7, the unvaccinated calves shed 173 pfu/mL (95% CI 125 to 220) more viral particles than the vaccinated calves (P < 0.001).
Figure 4.

Scatter plot of nasal shedding of bovine respiratory syncytial virus (BRSV) after challenge on day 0. Symbols represent BRSV shed from individual control calves. Vaccinated calves did not shed BRSV before or after challenge. Bars represent group mean values.
Antibody titers
All calves had minimal VN (< 1:6) and ELISA (< 15 units) titers on study day 0. None of the control calves developed antibody responses before study day 46 or after infection, whereas all of the vaccinated calves had an increase in BRSV-neutralizing antibodies (median, 64; IQR, 26 to 224; Figure 5) and BRSV-specific IgG (Figure 6) after vaccination and prior to challenge.
Figure 5.

Scatter plot of bovine respiratory syncytial virus (BRSV) neutralizing antibody titers after vaccination on day 0. Symbols represent antibody titers from individual vaccinated calves. Control calves did not have BRSV neutralizing antibodies before or after challenge. Bars represent group geometric mean values.
Figure 6.

Mean (± standard deviation) bovine respiratory syncytial virus (BRSV)-specific immunoglobulin (Ig)G antibody titers as determined by enzyme-linked immunosorbent assay (ELISA) after vaccination on day 0. Control calves did not have BRSV-specific IgG antibody titers before or after challenge.
Mortality and percentage of pneumonic lung
Two control calves were euthanatized on study day 53 (7 d after challenge) because of severe respiratory distress and low arterial PO2 values. Vaccinated calves had no or minimal pneumonic changes (< 3%; Figure 7), while all unvaccinated control calves had variable lung lesions (Figure 3B). Median percentage of the lung that was pneumonic was significantly (P < 0.001) higher for the control group (46.5; interquartile range [IQR], 42.2 to 50.8) than for vaccinated calves (0.5; IQR, 0.0 to 1.5). Gross lung lesions consisted of atelectasis of the cranioventral regions of the lung lobes and edema and emphysema, often with the formation of bullae, in the dorsal regions of the lung lobes (Figure 8). Histologically in the 2 calves that were euthanatized on study day 53, affected areas contained regions of necrotizing bronchiolitis and bronchointerstitial pneumonia with immunohistochemical evidence of BRSV in bronchiolytic and parenchymal lesions.
Figure 7.
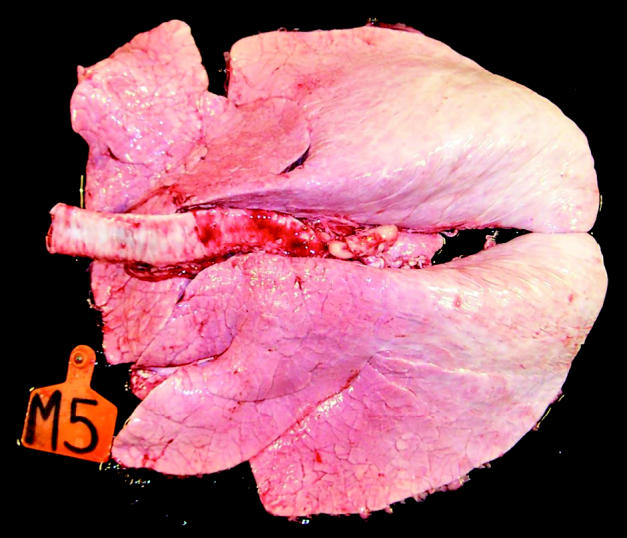
Lungs from representative vaccinated calf.
Figure 8.

Lungs from representative control calf note cranioventral atelectasis and emphysema in the dorsal areas of the lung.
Discussion
The results of this study provide further evidence that inactivated BRSV vaccines, when properly administered, can provide significant protection from BRSV-associated clinical disease and pulmonary lesion development in calves. Similar to several other studies with experimental and commercially available inactivated BRSV vaccines (8–12), cattle in this study developed high serum BRSV-specific IgG concentrations, as measured by ELISA; however, in contrast to other inactivated vaccines tested, this vaccine also stimulated the production of VN antibodies at concentrations similar to those stimulated by MLV vaccines (9–12). In contrast to the studies involving HRSV (1–3) in laboratory rodents and in some studies involving cattle (13), where there was enhanced respiratory disease subsequent to natural or experimental exposure to respiratory syncytial virus, the calves that were immunized with this inactivated BRSV vaccine had no or minimal clinical disease and no or minimal pulmonary lesions compared with unvaccinated controls.
