Abstract
The objectives of this study were to evaluate behavior of sternocleidomastoid muscle (SCM) electromyogram (EMG) related to impact velocity, gender, awareness and kinematics of head movement in simulated low velocity rear-end impacts. Twenty-nine healthy adults (17 male) were subjected in random order to three rear-end impacts: Two unexpected impacts causing chair accelerations of 4.5 m/s2 (slow) and 10.1 m/s2 (fast) and one 10.1 m/s2 expected impact. Normalized left and right SCM EMG, linear head acceleration, angular head acceleration and maximum angular head displacement were recorded. The magnitude of normalized SCM EMG peak response ranged 2–3 times higher (P< 0.001) in female subjects than their counterpart male subjects. SCM EMG magnitude was 3–4 times higher (P< 0.001) for the fast unexpected than slow unexpected impacts, but there was no significant difference (P> 0.05) for the fast expected compared to the fast unexpected impacts. The onset time of SCM peak EMG ranged from 78 ms to 114 ms later than peak of linear head acceleration for all groups. Onset time of peak SCM EMG was not significantly different (P> 0.05) than onset of angular acceleration for the slow and fast-unexpected impacts, but onset peak SCM EMG was significantly earlier than peak angular head acceleration (30 ms) (P ≤ 0.05) for the fast expected impact. SCM EMG magnitude increased with increased impact velocity. Gender differences exist for SCM EMG magnitude. Temporal and amplitude awareness of a simulated impact do not produce different magnitude of SCM EMG response. The temporal relationship between the SCM and angular head acceleration is different from the temporal relationship between the SCM and linear head acceleration.
Keywords: Whiplash, Head kinematics, Electromyogram, Sternocleidomastoid muscle
Introduction
Rear-end impacts and whiplash injuries are highly associated [17]. Whiplash injuries are a major health problem and they have significant economical consequences in most industrialized countries [3, 12, 24]. Recently, researchers have focused on the role of the cervical muscles in whiplash injury [2, 9, 11, 19, 20]. Kumar et al. [9] suggested that the first injury is to the muscle, followed by injury to the ligaments, the facet joints, and the brain. “The common perception that muscles activate too late in a rear-end collision to affect whiplash injury is incorrect” [20]. Castro et al. [3] reported that electromyography (EMG) signals of the neck muscles started before the head movement took place. However, no EMG data was presented and they did not specify which muscles were studied.
Among the cervical muscles, the sternocleidomastoid (SCM) muscle might play a significant role in whiplash injury [2, 9, 11, 13, 16]. Magnusson et al. [11] did not report significant differences in cervical muscle reaction times between expected and unexpected conditions. Kumar et al. [9] reported lower SCM EMG activity in expected impacts. Differences by gender have been reported by Siegmund et al. [21] and by Brault et al. [2], but not by Kumar et al. [9].
As a result of the controversial reported findings, the present study was designed to determine the SCM muscle response and its temporal relationship with the kinematics of head movement in human volunteers exposed to simulated rear-end impacts at low velocities. The objectives of this study were to evaluate:
Behavior of SCM EMG activity at two impact magnitudes.
Temporal relationship between SCM muscle activity and kinematics of head movement.
Relationship of impact awareness with muscle response.
Gender differences for muscle response.
Methods and materials
The Human Research Ethics Board at the University of Alberta approved the protocol for this study. Participants were recruited by poster advertisement on the University of Alberta campus. Thirty individuals were screened and deemed eligible to participate in this study; however, only 29 completed the experiment. One male subject had a panic attack and withdrew. Demographic data is presented in Table 1.
Table 1.
Descriptive statistics of demographic data
| Number | Age (SD)(years) | Height (SD)(cm) | Weight (SD)(kg) | |
|---|---|---|---|---|
| Females | 12 | 25 (2.4) | 167 (8.9) | 58 (8.9) |
| Males | 18 | 25 (2.9) | 179 (6.6) | 76 (11.9) |
Participants were between 18 years and 35 years old, healthy and free of any signs or symptoms in the cervical or orofacial regions. Exclusion criteria included history of car accident or trauma to the back or neck within the last 12 months, more than one missing tooth by quadrant with the exception of the third molar, and the wearing of any type of occlusal appliance.
Participants attended two appointments. At the first appointment, subjects read and signed the information sheet, completed a medical history form and gave informed consent. Clinical examination of the head and neck was performed on each subject. The second appointment was the experimental phase: each subject underwent three impacts. SCM EMG activity and kinematics of head movement were recorded.
Experimental set-up
Two recording systems were used in the present study. One system recorded the chair acceleration and SCM EMG activity, and the other the kinematics of head movement. Accelerometers were used to measure head and chair acceleration.
Acceleration sled set up
The sled system consisted of a 250 cm by 125 cm raised wooden platform, with two 200 cm long parallel tracks mounted along the length of the platform. A Volvo car seat with a headrest was sturdily mounted on a rectangular sliding board coupled with the tracks for friction-reduced travel upon impact. The sled was accelerated with a pneumatic cylinder of 7.5 mm diameter and 30 cm stroke. The piston of the cylinder was connected to an air supply through a pressure regulator calibrated for delivery of known forces causing desired acceleration. One uniaxial accelerometer (25 g) (Crossbow Technology, San José, CA, USA) was located in the car seat to measure acceleration of the sled relative to the floor.
