Abstract
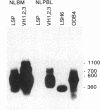
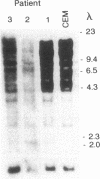
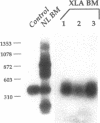
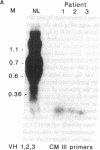
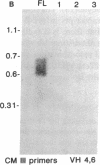
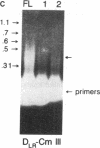
X-linked agammaglobulinemia (XLA) results from a failure of B lymphoid development. We have previously examined pre-B cell hybrids from three patients with XLA and found them to be limited to production of a novel germ line transcript of the Ig H chain locus composed of a leader sequence (LS) spliced to the constant region of mu chain (C mu) as mRNA and polypeptide. These transcripts result from transcriptional activation of the germ line heavy chain locus from an LS exon upstream of the embryonic JH locus. Germ line LS-C mu transcripts are produced by pre-B cells from normal bone marrow and fetal liver, indicating that they are products of normal pre-B cell development, as part of the process of transcriptional activation to provide access for the recombinase. Bone marrow from three patients with XLA has been examined directly by polymerase chain reaction amplification to determine whether the exclusive production of LS-C mu by XLA pre-B cell hybrids is representative of XLA pre-B cells. I report that LS-C mu is the predominant Ig molecule produced by XLA pre-B cells, with limited production of the D mu product of DJH intermediate stage of V(D)J recombination. Mature VHDJH recombinations were not detected with a variety of primers that amplify VH sequences. I conclude that XLA is associated with a limitation in V(D)J recombination that may cause the failure of pre-B cell development.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Blackwell T. K., DePinho R. A., Reth M. G., Yancopoulos G. D. Regulation of genome rearrangement events during lymphocyte differentiation. Immunol Rev. 1986 Feb;89:5–30. doi: 10.1111/j.1600-065x.1986.tb01470.x. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Yancopoulos G. D., Blackwell T. K., Wood C., Thomas E., Boss M., Coffman R., Rosenberg N., Tonegawa S., Baltimore D. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984 Jun;3(6):1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker R., Conley M. E., Pollok B. A. Clonal diversity in the B cell repertoire of patients with X-linked agammaglobulinemia. J Exp Med. 1989 Jun 1;169(6):2109–2119. doi: 10.1084/jem.169.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T. K., Moore M. W., Yancopoulos G. D., Suh H., Lutzker S., Selsing E., Alt F. W. Recombination between immunoglobulin variable region gene segments is enhanced by transcription. Nature. 1986 Dec 11;324(6097):585–589. doi: 10.1038/324585a0. [DOI] [PubMed] [Google Scholar]
- Bosma G. C., Fried M., Custer R. P., Carroll A., Gibson D. M., Bosma M. J. Evidence of functional lymphocytes in some (leaky) scid mice. J Exp Med. 1988 Mar 1;167(3):1016–1033. doi: 10.1084/jem.167.3.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Conley M. E. B cells in patients with X-linked agammaglobulinemia. J Immunol. 1985 May;134(5):3070–3074. [PubMed] [Google Scholar]
- Conley M. E., Puck J. M. Carrier detection in typical and atypical X-linked agammaglobulinemia. J Pediatr. 1988 May;112(5):688–694. doi: 10.1016/s0022-3476(88)80683-8. [DOI] [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Winkelstein J. A., Civin C. I., Pardoll D. M., Vogelstein B. Carrier detection in X-linked agammaglobulinemia by analysis of X-chromosome inactivation. N Engl J Med. 1987 Feb 19;316(8):427–431. doi: 10.1056/NEJM198702193160802. [DOI] [PubMed] [Google Scholar]
- Hoch S., Schwaber J. Identification and sequence of the VH gene elements encoding a human anti-DNA antibody. J Immunol. 1987 Sep 1;139(5):1689–1693. [PubMed] [Google Scholar]
- Honjo T. Immunoglobulin genes. Annu Rev Immunol. 1983;1:499–528. doi: 10.1146/annurev.iy.01.040183.002435. [DOI] [PubMed] [Google Scholar]
- Ichihara Y., Matsuoka H., Tsuge I., Okada J., Torii S., Yasui H., Kurosawa Y. Abnormalities in DNA rearrangements of immunoglobulin gene loci in precursor B cells derived from X-linked agammaglobulinemia patient and a severe combined immunodeficiency patient. Immunogenetics. 1988;27(5):330–337. doi: 10.1007/BF00395128. [DOI] [PubMed] [Google Scholar]