The mechanism of protection stimulated by this inactivated BRSV vaccine was not extensively examined herein. Neutralizing antibodies at the time of challenge that increased anamnestically after infection may have accounted for the low viral load in 2 of the 6 vaccinated calves. As in a recent study (15) with another commercially available inactivated BRSV vaccine, protection was associated with high concentrations of non-neutralizing antibodies (IgG). Conceptually, it is not apparent how non-neutralizing antibodies affect protection, although blocking of binding to target cells is one unexamined possibility. In previous studies documenting the clinical efficacy of MLV and inactivated BRSV vaccines, cell-mediated immune mechanisms, including CD8+ cytolytic T lymphocytes, interferon-γ activity, or both, were additional correlates of protective immunity (12,15). The vaccine tested herein is adjuvanted with saponin, which is a unique surface acting compound that theoretically affects processing of inactivated antigens through the “endogenous pathway,” mimicking infection by wild type or modified live (vaccine) viruses (23). As a result of this processing, viral peptides are presented in the context of class I major histocompatibility antigens (MHC), thereby stimulating CD8+ effector cells that are capable of directly lysing infected target cells (23). Supportive of this possibility are data demonstrating that the inclusion of the adjuvant, Quil A, another surface acting agent closely related to saponin, together with BRSV subunits, induces cytotoxic lymphocyte responses in vaccinated sheep (24). Additional studies are necessary to clarify which cell-mediated immune mechanisms are stimulated by this adjuvanted vaccine. In addition to more effectively stimulating T cell responses, the adjuvant, inactivation method, or both may have better preserved and presented epitopes on envelop glycoproteins that stimulate neutralizing antibodies.
There was no immune-mediated enhancement of disease in vaccinated calves. However, there continues to be speculation about the prevalence and mechanism of vaccine-enhanced disease in BRSV-infected cattle, despite there being relatively few data that document the occurrence of this phenomenon following the use of commercially available vaccines in the field. Although inactivated vaccines are generally considered to be the offending immunogen in both cattle and other species, in fact, the first documented case of apparent disease enhancement was following the use of a modified-live BRSV vaccine (25). In that case, it was suggested that the timing of vaccination and infection coincided, so that vaccinated calves had a predominately IgM response at the time of infection and viral replication. The interaction of this specific antibody isotype with virus infected cells was implicated in the pathogenesis of accelerated inflammation and tissue damage and resultant enhancement of clinical disease. The results of this study again emphasize that it is difficult to draw generic conclusions about the protective or disease-enhancing properties of inactivated BRSV vaccines.
More recent experience in the field (18,19) indicates that under certain conditions, perhaps related most directly to vaccine formulation, the administration of certain inactivated BRSV vaccines can result in apparent enhancement of respiratory disease in cattle that are subsequently naturally exposed to the virus. It is interesting that the vaccine that was implicated in these incidents in Europe was also adjuvanted with a saponin together with an aluminum salt (aluminium hydroxychloride); however, differences in adjuvant composition, or other differences in formulation, such as antigenic mass, the inactivation process, or both, may be responsible for induction of disparate immune responses by 2 apparently similarly adjuvanted vaccines. Available data from the European incidents in which there was a lack of protection, or disease enhancement, suggest that the vaccine used may have induced low titers of BRSV neutralizing antibodies (19). Previous studies have suggested that the dose of BRSV antigen, as well as the method of inactivation, can affect the bovine immune response to BRSV vaccines (13–15). The types of immune responses responsible for apparent disease-enhancing properties of some inactivated BRSV vaccines in cattle remain to be determined.