Head acceleration set up
A custom designed accelerometer system was developed to measure angular head acceleration. The system included: a multipurpose circuit board, a 16 channel 12 bit A/D converter, ±5 V input range (National Instruments Corporation, Austin, TX, USA) and two biaxial accelerometers ±10 g (item model ADXL 210, Analog Devices, Norwood, MA, USA). The accelerometer board was attached to the maxillary teeth with a custom dental tray. A clear plastic extension attached to the tray, positioned the accelerometer system outside of the mouth and it aligned with the facial midline. Photographs of the sled and accelerometer set up are provided in Figs. 1a, 1b.
Fig. 1.
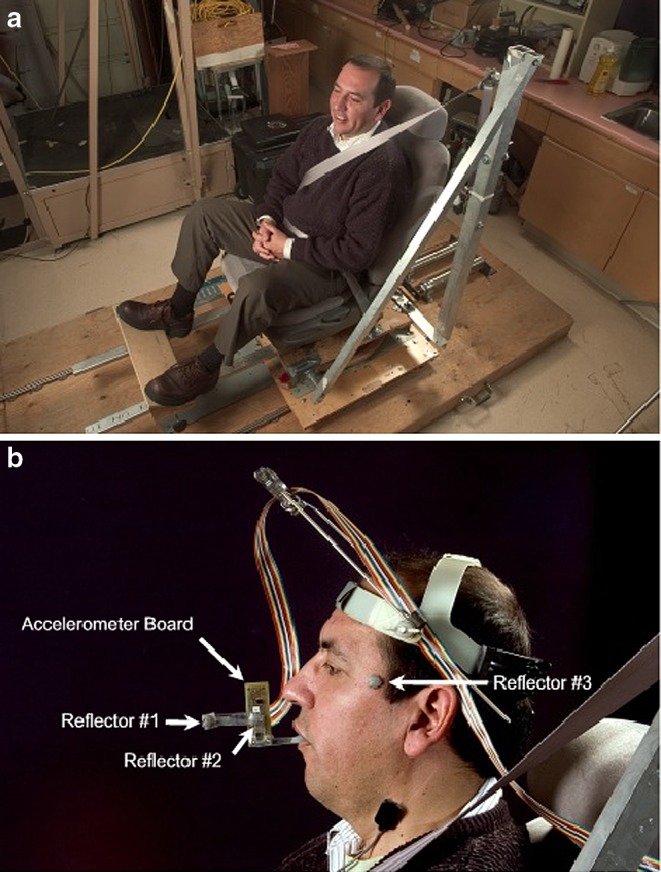
a Sled set up. Subject is shown in the position of the impact. The pneumatic cylinder is located on the platform behind the car seat b Accelerometer set up
High speed video cameras
Three spherical reflectors in conjunction with three ProReflex Cameras (item model MCO 240, Qualisys, Savedalen, Sweden) were also used to record head motion. Two reflectors were placed on the plastic extension attached to the dental tray and one reflector was placed in the anterior temporal region of the head. The video camera files were collected with the PCR reflex software and exported to a Tracker 3-D program (Qualisys, Savedalen, Sweden) in which the signals for the reflective balls were labeled and missing data identified. Also, the raw files were transformed in such a way that they could be read in a text editor. The data obtained from the video cameras were processed with the aid of MATLAB (MathWorks, MA, USA) software.
EMG system
The EMG system (Delsys Inc, Boston, MA, USA) included surface electrodes, electrode cables, preamplifiers, amplifiers and a screen where the recording was displayed. Bipolar electrodes with an interelectrode distance of 1 cm were used.
Data acquisition
It was assumed that most of the movement would be in the sagittal plane. Angular and linear head accelerations as well as angular head displacements were recorded. Kinematics of head movement was simultaneously recorded with the video camera and the custom designed accelerometer system. The recordings of head angular acceleration and displacement from two sources allowed verification of the quality of the data. Linear acceleration of the head in the X axis was recorded at the site of the anterior temporal region. Angular displacement of the head was obtained from the video cameras. Angular head acceleration was obtained from the accelerometer board.
Onset time and peak time for the EMG and kinematics of head movement were determined. Onset and peak time were relative to the onset of chair movement. Onset time was defined as the time in which 5% of the magnitude value of the peak occurred. Peak time was defined as the time in which the maximum value of the variable was reached. Data acquisition was restricted to the first 750 ms after impact.
Experimental phase
The subject’s skin over the SCM muscles was vigorously cleaned with a paper towel and alcohol prior to application of the electrodes. Electrodes were placed parallel to the direction of muscle fibers and over the sternal belly (C4 level) of the left and right SCM muscles. A ground electrode was placed over the distal portion of the left clavicle.
