Abstract
Human cytomegalovirus carries a mitochondria-localized inhibitor of apoptosis (vMIA) that is conserved in primate cytomegaloviruses. We find that inactivating mutations within UL37x1, which encodes vMIA, do not substantially affect replication in TownevarATCC (Towne-BAC), a virus that carries a functional copy of the betaherpesvirus-conserved viral inhibitor of caspase 8 activation, the UL36 gene product. In Towne-BAC infection, vMIA reduces susceptibility of infected cells to intrinsic death induced by proteasome inhibition. vMIA is sufficient to confer resistance to proteasome inhibition when expressed independent of viral infection. Murine cytomegalovirus m38.5, whose position in the viral genome is analogous to UL37x1, exhibits mitochondrial association and functions in much the same manner as vMIA in inhibiting intrinsic cell death. This work suggests a common role for vMIA in rodent and primate cytomegaloviruses, modulating the threshold of virus-infected cells to intrinsic cell death.
Apoptosis is a cell death pathway important for removal of cells during development, in tissue homeostasis, and following infection by pathogens (50). Many viruses, including human cytomegalovirus (HCMV), encode proteins that counteract this cell-intrinsic response (1, 10, 23, 24, 41, 57, 58, 60). The initiation and execution of apoptosis is dependent upon caspases and, in many cell types, mitochondrial events (27). Other cellular proteases play regulatory roles in this process (22, 31, 34, 61). Caspases are triggered in a hierarchical order: initiator caspases 8 and 9 mediate the cleavage-activation of effector caspase 3, either directly or following release of mitochondrial factors that promote this cascade. Cellular proapoptotic functions that are activated by caspases ultimately lead to characteristic morphological changes and cell fragmentation (27, 32). Inhibitors of regulatory proteases, including calpains and proteasomal proteases that control a wide range of processes beyond cell death, generally increase stress and susceptibility to intrinsic cell death.
HCMV, the prototypic betaherpesvirus, remains an important cause of congenital disease as well as an opportunistic pathogen causing acute and chronic disease complications in immunocompromised hosts (45). The HCMV genome has been estimated to encode at least 165 genes (17, 43), although laboratory strains of the virus have accumulated a range of mutations that disrupt as many as 20 genes (13, 53). HCMV encodes two potent cell death suppressors whose mechanisms of action have been elucidated: viral inhibitor of caspase 8 activation (vICA), encoded by UL36, and viral mitochondria-localized inhibitor of apoptosis (vMIA), encoded by UL37x1 (25, 53). vICA binds to procaspase 8 and prevents activation of this initiator caspase in a manner analogous to FLICE inhibitory proteins, but vICA lacks death domains and sequence homology to this class of inhibitor. vICA has been associated with resistance to extrinsic inducers, such as activation of Fas, rather than to intrinsic inducers of cell death (53). Commonly used laboratory strains of virus all retain functional vMIA but vary in their retention of vICA. TownevarATCC encodes a functional vICA, but the AD169varATCC gene product carries an inactivating mutation (Cys131). Indeed, the vMIA protein sequence is highly conserved in all characterized clinical as well as in laboratory strains of HCMV, suggesting this gene product plays some role in viral replication (26). This expectation has been substantiated by three investigations (12, 48, 62) where AD169varATCC vMIA mutants failed to replicate or replicated very poorly.
vMIA, the product of HCMV UL37x1, prevents caspase activation by sequestering oligomerized Bax at mitochondria and preventing mitochondrial release of cytochrome c; activities that are functionally similar to but mechanistically distinct from analogous cellular antiapoptotic proteins, Bcl-2 and Bcl-xL, as well as other viral antiapoptotic proteins (3, 25, 46). vMIA does not share sequence similarity with these Bcl-2 family members but blocks similar steps of mitochondria-dependent amplification of caspase 8 signals and activation of procaspase 9. Primate and rodent CMVs encode homologs of vICA (37, 39). Primate CMVs also encode functional vMIA, but rodent CMVs lack a sequence homolog (37). A murine CMV (MCMV)-encoded protein that has activity analogous to vMIA may be predicted based on the behavior of the proapoptotic Bcl-2 family members Bax and Bak during infection (2). An open reading frame (ORF), denoted m38.5, maps to an analogous genomic position in MCMV and rat CMV but lacks substantial sequence identity with the UL37x1 ORF (11, 37). When tested, m38.5 failed to protect from Fas-mediated apoptosis, where vMIA has been a potent inhibitor (37).
AD169varATCC UL37x1 mutants exhibit severe growth impairment, 1,000- to 10,000-fold lower than parental virus (48); this defect is overcome by inhibiting caspases with zVAD during infection. These and other studies (12, 18, 62) have reinforced the expectation that vMIA provides an indispensable activity in viral replication similar to the role of adenovirus E1B functions in prevention of intrinsic apoptosis induced by viral regulatory proteins or stress that accompanies replication (16).
Although caspases play the central role in the apoptosis pathway, a number of additional proteases may augment cell susceptibility to induction of this and other death pathways. Calpains, in particular, are activated during HCMV infection and underlie modulation of the cell cycle regulator p21cip1 (15). Although constitutive proteasome constituents remain unaltered or increase during infection, immunoproteasome subunits LMP2, MECL1, and LMP7 are specifically downmodulated (33). Proteasome activity appears to be required for replication (47), possibly in conjunction with regulation of apoptosis via such targets as p53. Proteasomal degradation of vMIA is regulated by interaction with growth arrest and DNA damage response protein 45 (G. B. Smith and E. S. Mocarski, submitted for publication). These observations suggest that an investigation of the roles of calpain and proteasome proteases during infection may yield insights into processes that determine the apoptotic threshold of cells.
We initiated a study of protease inhibitors to determine how replication of and cell death induction by a vMIA mutant were affected in comparison to parental, vMIA-expressing virus. The vMIA mutant we employed here exhibited nearly wild-type growth characteristics under normal culture conditions. Our studies revealed that vMIA plays an important role in preventing caspase-dependent death during inhibition of proteasome activity. Thus, vMIA appears to control the apoptotic threshold of infected cells rather than to provide a direct role essential for the process of viral replication. These differences imply that vICA may have a broader role in cell death protection than previously appreciated. Our results further suggest that MCMV m38.5 is a vMIA, based on intracellular localization and mode of action.
MATERIALS AND METHODS
Cells, viruses, and BACmid clones.
Human fibroblast (HF), 293T (a gift of Jerry Crabtree), Phoenix (a gift of Gary Nolan), and HeLa (a gift of Karla Kirkegaard) cells were cultured as previously described (38), except medium was supplemented with 10% fetal clone III (HyClone, Logan, UT). Parental and mutant HCMVs were generated from Towne-BAC (36) or vMIA mutant ΔUL37x1 (18), respectively, by transfection into HFs or vMIA-HFs, as indicated. HCMV TownevarATCC obtained from George Kemble (MedImmune Vaccines, Mountain View, CA), AD169varATCC (lot 22) from the American Type Culture Collection (ATCC), and MCMV K181+ (55) were employed for some experiments.
Transfections.
Calcium phosphate transfections followed published protocols (49). The pp71 expression plasmid used in BACmid transfections was generated by cloning hemagglutinin epitope-tagged UL82 ORF into the LNCX (40)-derived retroviral vector LzeC, encoding zeocin resistance in place of the neomycin resistance gene. The retroviral construct LNCX-GFP, derived from LNCX by addition of enhanced green fluorescent protein (EGFP), was included in some experiments to identify transfected cells.
Generation of vMIA-HF, vMIAmut-HF, and m38.5-HF.
DNA fragments with the ORF for vMIA or vMIAmut, obtained from the plasmids pUL37x1 (25) and Δ115-130 (26), respectively, after digestion with HindIII and HpaI (pUL37x1) or HindIII and XhoI (Δ115-130), were ligated to HindIII-HpaI- or HindIII-XhoI-digested LNCX-3myc (pON2711) derived by insertion of three copies of the myc epitope into the LNCX retrovirus vector (40) to generate pON2708 and pON2709. PCR product including the m38.5 ORF was generated with primers 5′-GTTAACTCGAGCgaatgtgtaatctccatcttc-3′ and 5′-GGAAGATCTGCCATGGagagtgtgcgccgaccc-3′, including sequence corresponding to nucleotides (nt) 52347 to 52367 and 51780 to 51800 of MCMV (GenBank accession U69299) (lowercase portions of sequences), and K181+ viral DNA and digested with BglII and XhoI, then ligated to LNCX-3myc digested with the same enzymes to generate pON2710. Retroviral particles recovered from Phoenix cells transfected with LNCX-m38.5 or from 293T cells cotransfected with LNCX-vMIA or LNCX-vMIAmut together with plasmids JK3, LTRVSVG, and CMVtat (5) were applied to HFs, and transduced cells were selected using 400 μg/ml Geneticin (Gibco/Invitrogen, Carlsbad, CA).
