Abstract
Plants produce a plethora of structurally diverse natural products. The final step in their biosynthesis is often a glycosylation step catalyzed by a family 1 glycosyltransferase (GT). In biosynthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor, the UDP-glucosyltransferase UGT85B1 catalyzes the conversion of p-hydroxymandelonitrile into dhurrin. A structural model of UGT85B1 was built based on hydrophobic cluster analysis and the crystal structures of two bacterial GTs, GtfA and GtfB, which each showed approximately 15% overall amino acid sequence identity to UGT85B1. The model enabled predictions about amino acid residues important for catalysis and sugar donor specificity. p-Hydroxymandelonitrile and UDP-glucose (Glc) were predicted to be positioned within hydrogen-bonding distance to a glutamic acid residue in position 410 facilitating sugar transfer. The acceptor was packed within van der Waals distance to histidine H23. Serine S391 and arginine R201 form hydrogen bonds to the pyrophosphate part of UDP-Glc and hence stabilize binding of the sugar donor. Docking of UDP sugars predicted that UDP-Glc would serve as the sole donor sugar in UGT85B1. This was substantiated by biochemical analyses. The predictive power of the model was validated by site-directed mutagenesis of selected residues and using enzyme assays. The modeling approach has provided a tool to design GTs with new desired substrate specificities for use in biotechnological applications. The modeling identified a hypervariable loop (amino acid residues 156–188) that contained a hydrophobic patch. The involvement of this loop in mediating binding of UGT85B1 to cytochromes P450, CYP79A1, and CYP71E1 within a dhurrin metabolon is discussed.
A glycosyltransferase (GT) is an enzyme that attaches a sugar molecule to a specific acceptor and thereby creates a glycosidic bond. GTs are found in all phylae. The nature of the acceptor molecules used is highly diverse, whereas the donor molecules typically are restricted to monosaccharides with either d- or l-configuration linked to a nucleotide (UDP/GDP/CMP/(d)TDP; Leloir, 1964). Glycosylation reactions constitute an integral part of both primary and secondary metabolism. The final step in plant natural product synthesis is often a glycosylation reaction that serves to stabilize, solubilize, and provide a safe storage form of potentially toxic aglycones. In Sorghum bicolor, the GT UGT85B1 catalyzes glucosylation of Tyr-derived p-hydroxymandelonitrile to yield the cyanogenic glucoside dhurrin (Jones et al., 1999). In planta, neither p-hydroxymandelonitrile nor its degradation products are detectable, indicating that the glucosylation reaction is highly efficient and channeled (Møller and Conn, 1980). This observation has been substantiated by metabolome and transcriptome analyses of transgenic Arabidopsis (Arabidopsis thaliana) plants expressing the entire dhurrin pathway (Tattersall et al., 2001; Jørgensen et al., 2005; Kristensen et al., 2005), as well as by confocal laser-scanning microscopy studies showing that the three enzymes CYP79A1, CYP71E1, and UGT85B1 catalyzing dhurrin formation are physically associated within a tight metabolon (Nielsen and Møller, 2005). In contrast, UGT85B1 exhibits a rather broad specificity toward acceptors in vitro, a key determinant for glycosylation being the presence of a sterically unmasked acceptor hydroxyl group (Jones et al., 1999; Hansen et al., 2003).
GTs are divided into families according to sequence identity and the nature of the biochemical reaction they catalyze (Coutinho et al., 2003). Currently, GTs are classified into 76 families (http://afmb.cnrs-mrs.fr/CAZY/GT.html). According to the CAZy classification system, UGT85B1 belongs to family 1, the UGTs (Mackenzie et al., 1997). UGTs are defined by a C-terminally situated PROSITE consensus sequence (Mackenzie et al., 1997; Paquette et al., 2003). Plant UGTs comprise a highly divergent, polyphyletic multigene family that may be divided into one major and two minor clades. The two minor clades encompass lipid and sterol GTs and are more related to nonplant clades than to other plant clades. The major clade is vastly expanded and specific to plants, and is generally thought to be involved in biosynthesis of the vast array of glycosidic plant natural products. As documented for UGT85B1, plant UGTs involved in natural product synthesis generally exhibit a broad substrate specificity with respect to the aglycon (sugar acceptor; Jones et al., 1999; Taguchi et al., 2000; Jackson et al., 2001; Lim et al., 2001a, 2001b, 2003a, 2003b; Vogt, 2002; Hansen et al., 2003; Paquette et al., 2003). Most animal UGTs utilize UDP-GlcUA as a sugar donor, whereas plant UGTs typically utilize UDP-Glc. UGT78D1 is the first plant UGT shown to utilize UDP-Rha and is involved in biosynthesis of kaempferol-3-O-rhamnoside and quercetin-3-O-rhamnoside (Jones et al., 2003). UGT94B1 is the first plant UGT shown to catalyze UDP-GlcUA-dependent glucuronylation of the 2″-hydroxyl group of the 3-glucosyl moiety of cyanidin 3-O-6″-O-malonylglucoside (Sawada et al., 2005). This demonstrates that the sugar donor specificity of plant UGTs is not restricted to UDP-Glc.