Each participant was seated in an upright position with her/his legs uncrossed, and head straight. The participants were asked to perform a maximum isometric flexion force using the SCM muscles. Each strength test was 5 s in duration. The corresponding peak and average magnitude of EMG generated in this exercise were recorded for each subject.
Each subject underwent three impacts: Two unexpected impacts–slow and fast, and one expected impact of the same magnitude of the unexpected fast impact. The mean chair acceleration peaks (maximum acceleration) in the anterior posterior direction for each acceleration level are presented in Table 2. The average time for the sled to reach maximum acceleration in the X axis was 9.2 ms from acceleration onset.
Table 2.
Mean of the linear chair acceleration peaks in the X axis
| Slow impact (SD) | Fast impact (SD) | Slow–fast P value and power | |
|---|---|---|---|
| Acceleration (m/s2) | 4.47 (0.73) | 10.07 (1.84) | 0.001* 0.999 |
| Sample | 29 | 58 |
One way ANOVA. Magnitude of the impacts expressed as mean of the linear acceleration peaks of the sled
*The mean difference is significant at the 0.05 level
The order of impacts was randomized. For the unexpected impacts, each subject listened to loud music and fabric blindfold was used to cover the participant’s eyes. Subjects were aware that there would be an impact, but were not advised of timing or impact magnitude. There was no attempt to deceive the subject with a “surprise” impact. In the expected impact, subjects were told the magnitude of the impact in qualitative terms, i.e. a fast impact, and when the impact would happen.
Data processing
A mechanical engineer, not a member of this research team, processed the raw EMG, accelerometer and video camera files. The raw files from the EMG data were analyzed using the Root Mean Square technique [1]. The EMG corresponding to the peak force during maximum voluntary contraction was given a value of 100%. The EMG amplitudes for the right and left SCM muscles recording during the acceleration trials were normalized against this maximum value and expressed as a percentage of maximum voluntary EMG.
The video camera raw files were processed in such a way that the reflective balls were labeled, missing data were identified and the data could be read in a text editor. The three markers had their position monitored at a sampling rate of 200 Hz for 5 s.
The accelerometer board system incorporated a low pass filter with a frequency cut-off of 50 Hz. The raw files from the accelerometer system recorded 10 s: 5 s pre-impact and 5 s post-impact. However, a 750-ms window at a sampling rate of 4,000 Hz with 5 s of pre-recorded data was analyzed. The high frequency used for sampling these data was used for a secondary engineering-related investigation.
Files for the different impacts were coded for blinding. Missing data points and data points presenting a technical error were identified and eliminated. From the original sample, full left SCM EMG data was available for 26 subjects and full right SCM EMG data was available for all 29 subjects. Missing kinematic data points further reduced the sample size for analysis of timing of SCM EMG and head movement. Special care was taken to integrate the data files for consistent timing of impact; the two recording systems had as time 0 the firing of the pneumatic cylinder piston, which caused the acceleration of the chair. In a second step, the timing of the response variables was adjusted so the onset of acceleration of the chair represented time 0 in each impact and for each subject.
Statistical analysis
The data was organized in an Excel spreadsheet and analyzed using SPSS (SPSS, Chicago, IL, USA). Repeated measures statistical method was used to analyze the muscle response with an increase of the magnitude of the impact as well as its behavior in the expected and unexpected impacts. Gender was included in the analysis. The same statistical method was used to determine whether the EMG muscle activity or the movement of the head was initiated first.
The significance level of alpha equal 0.05 was used to determine the level of significance of the data. Transformation of the raw data to natural logarithm was performed in order to obtain a normal data distribution and homogenize the variance between the variables. Statistical power was determined for all repeated measures statistical comparisons.