- Mensink E. J., Schuurman R. K., Schot J. D., Thompson A., Alt F. W. Immunoglobulin heavy chain gene rearrangements in X-linked agammaglobulinemia. Eur J Immunol. 1986 Aug;16(8):963–967. doi: 10.1002/eji.1830160815. [DOI] [PubMed] [Google Scholar]
- Pearl E. R., Vogler L. B., Okos A. J., Crist W. M., Lawton A. R., 3rd, Cooper M. D. B lymphocyte precursors in human bone marrow: an analysis of normal individuals and patients with antibody-deficiency states. J Immunol. 1978 Apr;120(4):1169–1175. [PubMed] [Google Scholar]
- Ravetch J. V., Siebenlist U., Korsmeyer S., Waldmann T., Leder P. Structure of the human immunoglobulin mu locus: characterization of embryonic and rearranged J and D genes. Cell. 1981 Dec;27(3 Pt 2):583–591. doi: 10.1016/0092-8674(81)90400-1. [DOI] [PubMed] [Google Scholar]
- Reth M. G., Alt F. W. Novel immunoglobulin heavy chains are produced from DJH gene segment rearrangements in lymphoid cells. 1984 Nov 29-Dec 5Nature. 312(5993):418–423. doi: 10.1038/312418a0. [DOI] [PubMed] [Google Scholar]
- Rosen F. S., Cooper M. D., Wedgwood R. J. The primary immunodeficiencies (1). N Engl J Med. 1984 Jul 26;311(4):235–242. doi: 10.1056/NEJM198407263110406. [DOI] [PubMed] [Google Scholar]
- Sakano H., Kurosawa Y., Weigert M., Tonegawa S. Identification and nucleotide sequence of a diversity DNA segment (D) of immunoglobulin heavy-chain genes. Nature. 1981 Apr 16;290(5807):562–565. doi: 10.1038/290562a0. [DOI] [PubMed] [Google Scholar]
- Schlissel M. S., Baltimore D. Activation of immunoglobulin kappa gene rearrangement correlates with induction of germline kappa gene transcription. Cell. 1989 Sep 8;58(5):1001–1007. doi: 10.1016/0092-8674(89)90951-3. [DOI] [PubMed] [Google Scholar]
- Schlissel M. S., Corcoran L. M., Baltimore D. Virus-transformed pre-B cells show ordered activation but not inactivation of immunoglobulin gene rearrangement and transcription. J Exp Med. 1991 Mar 1;173(3):711–720. doi: 10.1084/jem.173.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Hillson J. L., Perlmutter R. M. Early restriction of the human antibody repertoire. Science. 1987 Nov 6;238(4828):791–793. doi: 10.1126/science.3118465. [DOI] [PubMed] [Google Scholar]
- Schuler W., Weiler I. J., Schuler A., Phillips R. A., Rosenberg N., Mak T. W., Kearney J. F., Perry R. P., Bosma M. J. Rearrangement of antigen receptor genes is defective in mice with severe combined immune deficiency. Cell. 1986 Sep 26;46(7):963–972. doi: 10.1016/0092-8674(86)90695-1. [DOI] [PubMed] [Google Scholar]
- Schwaber J., Molgaard H., Orkin S. H., Gould H. J., Rosen F. S. Early pre-B cells from normal and X-linked agammaglobulinaemia produce C mu without an attached VH region. 1983 Jul 28-Aug 3Nature. 304(5924):355–358. doi: 10.1038/304355a0. [DOI] [PubMed] [Google Scholar]
- Schwaber J., Rosen F. S. X chromosome linked immunodeficiency. Immunodefic Rev. 1990;2(3):233–251. [PubMed] [Google Scholar]
- Siu G., Kronenberg M., Strauss E., Haars R., Mak T. W., Hood L. The structure, rearrangement and expression of D beta gene segments of the murine T-cell antigen receptor. 1984 Sep 27-Oct 3Nature. 311(5984):344–350. doi: 10.1038/311344a0. [DOI] [PubMed] [Google Scholar]
- Treisman R., Orkin S. H., Maniatis T. Structural and functional defects in beta-thalassemia. Prog Clin Biol Res. 1983;134:99–121. [PubMed] [Google Scholar]
- Yancopoulos G. D., Alt F. W. Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell. 1985 Feb;40(2):271–281. doi: 10.1016/0092-8674(85)90141-2. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Blackwell T. K., Suh H., Hood L., Alt F. W. Introduced T cell receptor variable region gene segments recombine in pre-B cells: evidence that B and T cells use a common recombinase. Cell. 1986 Jan 31;44(2):251–259. doi: 10.1016/0092-8674(86)90759-2. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Desiderio S. V., Paskind M., Kearney J. F., Baltimore D., Alt F. W. Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature. 1984 Oct 25;311(5988):727–733. doi: 10.1038/311727a0. [DOI] [PubMed] [Google Scholar]