Viral yield, plating efficiency, growth following transfection, cell death during infection, and impact of zVAD.
For growth curves, vMIAmut-HFs or vMIA-HFs in triplicate cultures were infected by ΔUL37x1 or parental Towne-BAC at a multiplicity of infection (MOI) of 0.002 and harvested on days 1, 3, 5, 7, 10, and 14. Total virus included cell supernatant and sonicated infected cells (Sonicator 3000; Misomix, Inc., Farmingdale, NY), and titrations employed 0.16% pooled human gamma globulin (Baxter Healthcare, Deerfield, IL). Yield per plaque was determined from cell-associated virus recovered following growth in the presence of 0.16% pooled human gamma globulin. Plating efficiencies are the mean plaque numbers of three viral isolates, each titer determined in duplicate on HFs, vMIA-HFs, or vMIAmut-HFs, with the standard deviation determined between independent isolates. Viral growth following transfection of BACmid DNA was assessed by live cell microscopy on days 3, 4, 7, and 11, with the numbers of single GFP-positive (GFP+) cells and GFP-positive foci (>1 GFP+ cell) graphed. For assessment of virus-induced cell death, cultures were stained with ethidium homodimer 1 (Molecular Probes, Eugene, OR) following the manufacturer's recommendations for viability determination and then fixed with 3.7% formaldehyde (Sigma, St. Louis, MO). GFP fluorescence, cell fragmentation, and ethidium homodimer 1 staining were evaluated microscopically. Nonfragmented cells excluded ethidium homodimer. Cell fragmentation frequency was calculated using the number of fragmented cells divided by the total number of GFP+ cells. For the impact of zVAD on cell fragmentation or viral yield, inhibitor was added at 2 h postinfection (hpi) and replenished throughout infection by replacement of medium every 2 days. Morphology was assessed as described above on day 3 or 7, as indicated, and viral yield was determined from total virus recovered on day 7.
Viral genomic structure analysis.
DNA obtained from supernatant virus was amplified by PCR with primers 5′-CAGCAATAGCGGTAAAGTC-3′ and 5′-CGACGTGAGACCCACACGC-3′ corresponding to nt 51056 to 51075 and 51656 to 51638, respectively, of Towne-BAC sequence (GenBank accession AY315197) and sequenced using the same primers (Stanford PAN facility). Subcloning AD169 nt 51805 to 54014 (GenBank accession 17403) from cosmid 1049 (20) produced pON2707, which was used to generate two probes: the UL38-39 region probe was the virus-specific HindIII-EcoRI fragment, while the UL37x1-specific probe was PCR product from primers 5′-ATGTCTCCAGTCTACGTGA-3′ and 5′-TGAGACTGCTGGGGGCCG-3′ corresponding to nt 51614 to 51596 and 51130 to 51146 of Towne-BAC (GenBank accession AY315197). Both were labeled with [32P]dCTP (Amersham) by random priming and Klenow fragment DNA polymerase (Roche Applied Science, Indianapolis, IN) fill-in. Restriction fragment length polymorphism analysis was performed on viral DNA associated with virions in the supernatant, purified as described elsewhere (54), digested with EcoRI, HindIII, BamHI, EcoRV, KpnI, XbaI, or PstI, and visualized by agarose gel electrophoresis.
Antibodies, fluorescence reagents, immunodetection, and imaging.
Monoclonal mouse antibodies to Fas (7C11; Coulter), to CMV IE1 and IE2 proteins (MAb810; Chemicon, Temecula, CA), to a c-myc epitope (9E10; Santa Cruz Biotechnology, Santa Cruz, CA), and to mtHSP70 (JG1; from Susan Pierce, Northwestern University) were used. Antibody 9E10 was directly conjugated to fluorescein for colocalization studies; otherwise, secondary antibodies were fluorescein- or Texas Red-conjugated horse anti-mouse immunoglobulin G (IgG) or peroxidase-conjugated horse anti-mouse IgG (Vector, Burlingame, CA). Immunofluorescence assays (IFA), immunoblotting, and imaging were as previously described (38).
Cell death induction assays.
Protease inhibitors lactacystin, N-acetyl-Leu-Leu-Met (ALLM), N-acetyl-Leu-Leu-Nle-CHO (ALLN), Z-Leu-Nle-CHO (calpeptin), cathepsin L inhibitor I (Z-FF-FMK), Z-Leu-Leu-Leu-CHO (MG132), or Z-Val-Ala-Asp(OMe)-CH2F (zVAD) (all from Calbiochem, La Jolla, CA) were either purchased as a dimethyl sulfoxide (DMSO) stock (MG132 only) or resuspended in DMSO (all other inhibitors) (Sigma, St. Louis, MO). zVAD was added at the time of exposure to cell death inducers or at the times postinfection indicated.
For protease inhibitor-induced death, HFs plated at 50% confluence for 48 h were infected at MOIs of 0.001 to 0.01 for 48 h before addition of inhibitors at the appropriate concentration for 20 h. Cultures were stained with ethidium homodimer 1 (Molecular Probes, Eugene, Oregon) to determine viability and then fixed with 3.7% formaldehyde (Sigma, St. Louis, Mo.). Surviving cells were GFP+, nonfragmented, and excluded ethidium homodimer 1.
For Fas protection assays, HFs plated at 70% confluence for 24 h were infected for 24 h, and then medium containing anti-Fas antibody 7C11 (Coulter, Fullerton, CA) at 0.2 μg/ml and cycloheximide (CH; Sigma, St. Louis, MO) at 10 μg/ml was added for 20 h as described previously (25, 53). Survival was determined from cells fixed with 3.7% formaldehyde, reacted with MAb 810 and fluorescein-conjugated horse anti-mouse IgG, stained with Hoechst 33342, and processed as for immunofluorescence analysis.
For uninfected cells, HFs plated at 70% confluence on coverslips for 24 h were exposed to protease inhibitor for 20 or 44 h as indicated. Cultures were stained with ethidium homodimer 1, fixed with 3.7% formaldehyde, stained with Hoechst 33342, and processed as for immunofluorescence analysis. Surviving cells remained attached to the coverslip, excluded ethidium homodimer 1, and appeared nonapoptotic based on nuclear size and Hoechst stain (29, 52, 56). For transient-expression assays, HeLa cells were transfected with a GFP-expressing plasmid and a plasmid encoding m38.5, vMIA, or vMIAmut or the empty vector. At 24 h posttransfection, Fas-mediated apoptosis was induced as described above or cultures were exposed to 10 μM MG132 for 44 h before determining survival by counting GFP+ cells. Plasmid DNA quantities were varied over the range of 0.5 μg to 8 μg per 105 cells transfected for Fas protection assays.
RESULTS
Growth of Towne-BAC-derived ΔUL37x1 virus.
Although reported in the category “no growth,” indicating an essential gene (18), the BACmid clone ΔUL37x1 generated virus whether transfected into HFs or vMIA-expressing HFs. Initially, this surprising result led us to investigate the growth characteristics and structure of virus recovered from vMIA-expressing cells (vMIA-HFs) (Fig. 1). The pattern of mutant virus replication in a multiple round assay at an MOI of 0.002 was slightly lower on HFs or vMIAmut-HFs (Fig. 1A and B) than on vMIA-HFs (Fig. 1C). This difference was well outside of experimental variability (most standard deviations are within the symbols in Fig. 1) and suggested a modest level of complementation of mutant virus in vMIA-HFs with surprisingly close levels of replication without complementation. Mutant virus yields were identical to Towne-BAC on days 3 and 5 pi regardless of vMIA expression, suggesting that this viral protein provided little benefit during the first round of infection. Over the course of a 14-day assay, five- to eightfold-lower levels of mutant were produced on HFs or vMIAmut-HFs, respectively, compared to vMIA-HFs, suggesting a modest impact of the cell death suppressor on yields following multiple rounds of replication. As expected, multiple independent isolates of ΔUL37x1 showed patterns similar to the example in Fig. 1 (Fig. 2 and data not shown). Furthermore, as previously reported (36), the regenerated Towne-BAC virus used here replicated as well as noncloned TownevarATCC virus (data not shown). These results suggested that growth properties of Towne-derived UL37x1 mutants were very different than those described for AD169-derived mutants (12, 48, 62).