Results
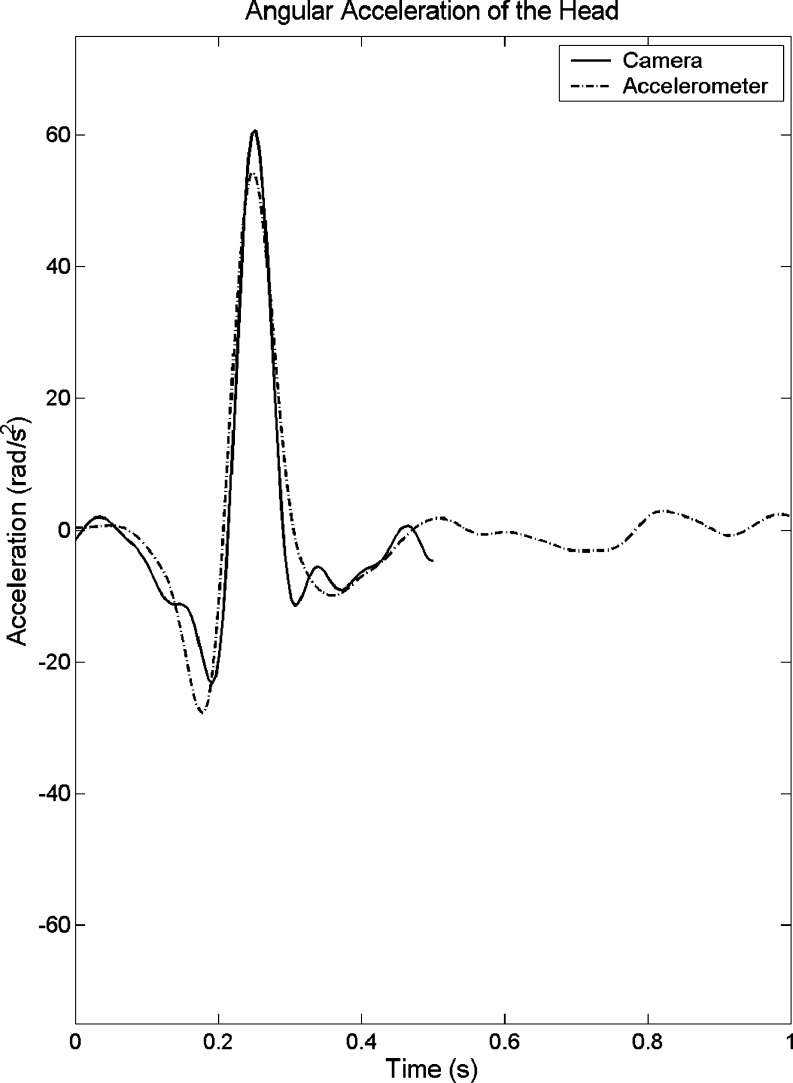
The video camera and accelerometer data presented a good agreement (Fig. 2). The natural logarithmic transformation of normalized EMG peaks (percentage of maximum voluntary contraction) for the SCM muscles in response to the unexpected and expected impacts are presented in Table 3. The corresponding raw data is presented in Table 4. The natural logarithmic transformation of onset time and peak time of the EMG peaks for the unexpected and expected impacts are presented in the Table 5. The corresponding raw data is presented in Table 6.
Fig. 2.
Comparison of video camera and accelerometer system recording
Table 3.
Mean normalized peak SCM muscle EMG in response to simulated rear-end impacts
| Sample | Slow unexpected (SD) | Gender P value power | Fast unexpected (SD) | Gender P value power | Fast expected (SD) | Gender P value power | Slow–fast unexpected P value, power | Fast: unexpected–expected P value, power | |
|---|---|---|---|---|---|---|---|---|---|
| Left | 15 M | 2.35 (0.85) | 0.014* | 3.85 (0.62) | 0.604 | 3.73 (0.63) | 0.031* | 0.001* | 0.929 |
| 11 F | 3.41 (1.19) | 0.714 | 4.03 (1.11) | 0.80 | 4.42 (0.89) | 0.597 | 0.999 | 0.999 | |
| 26 | 2.80 (1.12) | 3.93 (0.85) | 4.03 (0.81) | ||||||
| Right | 17 M | 2.68 (0.82) | 0.005* | 4.34 (0.46) | 0.651 | 4.24 (0.67) | 0.138 | 0.001* | 0.999 |
| 12 F | 3.63 (0.90) | 4.50 (1.09) | 4.61 (0.82) | 0.999 | 0.999 | ||||
| 29 | 3.07 (0.96) | 4.41 (0.77) | 4.39 (0.75) |
Normalized EMG represents the maximum voluntary contraction and it is presented as natural logarithm data. Repeated measures test for detecting gender differences in the left SCM (power values are included), and response differences between impacts. One way ANOVA analysis used for detecting gender differences in the right SCM. M refers to male and F to females
* The mean difference is significant at the 0.05 level
Table 4.
Raw data for combined gender mean normalized peak SCM muscle EMG in response to simulated rear-end impacts
| Sample | Slow unexpected (SD) | Fast unexpected (SD) | Fast expected (SD) | |
|---|---|---|---|---|
| Left | 26 | 32.82 (47.06) | 75.07 (82.78) | 82.00 (88.88) |
| Right | 29 | 34.53 (38.61) | 109.75 (91.21) | 104.74 (75.71) |
Normalized EMG represents the maximum voluntary contraction
Table 5.
Mean onset and peak times of the SCM muscle EMG in response to simulated rear-end impacts
| Slow unexpected impact (SD) | Fast unexpected Impact (SD) | Fast expected impact (SD) | Slow–fast unexpected P value, power | Fast: unexpected–expected P value, power | |
|---|---|---|---|---|---|
| Onset time Left SCM N=26 |
4.59 (0.48) | 4.56 (0.46) | 4.45 (0.56) | 0.999 0.136 |
0.844 0.136 |
| Onset time Right SCM N=29 |
4.70 (0.67) | 4.76 (0.56) | 4.55 (0.62) | 0.999 0.177 |
0.999 0.177 |
| Peak time Left SCM N=26 |
4.76 (0.40) | 4.81 (0.30) | 4.76 (0.34) | 0.999 0.071 |
0.999 0.071 |
| Peak time Right SCM N=29 |
4.90 (0.53) | 4.93 (0.45) | 4.82 (0.46) | 0.999 0.127 |
0.999 0.127 |
Repeated measures test. Time is presented as natural logarithm data. Combined male and female sample
Table 6.