Knowledge on the structure of plant UGTs is scarce and this hampers rational design of new UGTs with desired properties by molecular approaches. No plant GT crystal structure is available, but crystal structures of GTs from different CAZy families display pronounced tertiary structure similarities. Thus crystal structures available for GTs belonging to 14 different families exhibited only two different structural folds, designated GT-A and GT-B folds (Hu and Walker, 2002). The GT-A fold employs a DXD motif to bind a divalent metal ion and consists of one α/β/α-domain. In contrast, the GT-B fold is characterized by a two-domain structure where the N- and C-terminal domains are clearly distinct and joined by an α-helix linker structure. It has been proposed that the N- and C-terminal domains represent a result of gene duplication as they display similar patterns of fold with β-sheets interspersed between layers of α-helices in an α/β/α-sandwich resembling a Rossmann fold (Mulichak et al., 2001). The two domains are separated by a deep cleft where binding of UDP-Glc and aglycone as well as catalysis takes place (Vrielink et al., 1994; Breton et al., 2002; Hu and Walker, 2002).
It is assumed that GTs within a single CAZy family display the same structural fold (Coutinho et al., 2003). The only crystal structures that have been solved for the family 1 are GtfA, GtfB, and GtfD, which all serve to decorate the glycopeptide antibiotics of the vancomycin family in the bacterium Amycolatopsis orientalis (Mulichak et al., 2001, 2003, 2004). GtfA and GtfD utilize TDP-l-epivancosamine and TDP-l-vancosamine as sugar donors, respectively, and UDP-Glc is a donor in GtfB-mediated sugar transfer. Crystal structures reveal that all three enzymes display the GT-B fold; hence, plant UGTs are predicted to adopt this same fold. Crystal structures of GTs from six different CAZy families with GT-B folds are currently available (http://afmb.cnrs-mrs.fr/CAZY).
Recently, the betanidin 5-O-glucosyltransferase (UGT73A5) from Dorotheathus bellidiformis was modeled based on selected structural features of the crystal structure of A. orientalis GtfB (Hans et al., 2004) to delineate aglycone binding and the catalytic mechanism. In this study, we demonstrate that, based on hydrophobic cluster analysis (HCA) and the crystal structures of GtfA and GtfB, it was possible to use molecular modeling to construct a valid model of UGT85B1 despite an overall amino acid sequence identity of only approximately 15% between GtfA or GtfB and UGT85B1. The model provided detailed information on catalysis and geometry of the active site. Key amino acid residues involved in binding of aglycone and a sugar donor in the active site of UGT85B1 were identified. UDP-Glc was predicted to serve as the sole sugar donor and this was confirmed by biochemical analysis of recombinant UGT85B1 with UDP-Glc, UDP-Gal, UDP-GlcUA, and UDP-Xyl as sugar donors. To substantiate the model predictions, these were verified experimentally using mutant proteins generated by site-directed mutagenesis.