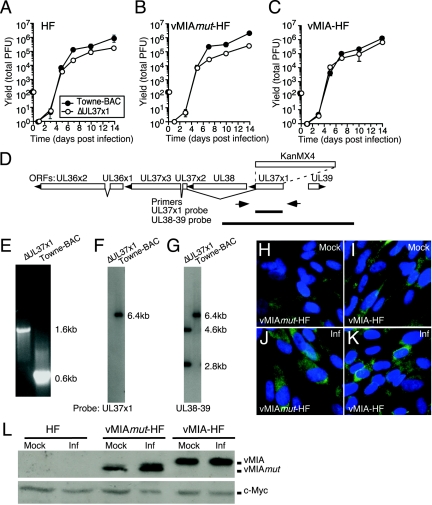
FIG. 1.
ΔUL37x1 virus yield and complementation. (A to C) Mean viral yields from triplicate cultures of HFs (A), vMIAmut-HFs (B), or vMIA-HFs (C) infected at an MOI of 0.002 by ΔUL37x1 or Towne-BAC, graphed with standard deviations (hidden behind the symbols in most cases). (D) Map of the UL36-39 region showing positions of oligonucleotide primers (arrows) and probes (thick lines) used in characterizing the ΔUL37x1 virus genome structure. The ORF map shows splicing patterns, transcript orientation (arrowheads), and the position of the KanMX4 cassette replacement of UL37x1 (dashed lines). (E) UL37x1 region PCR products from ΔUL37x1 or Towne-BAC viral DNA, as indicated, resolved by electrophoresis in agarose gels and visualized by ethidium bromide fluorescence. (F and G) Autoradiographs of EcoRI-digested ΔUL37x1 or Towne-BAC viral DNAs hybridized with UL37x1 probe (F) or UL38-39 probe, consistent with the expected additional EcoRI site due to the KanMX4 cassette in the mutant (G). (H to K) IFA of gene expression in vMIAmut-HF (H and J) or vMIA-HF (I and K) without infection (mock; H to I) or following Towne-BAC infection at an MOI of 3 for 24 h (Inf; J to K). Shown is a composite of vMIAmut and vMIA proteins (green) and nuclei (blue). (L) Immunoblot analysis of vMIAmut and vMIA in lysates of HFs, vMIAmut-HFs, and vMIA-HFs following infection (Inf) by Towne-BAC at an MOI of 3 for 24 h, as indicated. Shown are vMIAmut and vMIA proteins detected with anti-Myc antibody (upper panel) along with endogenous c-Myc protein (lower panel) as a loading control.
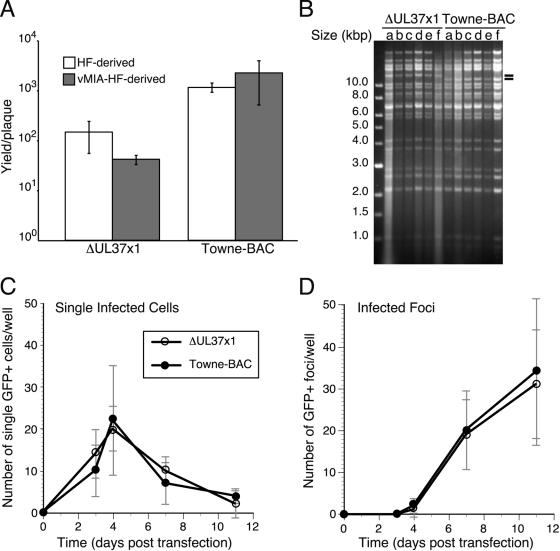
FIG. 2.
Growth and genomic structure properties of ΔUL37x1 viruses derived on complementing vMIA-HF and noncomplementing HF cells. (A) Mean viral yields with standard deviations from HF cultures infected for 14 days with three transfection-independent viruses derived on HF or vMIA-HF at an MOI of 0.001. (B) Restriction fragment length polymorphism analysis of EcoRV-digested ΔUL37x1 or Towne-BAC virion DNA recovered from independent transfections of HFs (isolates a, b, and c) and vMIA-HFs (isolates d, e, and f), with two dashes indicating the expected polymorphism due to insertion of the KanMX4 cassette. (C and D) Infected cell and focus formation by ΔUL37x1 and Towne-BAC following transfection of equivalent amounts of BAC DNA into noncomplementing HFs. The mean numbers of single GFP-positive (GFP+) cells (C) and GFP-positive foci (D), monitored by live cell microscopy, are graphed with standard deviations from four independent transfections of ΔUL37x1 or Towne-BAC.
The genomic structure of viral DNA was investigated to eliminate the possibility of contamination. First, the mutated UL37x1 locus was amplified from a ΔUL37x1 virus stock by PCR using CMV-specific primers (Fig. 1D and E). The electrophoretic mobilities of amplified products from the deletion mutant (1.6 kbp) and parental virus (0.6 kbp) differed as expected (14) due to replacement of UL37x1 ORF with the selectable marker KanMX4, which is larger in size than the UL37x1 ORF (18) (Fig. 1D). Sequence analysis of these PCR products was used to confirm precise KanMX4 replacement of the UL37x1 ORF (data not shown). Second, probes for the UL37x1 gene as well as for the UL38-39 region were used on DNA blots to be certain that ΔUL37x1 virus lacked any copies of this gene, as shown in Fig. 1D, F, and G. A UL37x1-specific probe failed to detect homology in mutant virus DNA, while it did hybridize with the expected 6.4-kbp EcoRI fragment of parental Towne-BAC (Fig. 1F). As expected, a UL38-39 probe also hybridized to a single 6.4-kbp fragment in Towne-BAC virus DNA and to two EcoRI fragments in ΔUL37x1 virus DNA, 2.8 and 4.6 kbp, with a combined size appropriate for replacement of the UL37x1 ORF with the larger KanMX4 cassette that contains an EcoRI site (Fig. 1G). These results confirmed that both viral DNAs had the correct structure and that ΔUL37x1 completely lacked any UL37x1 sequences at the natural site or elsewhere in the viral genome.
We also used IFA and immunoblotting to confirm that protein localization and quantities of vMIA and vMIAmut were similar in the retrovirus vector-transduced cells used for complementation studies (Fig. 1H to L). IFA patterns showed cells expressed these antigens in a mitochondrial pattern, as expected (25, 26) (Fig. 1H to K). Both IFA and immunoblotting detected similar amounts of vMIA and vMIAmut in infected cells, although uninfected vMIAmut-HFs had lower amounts than uninfected vMIA-HFs (Fig. 1H to L). Thus, the reduced ΔUL37x1 virus replication in vMIAmut-HFs suggested that the amino acids deleted from vMIA, 115 to 130, were required to maximize infection.
vMIA impacts viral yield after multiple rounds of replication.
Additional virus isolates of ΔUL37x1 and Towne-BAC were derived from independent transfections, three each on HFs and two each on vMIA-HFs, in order to determine whether growth properties might be attributed to the presence of vMIA in virions produced on complementing cells. All viral stocks, six mutants and six parents, showed the expected plating efficiencies on vMIA-HFs, vMIAmut-HFs, or HFs, without any indication that the presence of vMIA in the host cell contributed to behavior (Table 1). The viral yield per plaque was determined under conditions that prevented secondary spread of virus (Fig. 2A), reinforcing the data in Fig. 1 that show that viral replication was only slightly reduced in the absence of vMIA following multiple rounds of replication. We confirmed that the genomic structure was correct for all of these isolates by using assays described above (Fig. 1E to G) as well as by restriction fragment length polymorphism analysis (Fig. 2B and data not shown). In total, digestion by seven different restriction enzymes failed to detect any genomic alterations other than those that were intentionally engineered (18).
TABLE 1.
Plating efficiencies of ΔUL37x1 and Towne-BAC viruses on vMIA-HF and vMIAmut-HF
| Virus | Stock preparation | Titer | Plating efficiencya |
|---|---|---|---|
| ΔUL37x1 | HF | vMIA-HF | 1.1 ± 0.6 |
| ΔUL37x1 | HF | vMIAmut-HF | 1.3 ± 0.6 |
| ΔUL37x1 | vMIA-HF | vMIA-HF | 1.4 ± 0.4 |
| ΔUL37x1 | vMIA-HF | vMIAmut-HF | 1.5 ± 0.7 |
| Towne-BAC | HF | vMIA-HF | 0.9 ± 0.4 |
| Towne-BAC | HF | vMIAmut-HF | 1.4 ± 0.7 |
| Towne-BAC | vMIA-HF | vMIA-HF | 1.6 ± 0.7 |
| Towne-BAC | vMIA-HF | vMIAmut-HF | 1.6 ± 0.6 |
Plating efficiency = (number of plaques on vMIA-HF or vMIAmut-HF cells)/(number of plaques on HFs).