Raw data for mean onset and peak times of the SCM muscle EMG in response to simulated rear-end impacts
| Slow unexpected impact (SD) | Fast unexpected impact (SD) | Fast expected impact (SD) | |
|---|---|---|---|
| Onset time Left SCM N=26 |
116.46 (106.80) | 107.00 (49.55) | 100.03 (54.74) |
| Onset time Right SCM N=29 |
142.21 (128.58) | 142.41 (125.02) | 119.45 (106.13) |
| Peak time Left SCM N=26 |
132.50 (42.54)) | 128.62 (39.07) | 124.19 (46.15) |
| Peak time Right SCM N=29 |
159.62 (123.45) | 160.10 (120.68) | 141.90 (101.09) |
Time is expressed in milliseconds with onset of chair movement recorded as time zero. Combined male and female sample
Normalized SCM muscle EMG
Statistical power was adequate for EMG magnitude data. Gender differences existed in the EMG responses. Normalized SCM EMG activity of female subjects ranged between 2 and 3 times higher than male participants.
The magnitude of normalized SCM EMG activity was approximately 3–4 times higher for the fast unexpected impacts compared to the slow unexpected impacts. The magnitude of normalized SCM EMG was not significantly different for the fast unexpected and fast expected impacts.
No differences in onset or peak time of the left and right SCM EMG peaks were detected between the slow and fast-unexpected impacts. Significant difference for expectation was not identified for onset and timing of peak EMG response. Statistical power was low for EMG timing data.
Temporal relationship between the muscle activity and kinematics of head movement
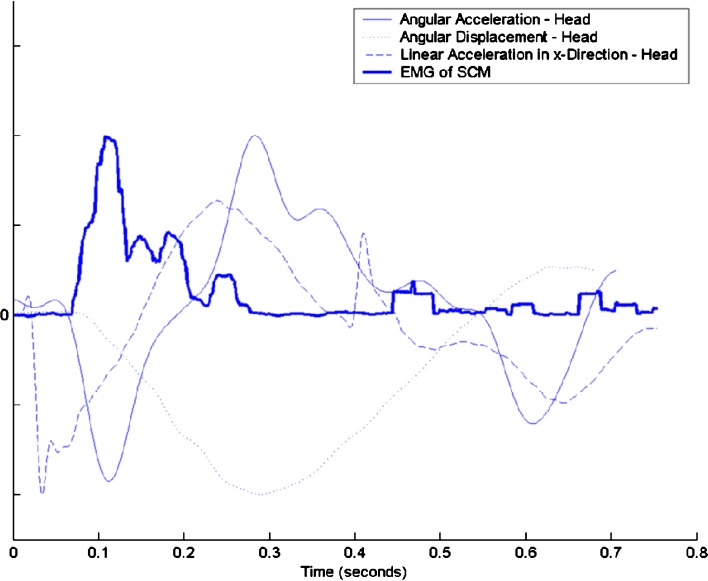
Temporal relationship was assessed as follows: a repeated measures test was used to determine whether the onset time of the EMG peak for the left and right SCM was prior or later than the peak time of kinematic events of head movement. No significant difference was detected between the right and left SMC muscles (P value = 0.999, power = 0.999). In addition, both muscles presented a similar behavior when compared with the kinematic events of the head. Only the left SCM data is presented due to a better signal and is provided in Tables 7 and Table 8. Normalized SCM EMG and head kinematics from a representative subject subjected to expected fast impact are presented in Figure 3.
Table 7.
Mean onset time of the left SCM EMG and mean peak time of the kinematic events of the head movement in response to simulated rear-end impacts
| Onset time of SCM EMG (SD) | Peak time of the kinematic events of the head movement (SD) | SCM - Linear head acc P value, Power | SCM - Angular head acc P value, Power | SCM - Angular head displacement P value, power | |||
|---|---|---|---|---|---|---|---|
| Linear head acceleration (x axis) | Angular head acceleration | Angular head displacement | |||||
| Slow impact N=16 |
4.6 (0.51) | 2.3 (0.83) | 4.6 (0.23) | 5.54 (0.50) | 0.001* 0.999 | 0.999 0.999 | 0.006* 0.999 |
| Fast unexpected impact N=14 |
4.49 (0.44) | 2.33 (0.09) | 4.92 (0.35) | 5.51 (0.45) | 0.001* 0.999 | 0.053 0.999 | 0.001* 0.999 |
| Fast expected impact N=18 |
4.32 (0.52) | 2.06 (0.29) | 4.73 (0.18) | 5.59 (0.21) | 0.001* 0.999 | 0.019* 0.999 | 0.001* 0.999 |
Time is presented as natural logarithm data
Repeated measures test. P value and power is presented for each analysis
* The mean difference is significant at the 0.05 level
Table 8.