Respiratory syncytial virus infections are often recurrent, suggesting that the duration of clinical immunity following natural infection and vaccination may be short (1,2,26,27). Results of this and a previous study (12,15) indicate that commercially available MLV vaccines, and at least 1 inactivated BRSV vaccine, can confer substantial clinical protection when vaccinated cattle are challenged with virulent virus within a month after immunization. These data do not address the issue of how differences in vaccine formulation may affect the generation of memory responses to the virus. Further studies are required to determine how vaccine design may determine the duration of immunity following administration of various BRSV vaccines.
In conclusion, results of the present study demonstrate the clinical efficacy of a commercially available inactivated BRSV vaccine in a challenge model that mimics severe naturally occurring disease (12,14,15). The possibility of inapparent variation that may affect results make it difficult to compare results among studies; however, clinical efficacy in the present study was similar to that reported for MLV vaccines and another inactivated vaccine tested using this model (12,15). This clinical efficacy was reflected in prevention of significant clinical respiratory disease, reduced viral shedding, reduced severity of pulmonary lesions, and enhanced antibody responses. Furthermore, these data demonstrate again that enhancement of BRSV-associated respiratory tract disease is not a generic response to all inactivated virus vaccines. CVJ
Footnotes
Financial assistance was provided by Wyeth Animal Health, Guelph, Ontario.
References
- 1.Van der Poel WH, Brand A, Kramps JA, et al. Respiratory syncytial virus infection in human beings and in cattle. J Infect. 1994;29:215–228. doi: 10.1016/s0163-4453(94)90866-4. [DOI] [PubMed] [Google Scholar]
- 2.Kimman TG, Westenbrink F. Immunity to human and bovine respiratory syncytial virus. Arch Virol. 1990;112:1–25. doi: 10.1007/BF01348982. [DOI] [PubMed] [Google Scholar]
- 3.Baker JC, Ellis JA, Clark EG. Bovine respiratory syncytial virus. Vet Clin North Am Food Anim Pract. 1997;13:425–454. doi: 10.1016/s0749-0720(15)30307-8. [DOI] [PubMed] [Google Scholar]
- 4.Bryson DG, McNulty MS, Logan EF, et al. Respiratory syncytial virus pneumonia in young calves: clinical and pathologic findings. Am J Vet Res. 1983;44:1648–1655. [PubMed] [Google Scholar]
- 5.Bryson DG. Necropsy findings associated with BRSV pneumonia. Vet Med 1993;88:894,896–899.
- 6.Kimman TG, Straver PJ, Zimmer GM. Pathogenesis of naturally acquired bovine respiratory syncytial virus infection in calves: morphologic and serologic findings. Am J Vet Res. 1989;50:684–693. [PubMed] [Google Scholar]
- 7.Ferguspm JD, Galligan DT, Cortese V. Milk production and reproductive performance in dairy cows given bovine respiratory synctyial virus vaccine prior to parturtition. J Am Vet Med Assoc. 1997;210:1779–1783. [PubMed] [Google Scholar]
- 8.Bowland SL, Shewen PE. Bovine respiratory diesase: commercial vaccines currently available in Canada. Can Vet J. 2000;41:33–48. [PMC free article] [PubMed] [Google Scholar]
- 9.Ellis JA, Russell HI, Cavender J, et al. Bovine respiratory syncytial virus-specific immune responses in cattle following immunization with modified-live and inactivated vaccines: analysis of specificity and activity of serum antibodies. Vet Immunol Immunopathol. 1992;34:33–37. doi: 10.1016/0165-2427(92)90150-o. [DOI] [PubMed] [Google Scholar]
- 10.Ellis JA, Hassard LE, Morley P. Bovine respiratory syncytial virus-specific immune responses in calves after inoculation with commercially available vaccines. J Am Vet Med Assoc. 1995;206:354–361. [PubMed] [Google Scholar]
- 11.West K, Ellis J. Functional analysis of antibody responses of feed-lot cattle to bovine respiratory syncytial virus following vaccination with mixed vaccines. Can J Vet Res. 1997;61:28–33. [PMC free article] [PubMed] [Google Scholar]