To follow replication and spread as precisely as possible, the relative infectivity of BACmid DNA and rate of spread of infection from the initial infected cell was followed over time in transfected cultures by monitoring GFP expression in living cells. Single cells and infected cell foci developed in parallel following transfection with either mutant or parental BACmid (Fig. 2C and D). The two BACmids exhibited equivalent patterns, with a peak of single GFP-positive cells on day 4 (Fig. 2C) and parallel focus formation starting at day 4 and continuing through days 7 and 11 (Fig. 2D). Thus, similar numbers of single GFP-positive cells progressed to form infected cell foci regardless of the potential to encode vMIA (Fig. 2C and D). Although these experiments were all performed with pp71 enhancement (4), parallel experiments in the absence of pp71 yielded comparable patterns but with overall lower numbers of positive cells and foci for both BACmids. These results demonstrated vMIA did not impact DNA infectivity or cell-to-cell spread of virus and suggested that the slight defect observed in low-MOI ΔUL37x1 infection (Fig. 1A to C) was due to events unique to multiple rounds of infection.
vMIA suppresses caspase-3-independent cell death of virus-infected cells.
Infection with AD169varATCC UL37x1 mutants sensitized infected cells to apoptosis, prematurely terminating replication (48). This defect was rescued by the broad caspase inhibitor zVAD. In contrast, Towne-BAC-derived ΔUL37x1 replicated efficiently without addition of caspase inhibitor. Despite having replicated efficiently, the plaques that formed contained more fragmented cells than did Towne-BAC plaques (Fig. 3A and B and data not shown), and cultures infected with the mutant virus showed more cell fragmentation as early as 72 hpi that increased with time compared to parental virus (Fig. 4A and B and data not shown), a pattern indistinguishable from apoptosis (32). We counted fragmented GFP-positive cells under conditions (72 hpi, using MOIs < 0.001) where detachment from the HF monolayer was minimized and single particle conditions that separated infected cells were ensured. Most fragmented cells appeared apoptotic based on viability dye exclusion, while a few, with more extensive changes, appeared necrotic. The number of such dead or dying cells reached 10% of total cells by 72 hpi (Fig. 3C). In contrast, only about 1% of vMIA-HFs appeared dead or dying at this time pi, about 10-fold lower than infected HFs or vMIAmut-HFs (Fig. 3C). Towne-BAC infection did not induce any fragmentation by 72 hpi on any of these cells (data not shown). To assess any antiapoptotic role of vMIA during infection that might account for the slight differences in viral yields (Fig. 1A), we assessed the ability of zVAD to reverse cell death and increase viral yield (Fig. 3D and E and data not shown). We tested a range of zVAD concentrations (6 μM, 12 μM, 25 μM, or 50 μM) added at 2 hpi and analyzed cultures at 72 hpi or later times (7 days pi); however, the caspase inhibitor did not alter the number of GFP-positive cells (data not shown) or suppress death (Fig. 3D and data not shown). In control experiments, 6 to 25 μM zVAD efficiently suppressed Fas-induced apoptosis in AD169varATCC-infected cells (data not shown).
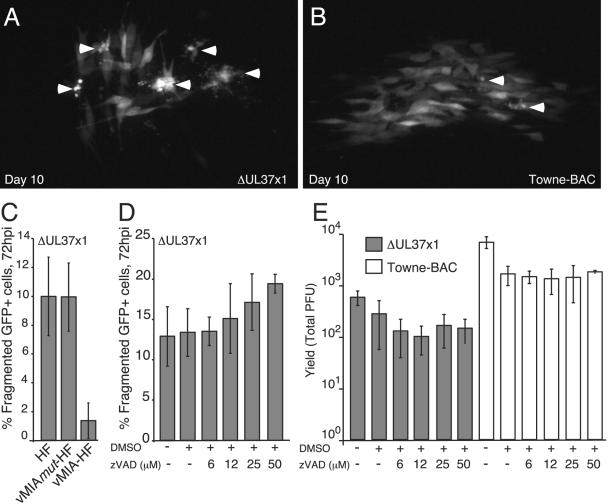
FIG. 3.
Cell death and vMIA suppression in ΔUL37x1 cultures. (A and B) Fluorescence images of GFP expression on day 10 pi of a ΔUL37x1 (A) or Towne-BAC (B) plaque. Arrowheads indicate fragmented cells. (C and D) ΔUL37x1 (GFP+) infected cell death frequency (fragmented cells) at 72 hpi on HF, vMIAmut-HF, or vMIA-HF (C) and on HFs exposed to zVAD (D), as indicated. Fragmented cells from four independent cultures were counted and are graphed as the mean number of fragmented cells relative to total GFP+ cells (percent fragmented GFP+ cells), with standard deviations indicated by error bars. Percent fragmentation was determined from >1,000 infected cells in total. (E) Impact of zVAD as indicated on day 7 viral yields of ΔUL37x1 or Towne-BAC.
FIG. 4.
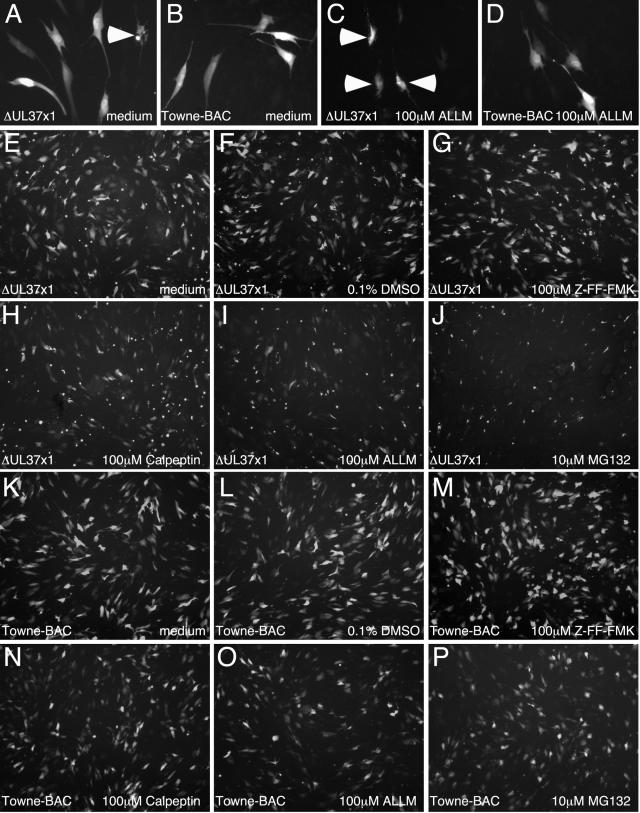
Protease inhibitor-induced cell death of ΔUL37x1-infected HFs. HFs were infected with ΔUL37x1 (A, C, and E to J) or Towne-BAC (B, D, and K to P) for 48 h, before replacement of medium with normal medium (A, B, E, and K), medium plus 0.1% DMSO (F and L), or medium including 0.1% DMSO and 100 μM ALLM (C, D, I, and O), 100 μM Z-FF-FMK (G and M), 100 μM calpeptin (H and N), or 10 μM MG132 (J and P). Images are from GFP fluorescence 20 h post-media replacement. Arrowheads are placed at examples of fragmented cells in ΔUL37x1-infected cultures (A and C).
We also evaluated the impact of zVAD on viral yields, because of the reported ability of this caspase inhibitor to rescue the growth defect of AD169varATCC UL37x1 mutants (48). zVAD at any concentration tested (6 to 50 μM) failed to improve the yield of ΔUL37x1 and did not alter (parental) Towne-BAC virus replication levels (Fig. 3E). This result suggests that increased death observed during ΔUL37x1 infection was independent of caspases 1, 3, 4, and 7. Although Towne-BAC and AD169varATCC both encode vMIA, the requirement for this mitochondrial cell death suppressor during replication clearly varies with viral strain. During infection with Towne-BAC, vMIA is only needed to suppress caspase-3-independent death, whereas in AD169varATCC replication is dependent on vMIA-mediated suppression of apoptosis (48).
vMIA increases the apoptotic threshold of infected cells.