Mean onset time of the left SCM EMG and mean peak time of the kinematic events of the head movement in response to simulated rear-end impacts
| Onset time of SCM EMG (SD) | Peak time of the kinematic events of the head movement (SD) | |||
|---|---|---|---|---|
| Linear head acceleration (x axis) | Angular head acceleration | Angular head displacement | ||
| Slow impact N=16 |
130.69 (132.47) | 16.25 (29.70) | 106.90 (21.54) | 276.12 (77.14) |
| Fast unexpected impact N=14 |
99.14 (51.05) | 10.35 (1.00) | 148.94 (79.12) | 269.28 (99.57) |
| Fast expected impact N=18 | 86.50 (49.49) | 8.22 (2.31) | 116.23 (24.92) | 274.16 (61.48) |
Time is presented as raw data
Time is presented in milliseconds with onset of chair movement recorded as time zero. Combined male and female sample
Fig. 3.
Timing of the left SCM EMG and kinematic parameters in response to simulated fast expected rear-end impact (10.1 m/s2 chair acceleration) for one representative subject
The onset of the SCM peak EMG ranged from 78 ms–114 ms later than the peak of the linear head acceleration in the unexpected slow, expected fast and unexpected fast impacts. The onset of the SCM peak EMG was not significantly different than the peak angular acceleration in the unexpected slow and unexpected fast impacts. For the expected fast impact onset SCM EMG activity occurred significantly earlier (approximately 30 ms) than the peak angular head acceleration.
The onset of the SCM peak EMG ranged from 136 ms–188 ms earlier than the peak rearward angular head displacement in the slow, and fast unexpected and expected impacts.
Statistical power was adequate for analysis of timing of EMG and head kinematics.
Discussion
The current study analyzed the SCM muscle responses in simulated rear-end impacts. Increased EMG activity was observed at higher impact magnitude, and the results of the current study support the hypothesis that at higher levels of impacts the muscles may become injured [2]. The present study did not identify more rapid SCM muscle response with greater impact velocity. This finding is not in agreement with those of Kumar et al. [6, 7, 9], and Brault et al. [2]. Despite the relatively large sample size in the present study, there were large variances of the timing variables resulting in low statistical power (≈0.1). The Kumar et al. [9] (seven subjects) [6, 7] (ten subjects) studies were based on a smaller sample size and although the variance appears smaller than the present study, they did not report statistical power. The Brault et al. [2] study was based on a large sample size (42 subjects) with lower timing variable variance than the present study.
Brault et al. [2] reported that female subjects activated their cervical muscles at an average of 5% earlier than male subjects. Due to large variances and low statistical power, the present study does not allow conclusions regarding gender differences in SCM EMG onset and peak times. The present study did identify gender differences in magnitude of peak EMG with female subjects demonstrating a greater EMG response. This finding is consistent with higher prevalence of whiplash symptoms reported in females in low impact rear-end collisions [17]. Interpretation of results from previously published studies without differentiation of gender should be viewed with caution.
Awareness of an event refers to anticipation of such event. Temporal, event and amplitude awareness are the three dimensions in such anticipation. The first refers to whether the subject knows about the exact timing in which an event will occur, the second describes whether the subject knows an event will occur, and the third refers to the subject’s knowledge of the magnitude of the imminent event [21–23]. It has been reported that awareness of an impact influences the muscle response in a simulated whiplash event [9, 21]. Kumar et al. [6, 7, 9, 10], reported SCM EMG magnitude reduced with expectancy of impact. Likewise, Kumar et al. [9] reported reduced EMG latency in expected impacts. Magnusson et al. [11] did not report significant differences regarding temporal awareness in simulated impacts at 0.5 g. Siegmund et al. [21] reported that lack of event awareness significantly increased onset and peak latencies of the cervical muscles.
The current findings, as well as those of Siegmund et al. [21] and Magnusson et al. [11], did not report significant differences in muscle response regarding temporal awareness. The unexpected events in the present study are similar to the “unalerted” group in the Siegmund et al. [21] study. Subjects were aware that they would eventually sustain impact and; therefore, had event awareness. The expected impact events in the present study are similar to the “alerted” group in the Siegmund et al. [21] study. It is difficult to simulate actual rear-end collision conditions in a lab setting; participants are aware in advance of the magnitude of the impacts and the risks of experiencing symptoms due to impacts. Therefore, the participants of the present study might not have perceived the simulated impact sufficiently noxious to get injured. This might explain the lack of significant low muscle response when subjects were exposed to the expected impacts, and subsequently the discrepancy of results between the current study and those who reported different muscle responses with expected impacts.
Previous studies [2, 4, 9, 16] evaluated the temporal relationship between the muscle activity and kinematic events of the head movement. In the current study, the temporal relationship between the muscle activity and the acceleration of the head was different depending whether the EMG activity was compared with the linear or angular acceleration. If the analysis is made with the linear acceleration, it might be concluded that the muscle activates too late to have an influence on the head kinematics. On the other hand, the same comparison made with the angular acceleration is less definitive, and it supports the concept that the muscle may play a role in the head kinematics upon impact. Finally, the onset of the SCM EMG activity was always prior to the maximum rearward angular peak displacement time. This finding is in agreement with those of Ono et al. [16]. The initial linear (horizontal) acceleration of the head would occur as the entire body is pushed back into the cushion of the chair. Angular head acceleration represents the rotational movement of the head. SCM contraction would serve to restrain rotational movement of the head. The results of the present study suggest that the SCM is activated by bodily movement and occurs early enough to serve as a protective function for the cervical spine during angular acceleration of the head.