- 12.West K, Petrie L, Konoby C, et al. The efficacy of modified-live bovine respiratory syncytial virus vaccines in experimentally infected calves. Vaccine. 2000;18:907–919. doi: 10.1016/s0264-410x(99)00324-2. [DOI] [PubMed] [Google Scholar]
- 13.Gerschwin LJ, Schelegle ES, Gunther RA, et al. A model of vaccine enhanced respiratory syncytial virus pathophysiology. Vaccine. 1998;16:1225–1236. doi: 10.1016/s0264-410x(98)80123-0. [DOI] [PubMed] [Google Scholar]
- 14.West K, Petrie L, Haines DM, et al. The effect of formalin-inactivated vaccine on respiratory disease associated with bovine respiratory syncytial virus infection in calves. Vaccine. 1999;17:809–820. doi: 10.1016/s0264-410x(98)00265-5. [DOI] [PubMed] [Google Scholar]
- 15.Ellis J, West K, Konoby C, et al. Efficacy of an inactivated respiratory syncytial virus vaccine in calves. J Am Vet Med Assoc. 2001;218:1973–1980. doi: 10.2460/javma.2001.218.1973. [DOI] [PubMed] [Google Scholar]
- 16.Murphy BR, Prince GA, Walsh EE, et al. Dissociation between serum neutralizing and glycoprotein antibody responses in infants and children who received inactivated respiratory syncytial virus vaccine. J Clin Microbiol. 1986;24:197–202. doi: 10.1128/jcm.24.2.197-202.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim WH, Chanchola JG, Brandt CD, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 18.Schreiber P, Matheise JP, Dessy F, et al. High mortality rate associated with bovine respiratory syncytial virus (BRSV) infection in Belgian white blue calves previously vaccinated with an inactivated BRSV vaccine. J Vet Med B. 2000;47:535–500. doi: 10.1046/j.1439-0450.2000.00380.x. [DOI] [PubMed] [Google Scholar]
- 19.Larsen LE, Tegtmeier C, Pedersen E. Bovine respiratory syncytial virus (BRSV) pneumonia in beef calf herds despite vaccination. Acta Vet Scand. 2001;42:113–121. doi: 10.1186/1751-0147-42-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Will J, Bisgard G. Cardiac catheterization of unanesthetized large domestic animals. J Appl Physiol. 1972;33:400–401. doi: 10.1152/jappl.1972.33.3.400. [DOI] [PubMed] [Google Scholar]
- 21.West K, Bogdan J, Hamel A, et al. A comparison of diagnostic methods for the detection of bovine respiratory syncytial virus in experimental clinical specimens. Can J Vet Res. 1998;62:245–250. [PMC free article] [PubMed] [Google Scholar]
- 22.Haines DM, Clark EG, Chelack BJ. The detection of bovine respiratory syncytial virus in formalin fixed lung tissue with commercially available monoclonal antibodies and avidin biotin immunohistochemistry. Can J Vet Res. 1989;53:366–368. [PMC free article] [PubMed] [Google Scholar]
- 23.Tizard I. Dendritic cells and antigen processing. In: Veterinary Immunology, An Introduction. Philadelphia: WB Saunders, 2000:58–68.
- 24.Sharma AK, Woldehiwet Z, Walrevens K, et al. Immune responses of lambs to the fusion (F) glycoprotein of bovine respiratory syncytial virus expressed on insect cells infected with recombinant baculovirus. Vaccine. 1996;14:773–779. doi: 10.1016/0264-410x(95)00248-y. [DOI] [PubMed] [Google Scholar]
- 25.Kimman TG, Sol J, Westenbrink F, Straver PJ. A severe outbreak of respiratory tract disease associated with bovine respiratory syncytial virus probably enhanced by vaccination with modified live vaccine. Vet Q. 1989;11:250–253. doi: 10.1080/01652176.1989.9694231. [DOI] [PubMed] [Google Scholar]
- 26.Van der Poel WHM, Kramps JA, Middel WGJ, et al. Dynamics of bovine respiratory syncytial virus infections: a longitudinal epidemiological study in dairy herds. Arch Virol. 1993;133:309–321. doi: 10.1007/BF01313771. [DOI] [PubMed] [Google Scholar]
- 27.Breese Hall C, Walsh EE, Long CE, et al. Immunity and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163:693–698. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]