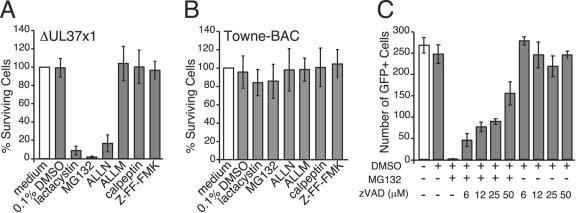
Although Towne-BAC-derived ΔUL37x1 virus infection did not induce caspase-3-dependent apoptosis, we investigated the impact of vMIA on the susceptibility of infected cells to apoptosis. Preliminary attempts to induce extrinsic apoptosis through Fas failed, presumably due to the presence of vICA (53), and so intrinsic cell death was evaluated using proteasomal and calpain protease inhibitors. ALLM, an inhibitor of both proteasomal proteases and calpains at the concentration employed (19), was found to differentially induce death in mutant-infected but not Towne-BAC virus-infected cells within 20 h after addition at a concentration of 100 μM starting 48 hpi (Fig. 4A to D). Thus, ΔUL37x1 virus-infected cells were sensitized to death induced by this protease inhibitor, which led us to test additional inhibitors (Fig. 4E to P). Addition of 100 μM calpeptin or 10 μM MG132 (Fig. 4H and J) at 48 hpi also induced mutant- but not Towne-BAC-infected cell death (Fig. 4N and P), whereas 100 μM Z-FF-FMK (Fig. 4G and M), an inhibitor that more specifically targets cathepsins, had no effect on either virus. We observed differences in the sensitivity of mutant virus to calpain and proteasome inhibitors such that 10 μM MG132 had a more dramatic impact (Fig. 4J) than either 100 μM calpeptin or 100 μM ALLM (Fig. 4H and I). These data suggested that the inhibition of proteasomal proteases was most likely sensitizing cells to apoptosis. A 0.1% concentration of DMSO, the carrier used for protease inhibitors, did not have any effect (Fig. 4F and L). These results suggest that vMIA plays a role in resistance to apoptosis that is mediated by intrinsic cell death pathways. Inhibition of proteasomal proteases, a class of proteases that have previously been implicated in HCMV replication (47), likely increase infected cell stress.
To more precisely determine the relative impact of calpain and proteasomal protease inhibition in ΔUL37x1-infected cells, we evaluated protease inhibitors at a low (10 μM) concentration and included ALLN (19) as well as lactacystin, a natural inhibitor specific for the proteasome (35) (Fig. 5A and B). Lactacystin, MG132, or ALLN induced cell death in ΔUL37x1- but not Towne-BAC virus-infected cultures, while ALLM and calpeptin inhibition of calpains or Z-FF-FMK inhibition of cathepsins failed to induce death of mutant virus-infected cells (Fig. 5A and B). This result showed that inhibition of the proteasome was responsible for increased cell death in the absence of vMIA and, further, that inhibition of the proteasome at 100 μM calpeptin or ALLM (19) was likely to have been responsible for inducing cell death at the higher concentration (Fig. 4). These data showed that resistance of Towne-BAC to proteasome inhibitor-induced apoptosis was lost in the absence of vMIA. Mutant virus sensitivity to MG132 was dramatically reduced when assayed on vMIA-HF (data not shown), confirming the work with Towne-BAC in showing that vMIA was responsible for resistance. Replication of either virus was severely impacted by proteasome inhibition (data not shown). Thus, resistance to apoptosis was vMIA dependent, but susceptibility to antiviral effects was vMIA independent.
FIG. 5.
Caspase-dependent death induced in ΔUL37x1-infected cells by 10 μM proteasome inhibitors. (A and B) HFs infected with ΔUL37x1 (A) or Towne-BAC (B) for 48 h were exposed to 10 μM concentrations of lactacystin, MG132, ALLN, ALLM, calpeptin, or Z-FF-FMK for 20 h in medium containing 0.1% DMSO, control medium with 0.1% DMSO), or normal medium without DMSO (open bar). Surviving cells from three independent experiments were counted and are graphed as the mean percent survival relative to the mean number of viable cells in the medium-only cultures (percent surviving cells), with standard deviations indicated by error bars. Percent survival was determined from >1,000 infected cells per inhibitor. (C) HFs infected with ΔUL37x1 virus were exposed for 20 h to 10 μM MG132, zVAD at the concentrations indicated, control medium (open bar), or medium plus 0.1% DMSO. Graphed are the mean numbers of viable GFP-positive cells determined in three separate cultures, with error bars indicating the standard deviations between cultures.
To determine whether proteasomal protease inhibitors were inducing caspase-dependent apoptosis, ΔUL37x1-infected cultures were incubated with 10 μM MG132 in the presence or absence of the broad caspase inhibitor zVAD. At 20 h posttreatment, viable, GFP-positive cells were counted (Fig. 5C). This dose of MG132 induced death, as expected, and zVAD suppressed death when added at 6 μM, 12 μM, 25 μM, or 50 μM. zVAD alone at any of these concentrations did not suppress virus-induced death, consistent with results of Fig. 3. This result clearly showed that MG132 induced ΔUL37x1-infected cells to undergo caspase-dependent apoptosis. zVAD also suppressed death induced by 100 μM ALLM (data not shown). These results imply that, although ΔUL37x1-infected cells undergo very little cell death at 72 hpi under normal culture conditions, a dramatically reduced apoptotic threshold is revealed by induction of intrinsic cell death by proteasome inhibition.
vMIA inhibits proteasome-induced cell death independent of viral infection.
Although vMIA has been shown to be a potent cell death suppressor in many contexts (6, 8, 9, 25, 30, 51, 59), the ability to independently block proteasome inhibitor-induced apoptosis has not been investigated. A 10 μM concentration of MG132 induced a significant level of HF cell death within 44 h after addition (Fig. 6A and data not shown); however, uninfected HFs were resistant to doses of ALLM that induced infected cell death. A dose of 300 μM ALLM added for 44 h was found to induce death in 90% of cells and was used here (Fig. 6A). HFs appeared intact after 24 h of treatment with either inhibitor, although nuclear changes consistent with apoptosis were evident at this time in a few cells (data not shown). Hence, sensitivity and kinetics of uninfected cell death differed from that observed in ΔUL37x1-infected cells, where apoptosis was complete by 20 h postaddition of either 10 μM MG132 or lower doses of ALLM. Thus, viral infection appears to increase the susceptibility of HFs to induction of intrinsic cell death by proteasome inhibitors.
FIG. 6.
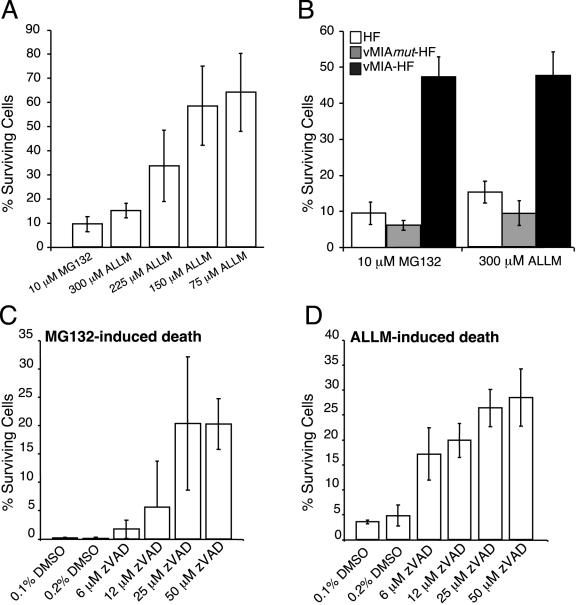
Kinetics, dose dependence, and protection by vMIA of death induced by proteasome inhibition. (A and B) HFs, vMIAmut-HFs, and vMIA-HF were exposed to 10 μM MG132 or ALLM at 300 μM, 225 μM, 150 μM, or 75 μM as indicated. At 44 h postaddition, surviving cells from five microscopic fields were counted and are graphed as the mean percent survival relative to controls, with standard deviations indicated by error bars. Each field of untreated control cells included approximately 1,000 cells. (C and D) HFs were exposed to 10 μM MG132 (C) or 300 μM ALLM (D) in the presence or absence of zVAD at the concentrations indicated. A 0.1% DMSO concentration was equivalent to the solvent from proteasome inhibitors, and 0.2% DMSO was equivalent to the final concentration in all cultures that received proteasome inhibitor plus zVAD at the concentrations indicated. Analysis followed as for panels A and B.
To determine whether vMIA was sufficient to provide protection from MG132- or ALLM-induced cell death, vMIA-HFs and vMIAmut-HFs were exposed to 10 μM MG132 or 300 μM ALLM and viable cells were counted 44 h later (Fig. 6B). vMIAmut-HFs were as sensitive as control HFs to death induced by the protease inhibitors; however, vMIA-HFs resisted proteasome inhibitor-induced cell death. Death under these circumstances appeared to be caspase dependent, because zVAD blocked death in HFs exposed to MG132 or ALLM (Fig. 6C and D). Thus, apoptosis is induced in uninfected HFs by inhibition of the proteasome and vMIA alone is sufficient to inhibit this process.
m38.5 inhibits proteasome-induced cell death.