The extent to which the central and peripheral mechanisms play a role in whiplash injury is still open to debate. Siegmund et al. [19, 20, 23] suggested that whiplash injuries resemble reflex muscle responses. They have reported habituation of the cervical muscle response upon multiple whiplash-like perturbations [22]. In addition, they stated that the early onset time of the muscle response reported by simulated impact studies suggests a muscle reflex response [20]. The numerical values for event timing in the present study cannot be compared directly with previous studies because the current study used chair movement as a base reference. Siegmund et al. [23] and Brault et al. [2] used the bumper contact between the two cars as time zero. Kumar et al. in all his studies [6–10] used the firing of the solenoid as time zero; therefore, the actual movement of the chair varies for each impact. It remains unknown as to what extent the finding of reduced onset of head movement and muscle activity with increased impact magnitude is due to the significantly reduced onset of chair movement.
Although the time interval between onset of chair movement and SCM EMG onset was larger than reported by Brault et al. [2] and more similar to Szabo and Welcher [25], the sequence of horizontal head movement, SCM EMG and angular head movement still supports the muscle reflex hypothesis. Furthermore, the fact that the magnitude of muscle response increases with an increased impact magnitude strongly supports a role of a peripheral mechanism.
The mechanism of impact delivery and physical properties of the car seat may influence the timing of body and in particular head movement. Kumar et al. [6–10] used a molded plastic chair rather than an automobile seat. Siegmund et al. [21] suggest that the use of a molded plastic chair may not allow direct comparison between these studies and those that use a car seat.
The muscle reaction generated in a simulated whiplash event affects the whiplash biomechanics and could possibly offer protection to the cervical spine. However, the magnitude of normalized SCM may exceed the maximum voluntary contraction potentially resulting in failure within the muscle [18]. Brault et al. [2] reported a lengthening by 3–6% of the SCM upon simulated impacts. This eccentric muscle contraction mechanism is consistent with the delayed onset of muscle symptoms observed in whiplash injuries [14, 15]. This mechanism also supports the generalized muscular hyperalgesia present in subjects who have experienced whiplash trauma; this generalized central hyperexcitability might be caused through tissue injury [5].
This study presents some limitations. It is possible that the mechanism by which the accelerometer board was attached to the subject created an additional sensory input altering the muscle response. The low magnitude of impacts in this study (linear chair acceleration of 4.5 and 10.1 m/s2) might have not been sufficient to create an actual simulation of a whiplash event; and therefore, to be perceived sufficiently noxious by the participants in the expected condition. The results of the current study should not be extrapolated to older people since the composition of the muscle tissue is different, and therefore the muscle response might be different.
Conclusions
Based on the findings of the current study, the following can be concluded: There is increased SCM EMG activity with an increased impact magnitude. Gender differences for SCM EMG magnitude were identified. Conclusions regarding gender differences related to timing of EMG response could not be drawn due to large variances of the variables and low power associated with those findings. Temporal and amplitude awareness of a simulated impact do not produce differences in the magnitude of muscle response. The temporal relationship between the SCM and angular head acceleration is different from the temporal relationship between the SCM and the linear head acceleration.
Acknowledgements
The authors would like to thank Dr. S Kumar for use of his lab, equipment and technical support. This research was supported by the University of Alberta, Fund for Dentistry Grant# 2002-02 and the McIntyre Memorial Research Fund. The Human Research Ethics Board at the University of Albert approved the protocol for this study
Footnotes
The Human Research Ethics Board at the University of Alberta approved the protocol for this study
References
- 1.Basmajian J, de Luca C (1985) Description and analysis of the EMG signal. In: Muscles alive: their functions revealed by electromyography, 5th edn. Williams and Wilkins, Baltimore, pp 65–100
- 2.Brault JR, Siegmund GP, Wheeler JB. Cervical muscle response during whiplash: evidence of a lengthening muscle contraction. Clin Biomech (Bristol, Avon) 2000;15:426–435. doi: 10.1016/s0268-0033(99)00097-2. [DOI] [PubMed] [Google Scholar]