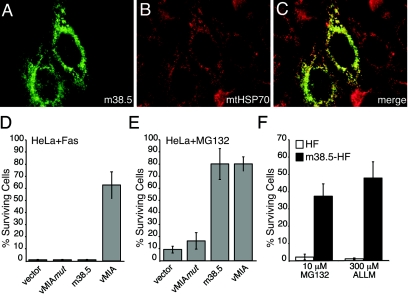
The finding that vMIA increased resistance to proteasome inhibition suggested this assay should be used with the predicted MCMV gene product, m38.5. Previously (37), m38.5 was identified as a potential analog of vMIA based on position and size of the ORF, but this protein failed to protect HeLa cells against extrinsically induced apoptosis. We determined protein localization and protection from proteasome-induced death. First, we found the myc-tagged m38.5 fluorescence pattern consistent with mitochondrial localization (Fig. 7A to C), but not entirely overlapping with a mitochondria resident protein, mtHSP70. This pattern resembled that previously observed for vMIA (38) (data not shown). Transient expression of m38.5 protected HeLa cells from MG132-induced death but, as previously observed (37), failed to inhibit Fas-induced apoptosis (Fig. 7D and E) even when quantities of transfected DNA were increased (data not shown). HeLa cells are known to undergo caspase-dependent apoptosis in response to MG132 (44) and Fas activation (24, 25, 37). Death was suppressed in our experiments by addition of zVAD (data not shown). Next, we evaluated myc-tagged m38.5 protection of HFs derived by retroviral transduction (see Materials and Methods) with expression confirmed by immunoblotting (data not shown). Similar to the results with vMIA-HFs, m38.5-HFs resisted 44 h of treatment with 10 μM MG132 or 300 μM ALLM (Fig. 7F), suggesting that m38.5 functions in a fashion entirely analogous to vMIA. Given that the presence of vICA has already been functionally confirmed in both MCMV and HCMV (37) and that both MCMV and rat CMV conserve a homolog of m38.5 (11, 37), our results reveal the conservation of a functioning positional homolog of vMIA in all of the cytomegaloviruses.
FIG. 7.
Subcellular localization of m38.5 and function in cell death assays. (A to C) m38.5 (green) (A), detected with antibodies to the myc epitope, and mitochondria (red) (B), detected with antibodies to mtHSP70, 24 h posttransfection of HeLa cells with LNCX-m38.5. Individual images were merged for panel C. (D and E) Survival of HeLa cells transfected with LNCX-GFP and LNCX-3myc (vector), LNCX-vMIAmut, LNCX-m38.5, or LNCX-vMIA exposed to anti-Fas antibody plus CH or MG132 added to culture wells for 20 h (Fas) or 44 h (MG132). Surviving GFP-positive cells from three independent transfections (nine fields) were counted and graphed as described for Fig. 6. Each field of untreated cells included an average of 90 GFP-positive cells. (F) m38.5-HFs or control HFs were exposed to 10 μM MG132 or 300 μM ALLM for 44 h. Surviving cells from five fields were counted and graphed as described for Fig. 6. Each field of untreated cells included an average of 1,000 cells.
DISCUSSION
vMIA is a potent suppressor of cell death (6, 8, 9, 25, 30, 51, 59) and can block apoptosis induced by proteasome inhibitors. This form of intrinsic cell death can be used to differentiate the susceptibility of HCMV that expresses or lacks this inhibitor. Without this added stress, UL37x1 mutant Towne has growth properties that are nearly the same as parental virus. These studies follow evidence of a severe growth defect due to induction of caspase-dependent apoptosis of UL37x1 mutants in AD169varATCC (12, 48, 62). We believe that a viral function(s) outside of the UL37x1 gene likely underlies differences in viral growth that have been observed, and the best candidate for an antiapoptotic effect is vICA, which has long been known to be mutated in AD169varATCC but to be functional in TownevarATCC (53). Thus, UL37x1 is completely dispensable for viral growth under standard conditions, but mutant infected cells become more susceptible to apoptosis induced intrinsically by proteasome inhibition. Neither complementation of growth nor resistance to apoptosis required UL37x2 or UL37x3, consistent with reports of engineered (7, 18, 62) and adventitious (25) mutations. The BACmid clone we used for these studies was previously reported to fail to produce virus (18), a result that was likely influenced by expectations from studies on AD169varATCC and the demands of characterizing over 100 viral mutants in this otherwise incisive work. The completeness of our characterization and demonstration that multiple isolates exhibit identical behavior suggests the possibility that this gene should be reclassified into their “growth like wild type” category due to the identical transfection efficiency, initial rate of spread, and <10-fold defect in growth at low MOIs relative to parental Towne-BAC. It is also possible that HFs used in our studies are less susceptible to viral apoptosis; however, over the course of our work HFs pooled from multiple donors have been used to eliminate individual variation in response to Fas or protease inhibitors. Further, neither cell cycle status at the time of infection nor serum sources prevented growth of ΔUL37x1 virus on noncomplementing cells.
Our data suggest that UL37x1 mutants in different strain backgrounds may now be expected to exhibit highly variable degrees of growth defects dependent on the constellation of genes that are retained. Towne-BAC itself lacks several genes that are found in wild-type HCMV (17, 18), particularly in the previously characterized UL-b′ region (13), although none of the genes in this region has yet been assigned any role in suppression of cell death. This suggests two alternative hypotheses concerning the level of proapoptotic stress in cells infected with different strains of virus. On the one hand, a viral factor(s) encoded by Towne-BAC may disarm the apoptotic response either prior to or after mitochondrial signaling. On the other hand, Towne-BAC may fail to sensitize cells to intrinsic cell death. vICA is a likely candidate factor, because this is the best-characterized difference between BACmids of Towne and AD169 (18, 43). Evidence that AD169varATCC impacts the cell in a manner that produces a stronger or different proapoptotic signal comes from the fact that AD169varATCC infection induces cells into pseudomitosis (28) at levels at least 10-fold higher than TownevarATCC (L. Hertel and E. S. Mocarski, unpublished).
We were surprised that zVAD failed to rescue the slight replication defect of ΔUL37x1, despite our evidence showing increased cell death in mutant virus-infected cultures that is insensitive to zVAD and the obvious effectiveness of zVAD in blocking exogenous induction of apoptosis in the presence of proteasome inhibitors. Sensitivity to zVAD provided the best evidence that vMIA can play multiple roles during infection. Although yields and cell survival of the AD169varATCC mutants were dramatically improved by zVAD (48), this treatment did not fully restore replication levels, leaving open the possibility that caspase-3-independent death is contributing to the growth defects in both strains of virus. Because the work on AD169varATCC mutants focused on apoptosis, two caspase-3-dependent assays were employed (48), poly(ADP-ribose) polymerase cleavage and terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling assays, but these were not appropriate to study the caspase-3-independent death we observed in Towne-BAC mutants. The nature of this process remains to be elucidated.
We have used the ΔUL37x1 virus to investigate the role of continued proteolysis by the proteasome and calpains during infection. Consistent with previous reports (47), we found that proteasome activity is required for viral replication, and we have demonstrated that in the context of a UL37x1 mutant, proteasome inhibition induces rapid apoptosis. This implies that the proteasome reduces proapoptotic proteins during CMV replication, allowing viral exploitation of this prosurvival system. The Bcl-2 family of proteins, p53, inhibitor of apoptosis proteins, and regulators of nuclear factor-κB kinase have all been identified as proteasome targets (31) and are implicated as potential regulators of apoptosis induced by CMV infection. CMV-infected HFs sequester Bax (3) at mitochondria and p53 in replication compartments (21). vMIA is associated with Bax at mitochondria (3, 46), but overexpression of Bax impedes vMIA protection (Smith and Mocarski, submitted). Viral factors required for p53 sequestration are not known, but overexpression of p53 may likewise overcome viral resistance to p53-dependent apoptosis.
We have demonstrated that vMIA is necessary and sufficient to prevent intrinsic apoptosis induced by proteasome inhibition, and we developed an assay based on this result that revealed cell death suppression by m38.5. This implies that m38.5 and vMIA likely fulfill the same role within the context of virus infection, despite different potencies in some assays executed in the context of human-derived cells. Continued mechanistic studies with these two evolutionarily related proteins, vMIA and m38.5, will provide further insight into events occurring at mitochondria during apoptosis. Further, revealing m38.5 antiapoptotic function suggests MCMV will be a useful model to probe the biological significance of this function in the context of the natural host.
Acknowledgments
This work was supported by PHS grants RO1 AI20211 to E.S.M., 5F32AI056959 to C.D.M., and T32GM07365 and F30NS051109 to G.B.S.
We acknowledge Yin Dong and Leena Bashyam for expert technical assistance, Victor Goldmacher for continued interest and advice, and Marcy Vana for critical reading of the manuscript. This work would not have been possible without the generous sharing of materials by Fenyong Liu and Walter Dunn.
REFERENCES
- 1.Alcami, A., and U. H. Koszinowski. 2000. Viral mechanisms of immune evasion. Trends Microbiol. 8:410-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andoniou, C., D. Andrews, M. Manzur, P. Ricciardi-Castagnoli, and M. Degli-Esposti. 2004. A novel checkpoint in the Bcl-2-regulated apoptotic pathway revealed by murine cytomegalovirus infection of dendritic cells. J. Cell Biol. 166:827-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnoult, D., L. Bartle, A. Skaletskaya, D. Poncet, N. Zamzami, P. Park, J. Sharpe, R. Youle, and V. S. Goldmacher. 2004. Cytomegalovirus cell death suppressor vMIA blocks Bax- but not Bak-mediated apoptosis by binding and sequestering Bax at mitochondria. Proc. Natl. Acad. Sci. USA 101:7988-7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldick, C. J., Jr., A. Marchini, C. E. Patterson, and T. Shenk. 1997. Human cytomegalovirus tegument protein pp71 (ppUL82) enhances the infectivity of viral DNA and accelerates the infectious cycle. J. Virol. 71:4400-4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartz, S., and M. Vodicka. 1997. Production of high-titer human immunodeficiency virus type 1 pseudotyped with vesicular stomatitis virus glycoprotein. Methods 12:337-342. [DOI] [PubMed] [Google Scholar]
- 6.Belzacq, A. S., C. El Hamel, H. L. Vieira, I. Cohen, D. Haouzi, D. Metivier, P. Marchetti, C. Brenner, and G. Kroemer. 2001. Adenine nucleotide translocator mediates the mitochondrial membrane permeabilization induced by lonidamine, arsenite and CD437. Oncogene 20:7579-7587. [DOI] [PubMed] [Google Scholar]
- 7.Borst, E. M., G. Hahn, U. H. Koszinowski, and M. Messerle. 1999. Cloning of the human cytomegalovirus (HCMV) genome as an infectious bacterial artificial chromosome in Escherichia coli: a new approach for construction of HCMV mutants. J. Virol. 73:8320-8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boya, P., R. Gonzalez-Polo, N. Casares, J. Perfettini, P. Dessen, N. Larochette, D. Metivier, D. Meley, S. Souquere, T. Yoshimori, G. Pierron, P. Codogno, and G. Kroemer. 2005. Inhibition of macroautophagy triggers apoptosis. Mol. Cell. Biol. 25:1025-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boya, P., M. C. Morales, R. A. Gonzalez-Polo, K. Andreau, I. Gourdier, J. L. Perfettini, N. Larochette, A. Deniaud, F. Baran-Marszak, R. Fagard, J. Feuillard, A. Asumendi, M. Raphael, B. Pau, C. Brenner, and G. Kroemer. 2003. The chemopreventive agent N-(4-hydroxyphenyl)retinamide induces apoptosis through a mitochondrial pathway regulated by proteins from the Bcl-2 family. Oncogene 22:6220-6230. [DOI] [PubMed] [Google Scholar]
- 10.Boya, P., T. Roumier, K. Andreau, R. A. Gonzalez-Polo, N. Zamzami, M. Castedo, and G. Kroemer. 2003. Mitochondrion-targeted apoptosis regulators of viral origin. Biochem. Biophys. Res. Commun. 304:575-581. [DOI] [PubMed] [Google Scholar]
- 11.Brocchieri, L., T. Kledal, S. Karlin, and E. S. Mocarski. 2005. Predicting coding potential from genome sequence: application to betaherpesviruses infecting rats and mice. J. Virol. 79:7570-7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brune, W., M. Nevels, and T. Shenk. 2003. Murine cytomegalovirus m41 open reading frame encodes a Golgi-localized antiapoptotic protein. J. Virol. 77:11633-11643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cha, T. A., E. Tom, G. W. Kemble, G. M. Duke, E. S. Mocarski, and R. R. Spaete. 1996. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J. Virol. 70:78-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chee, M. S., A. T. Bankier, S. Beck, R. Bohni, C. M. Brown, R. Cerny, T. Horsnell, C. A. I. Hutchison, T. Kouzarides, J. A. Martignetti, E. Preddie, S. C. Satchwell, P. Tomlinson, K. M. Weston, and B. G. Barrell. 1990. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr. Top. Microbiol. Immunol. 154:125-170. [DOI] [PubMed] [Google Scholar]
- 15.Chen, Z., E. Knutson, A. Kurosky, and T. Albrecht. 2001. Degradation of p21cip1 in cells productively infected with human cytomegalovirus. J. Virol. 75:3613-3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuconati, A., K. Degenhardt, R. Sundararajan, A. Anschel, and E. White. 2002. Bak and Bax function to limit adenovirus replication through apoptosis induction. J. Virol. 76:4547-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolan, A., C. Cunningham, R. Hector, A. Hassan-Walker, L. Lee, C. Addison, D. Dargan, D. McGeoch, D. Gatherer, V. Emery, P. Griffiths, C. Sinzger, B. McSharry, G. Wilkinson, and A. Davison. 2004. Genetic content of wild-type human cytomegalovirus. J. Gen. Virol. 85:1301-1312. [DOI] [PubMed] [Google Scholar]
- 18.Dunn, W., C. Chou, H. Li, R. Hai, D. Patterson, V. Stolc, H. Zhu, and F. Liu. 2003. Functional profiling of a human cytomegalovirus genome. Proc. Natl. Acad. Sci. USA 100:14223-14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Figueiredo-Pereira, M., N. Banik, and S. Wilk. 1994. Comparison of the effect of calpain inhibitors on two extralysosomal proteinases: the multicatalytic proteinase complex and m-calpain. J. Neurochem. 62:1989-1994. [DOI] [PubMed] [Google Scholar]
- 20.Fleckenstein, B., I. Muller, and J. Collins. 1982. Cloning of the complete human cytomegalovirus genome in cosmids. Gene 18:39-46. [DOI] [PubMed] [Google Scholar]
- 21.Fortunato, E. A., and D. H. Spector. 1998. p53 and RPA are sequestered in viral replication centers in the nuclei of cells infected with human cytomegalovirus. J. Virol. 72:2033-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedman, J., and D. Xue. 2004. To live or die by the sword: the regulation of apoptosis by the proteasome. Dev. Cell 6:460-461. [DOI] [PubMed] [Google Scholar]
- 23.Goldmacher, V. 2005. Cell death suppression by cytomegaloviruses. Apoptosis 10:251-265. [DOI] [PubMed] [Google Scholar]
- 24.Goldmacher, V. S. 2002. vMIA, a viral inhibitor of apoptosis targeting mitochondria. Biochimie 84:177-185. [DOI] [PubMed] [Google Scholar]
- 25.Goldmacher, V. S., L. M. Bartle, A. Skaletskaya, C. A. Dionne, N. L. Kedersha, C. A. Vater, J. Han, R. J. Lutz, S. Watanabe, E. D. McFarland, E. D. Kieff, E. S. Mocarski, and T. Chittenden. 1999. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc. Natl. Acad. Sci. USA 96:12536-12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayajneh, W. A., A. M. Colberg-Poley, A. Skaletskaya, L. M. Bartle, M. M. Lesperance, D. G. Contopoulos-Ioannidis, N. L. Kedersha, and V. S. Goldmacher. 2001. The sequence and antiapoptotic functional domains of the human cytomegalovirus UL37 exon 1 immediate early protein are conserved in multiple primary strains. Virology 279:233-240. [DOI] [PubMed] [Google Scholar]
- 27.Hengartner, M. 2000. The biochemistry of apoptosis. Nature 407:770-776. [DOI] [PubMed] [Google Scholar]
- 28.Hertel, L., and E. Mocarski. 2004. Global analysis of host cell gene expression late during cytomegalovirus infection reveals extensive dysregulation of cell cycle gene expression and induction of pseudomitosis independent of US28 function. J. Virol. 78:11988-12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaattela, M. 2004. Multiple cell death pathways as regulators of tumour initiation and progression. Oncogene 23:2746-2756. [DOI] [PubMed] [Google Scholar]