- 3.Castro WH, Schilgen M, Meyer S, Weber M, Peuker C, Wortler K. Do “whiplash injuries” occur in low-speed rear impacts? Eur Spine J. 1997;6:366–375. doi: 10.1007/BF01834062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaneoka K, Ono K, Inami S, Hayashi K. Motion analysis of cervical vertebrae during whiplash loading discussion 770. Spine. 1999;24:763–679. doi: 10.1097/00007632-199904150-00006. [DOI] [PubMed] [Google Scholar]
- 5.Koelbaek Johansen M, Graven-Nielsen T, Olesen A, Arendt-Nielsen L. Generalised muscular hyperalgesia in chronic whiplash syndrome. Pain. 1999;83:229–234. doi: 10.1016/S0304-3959(99)00106-2. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S, Ferrari R, Narayan Y. Cervical muscle response to right posterolateral impacts. Clin Biomech (Bristol, Avon) 2004;19:543–50. doi: 10.1016/j.clinbiomech.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Kumar S, Ferrari R, Narayan Y. Cervical muscle response to whiplash-type right anterolateral impacts. Eur Spine J. 2004;13:398–407. doi: 10.1007/s00586-004-0700-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar S, Ferrari R, Narayan Y. Cervical muscle response to posterolateral impacts–effect of head rotation. Clin Biomech (Bristol, Avon) 2004;19:899–905. doi: 10.1016/j.clinbiomech.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Kumar S, Narayan Y, Amell T. An electromyographic study of low-velocity rear-end impacts. Spine. 2002;27:1044–1055. doi: 10.1097/00007632-200205150-00009. [DOI] [PubMed] [Google Scholar]
- 10.Kumar S, Narayan Y, Amell T. Analysis of low velocity frontal impacts. Clin Biomech (Bristol, Avon) 2003;18:694–703. doi: 10.1016/s0268-0033(03)00137-2. [DOI] [PubMed] [Google Scholar]
- 11.Magnusson ML, Pope MH, Hasselquist L, Bolte KM, Ross M, Goel VK, et al. Cervical electromyographic activity during low-speed rear impact. Eur Spine J. 1999;8:118–125. doi: 10.1007/s005860050140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malleson A. Chronic whiplash syndrome Psychosocial epidemic. Can Fam Physician. 1994;40:1906–1909. [PMC free article] [PubMed] [Google Scholar]
- 13.McConnell W, Howard RP, Poppel JV, Krause R, Guzman HM, Bomar JB et al (1995) Paper 952724 Human head and neck kinematics after low velocity rear-end impacts-understanding “whiplash”. In: 39th Stapp car crash conference. Society Automotive Engineers, Inc, Warrendale, pp 3106–3129
- 14.McCully K, Faulkner J. Injury to skeletal muscle fibers of mice following lengthening contractions. J Appl Physiol. 1985;59:119–126. doi: 10.1152/jappl.1985.59.1.119. [DOI] [PubMed] [Google Scholar]
- 15.Newham DJ. The consequences of eccentric contractions and their relationship to delayed onset muscle pain. Eur J Appl Physiol. 1988;57:353–359. doi: 10.1007/BF00635995. [DOI] [PubMed] [Google Scholar]
- 16.Ono K, Kaneoka K, Wittek A, Kazjer J (1997) Paper 973340 Cervical injury mechanism based on the analysis of human cervical vertebral motion and head-neck-torso kinematics during low speed rear impacts. In: 41st Stapp car crash conference. Society of Automotive Engineers, Inc., Warrendale, pp 339–356
- 17.Otremski I, Marsh JL, Wilde BR, McLardy Smith PD, Newman RJ. Soft tissue cervical spinal injuries in motor vehicle accidents. Injury. 1989;20:349–351. doi: 10.1016/0020-1383(89)90011-9. [DOI] [PubMed] [Google Scholar]
- 18.Pope M, Magnusson M, Aleksiev A, Hasselquist L, Spratt K, Szpalski M, Goel VK, Panagiotacopulos N, et al. Electromyographic changes under whiplash loading. In: Yoganadan N, et al., editors. Frontiers in head and neck trauma. Amsterdam: IOS Press; 1998. pp. 338–343. [Google Scholar]
- 19.Siegmund G, Brault J, Chimich D. Do cervical muscles play a role in whiplash injury? J Whiplash Relat Disord. 2002;1:23–40. doi: 10.1300/J180v01n01_03. [DOI] [Google Scholar]
- 20.Siegmund GP, Brault JR. Role of cervical muscles during whiplash. In: Yoganadan N, Pintar FA, editors. Frontiers in whiplash trauma: clinical and biomechanical. Washington: IOS Press; 2000. pp. 295–320. [Google Scholar]
- 21.Siegmund GP, Sanderson DJ, Myers BS, Inglis JT. Awareness affects the response of human subjects exposed to a single whiplash-like perturbation. Spine. 2003;28:671–679. doi: 10.1097/00007632-200304010-00010. [DOI] [PubMed] [Google Scholar]
- 22.Siegmund GP, Sanderson DJ, Myers BS, Inglis T. Rapid neck muscle adaptation alters the head kinematics of aware and unaware subjects undergoing multiple whiplash-like perturbations. J Biomech. 2003;36:473–482. doi: 10.1016/S0021-9290(02)00458-X. [DOI] [PubMed] [Google Scholar]
- 23.Siegmund G (2001) The reflex response of human neck muscles to whiplash-like perturbations [Doctoral Thesis]. University of British, Columbia, Vancouver, British Columbia, Canada
- 24.Spitzer Spine. 1995;20:1S. [PubMed] [Google Scholar]
- 25.Szabo T, Welcher J (1996) Paper 962432 Human subject kinematics and electromyographic activity during low speed rear impacts. In: 40th Stapp car crash conference. Society of Automotive Engineers, Inc, Warrendale, pp 295–314