- 30.Jan, G., A. S. Belzacq, D. Haouzi, A. Rouault, D. Metivier, G. Kroemer, and C. Brenner. 2002. Propionibacteria induce apoptosis of colorectal carcinoma cells via short-chain fatty acids acting on mitochondria. Cell Death Differ. 9:179-188. [DOI] [PubMed] [Google Scholar]
- 31.Jesenberger, V., and S. Jentsch. 2002. Deadly encounter: ubiquitin meets apoptosis. Nat. Rev. Mol. Cell Biol. 3:112-121. [DOI] [PubMed] [Google Scholar]
- 32.Kerr, J. F., A. H. Wyllie, and A. R. Currie. 1972. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26:239-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan, S., A. Zimmermann, M. Basler, M. Groettrup, and H. Hengel. 2004. A cytomegalovirus inhibitor of gamma interferon signaling controls immunoproteasome induction. J. Virol. 78:1831-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LeBlanc, A. 2003. Natural cellular inhibitors of caspases. Prog. Neuropsychopharmacol. Biol. Psych. 27:215-229. [DOI] [PubMed] [Google Scholar]
- 35.Lee, D., and A. L. Goldberg. 1998. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 8:397-403. [DOI] [PubMed] [Google Scholar]
- 36.Marchini, A., H. Liu, and H. Zhu. 2001. Human cytomegalovirus with IE-2 (UL122) deleted fails to express early lytic genes. J. Virol. 75:1870-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCormick, A. L., A. Skaletskaya, P. A. Barry, E. S. Mocarski, and V. S. Goldmacher. 2003. Differential function and expression of the viral inhibitor of caspase 8-induced apoptosis (vICA) and the viral mitochondria-localized inhibitor of apoptosis (vMIA) cell death suppressors conserved in primate and rodent cytomegaloviruses. Virology 316:221-233. [DOI] [PubMed] [Google Scholar]
- 38.McCormick, A. L., V. L. Smith, D. Chow, and E. S. Mocarski. 2003. Disruption of mitochondrial networks by the human cytomegalovirus UL37 gene product viral mitochondrion-localized inhibitor of apoptosis. J. Virol. 77:631-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menard, C., M. Wagner, Z. Ruzsics, K. Holak, W. Brune, A. E. Campbell, and U. H. Koszinowski. 2003. Role of murine cytomegalovirus US22 gene family members in replication in macrophages. J. Virol. 77:5557-5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller, A. D., and G. J. Rosman. 1989. Improved retroviral vectors for gene transfer and expression. BioTechniques 7:980-990. [PMC free article] [PubMed] [Google Scholar]
- 41.Mocarski, E. S. 2002. Immunomodulation by cytomegaloviruses: manipulative strategies beyond evasion. Trends Microbiol. 10:332-339. [DOI] [PubMed] [Google Scholar]
- 42.Mocarski, E. S., Jr., and C. T. Courcelle. 2001. Cytomegaloviruses and their replication, p. 2629-2673. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 43.Murphy, E., D. Yu, J. Grimwood, J. Schmutz, M. Dickson, M. A. Jarvis, G. Hahn, J. A. Nelson, R. M. Myers, and T. E. Shenk. 2003. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc. Natl. Acad. Sci. USA 100:14976-14981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nencioni, A., F. Hua, C. Dillon, R. Yokoo, C. Scheiermann, M. Cardone, E. Barbieri, I. Rocco, A. Garuti, S. Wesselborg, C. Belka, P. Brossart, F. Patrone, and A. Ballestrero. 2005. Evidence for a protective role of Mcl-1 in proteasome inhibitor-induced apoptosis. Blood 105:3255-3262. [DOI] [PubMed] [Google Scholar]
- 45.Pass, R. F. 2001. Cytomegalovirus, p. 2675-2705. In D. Knipe and P. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 46.Poncet, D., N. Larochette, A. Pauleau, P. Boya, A. Jalil, P. Cartron, F. Vallette, C. Schnebelen, L. Bartle, A. Skaletskaya, D. Boutolleau, J. Martinou, V. Goldmacher, G. Kroemer, and N. Zamzami. 2004. An anti-apoptotic viral protein that recruits Bax to mitochondria. J. Biol. Chem. 279:22605-22614. [DOI] [PubMed] [Google Scholar]
- 47.Prosch, S., C. Priemer, C. Hoflich, C. Liebenthaf, N. Babel, D. Kruger, and H. Volk. 2003. Proteasome inhibitors: a novel tool to suppress human cytomegalovirus replication and virus-induced immune modulation. Antivir. Ther. 8:555-567. [PubMed] [Google Scholar]
- 48.Reboredo, M., R. F. Greaves, and G. Hahn. 2004. Human cytomegalovirus proteins encoded by UL37 exon 1 protect infected fibroblasts against virus-induced apoptosis and are required for efficient virus replication. J. Gen. Virol. 85:3555-3567. [DOI] [PubMed] [Google Scholar]
- 49.Reinhardt, J., G. Smith, C. Himmelheber, J. Azizkhan-Clifford, and E. Mocarski. 2005. The carboxyl-terminal region of human cytomegalovirus IE1491aa contains an acidic domain that plays a regulatory role and a chromatin-tethering domain that is dispensable during viral replication. J. Virol. 79:225-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roulston, A., R. C. Marcellus, and P. E. Branton. 1999. Viruses and apoptosis. Annu. Rev. Microbiol. 53:577-628. [DOI] [PubMed] [Google Scholar]
- 51.Roumier, T., H. L. Vieira, M. Castedo, K. F. Ferri, P. Boya, K. Andreau, S. Druillennec, N. Joza, J. M. Penninger, B. Roques, and G. Kroemer. 2002. The C-terminal moiety of HIV-1 Vpr induces cell death via a caspase-independent mitochondrial pathway. Cell Death Differ. 9:1212-1219. [DOI] [PubMed] [Google Scholar]
- 52.Samejima, K., S. Tone, and W. Earnshaw. 2001. CAD/DFF40 nuclease is dispensable for high molecular weight DNA cleavage and stage I chromatin condensation in apoptosis. J. Biol. Chem. 276:45427-45432. [DOI] [PubMed] [Google Scholar]
- 53.Skaletskaya, A., L. M. Bartle, T. Chittenden, A. L. McCormick, E. S. Mocarski, and V. S. Goldmacher. 2001. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc. Natl. Acad. Sci. USA 98:7829-7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spaete, R. R., and E. S. Mocarski. 1987. Insertion and deletion mutagenesis of the human cytomegalovirus genome. Proc. Natl. Acad. Sci. USA 84:7213-7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stoddart, C. A., R. D. Cardin, J. M. Boname, W. C. Manning, G. B. Abenes, and E. S. Mocarski. 1994. Peripheral blood mononuclear phagocytes mediate dissemination of murine cytomegalovirus. J. Virol. 68:6243-6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Susin, S., E. Daugas, L. Ravagnan, K. Samejima, N. Zamzami, M. Loeffler, P. Costantini, K. Ferri, T. Irinopoulou, M. Prevost, G. Brothers, T. Mak, J. Penninger, W. Earnshaw, and G. Kroemer. 2000. Two distinct pathways leading to nuclear apoptosis. J. Exp. Med. 192:571-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tortorella, D., B. E. Gewurz, M. H. Furman, D. J. Schust, and H. L. Ploegh. 2000. Viral subversion of the immune system. Annu. Rev. Immunol. 18:861-926. [DOI] [PubMed] [Google Scholar]
- 58.Tschopp, J., M. Thome, K. Hofmann, and E. Meinl. 1998. The fight of viruses against apoptosis. Curr. Opin. Genet. Dev. 8:82-87. [DOI] [PubMed] [Google Scholar]
- 59.Vieira, H. L., A. S. Belzacq, D. Haouzi, F. Bernassola, I. Cohen, E. Jacotot, K. F. Ferri, C. El Hamel, L. M. Bartle, G. Melino, C. Brenner, V. Goldmacher, and G. Kroemer. 2001. The adenine nucleotide translocator: a target of nitric oxide, peroxynitrite, and 4-hydroxynonenal. Oncogene 20:4305-4316. [DOI] [PubMed] [Google Scholar]
- 60.White, E. 2001. Regulation of the cell cycle and apoptosis by the oncogenes of adenovirus. Oncogene 20:7836-7846. [DOI] [PubMed] [Google Scholar]
- 61.Wojcik, C. 2002. Regulation of apoptosis by the ubiquitin and proteasome pathway. J. Cell Mol. Med. 6:25-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu, D., M. C. Silva, and T. Shenk. 2003. Functional map of human cytomegalovirus AD169 defined by global mutational analysis. Proc. Natl. Acad. Sci. USA 100:12396-12401. [DOI] [PMC free article] [PubMed] [Google Scholar]