RESULTS
Comparative Modeling of UGT85B1
GtfA, GtfB, and UGT85B1 belong to family GT1 according to the CAZy classification (http://afmb.cnrs-mrs.fr/CAZY). An alignment of these three sequences was used to model UGT85B1. Secondary structures of UGT85B1 were predicted by structure prediction programs (Combet et al., 2000) and HCA (Gaboriaud et al., 1987) and used to construct an optimal structural alignment (Fig. 1). The amino acid sequences of GtfA and GtfB exhibited 62% overall identity and that of UGT85B1 displayed approximately 15% overall identity to both GtfA and GtfB. The structural alignment predicted that UGT85B1 consisted of 14 α-helices and 12 β-strands (Fig. 1). In agreement with the predicted two-domain structure of UGTs, the n-terminal domain consisted of six β-strands and seven α-helices, and the C-terminal domain contained six β-strands and seven α-helices. An α-helix positioned at the C terminus of the n-terminal domain end joined the two domains (Fig. 1).
Figure 1.
Structural amino acid sequence alignment of the S. bicolor GT UGT85B1 with the A. orientalis GTs GtfA and GtfB. Secondary structure elements are as follows: β-strands (turquoise coloring) and α-helices (purple coloring). Loops A, B, C, and D, which were removed in the UGT85B1 model, are shown with red bars. Amino acid residues further analyzed by mutagenesis are shown with a green background. Amino acid residues in red italics were not seen in the GtfB crystal structure, indicating conformational flexibility for these regions. The hydrophobic patch in loop B is shown with a yellow background. The PSPG motif is underlined. α-Helix 7 links the N- and C-terminal domains.
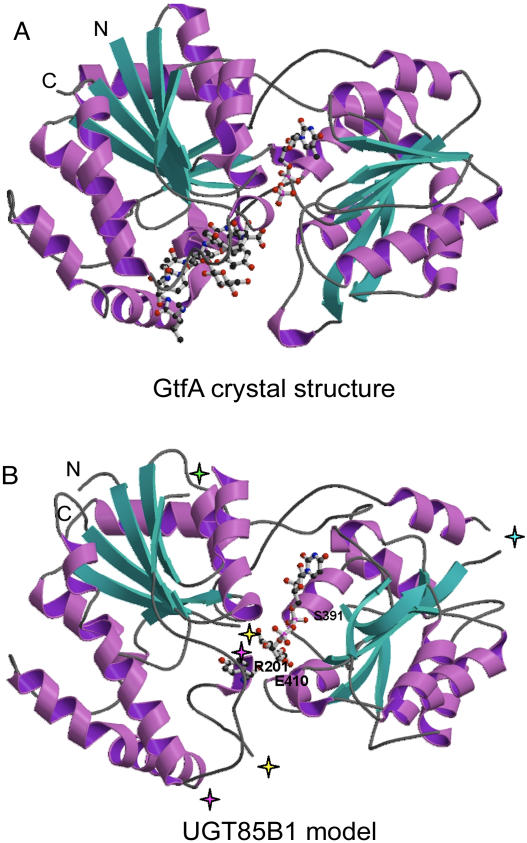
A molecular model of UGT85B1 was constructed from the structural alignment. Structurally conserved regions of the UGT85B1 model were built from crystal structures of GtfA and GtfB and subsequently loops were modeled. Four of 11 loops (A, residues 59–71; B, 156–188; C, 203–211; D, 288–300; Fig. 1) could not be modeled and were not included in the final UGT85B1 model. Loops A and D are positioned distant from the crevice constituting the active site (Fig. 2) and were therefore not considered important for understanding UGT85B1-mediated catalysis. Loops B and C are both positioned between β-strand 5 and α-helix 5 (Figs. 1 and 3). Multiple alignments showed that loop B is one of the most hypervariable regions in UGTs in both composition and length and often contains a hydrophobic patch (Fig. 3). Loop C is located on the back of the molecule pointing away from the active site and the corresponding region of GtfB is hypervariable (Mulichak et al., 2001), explaining why no single conformation has been determined for this loop. Loop B, the largest of all loops in the structural alignment (Figs. 1 and 3), could not be modeled because it is not present in GtfA and GtfB (Fig. 1) and because of an apparent lack of secondary structure. Furthermore, loop B points away from the active site (Fig. 2).
Figure 2.
Schematic presentation of the overall architecture of the GtfA crystal structure (A) and the UGT85B1 model (B). α-Helices are shown in purple and β-sheets in turquoise. N and C termini are indicated. Substrates are shown as ball-and-stick presentations in the catalytic pocket of both enzymes with GtfA, including both the TDP part of the donor molecule TDP-epi-vancosamine and the acceptor molecule vancomycin, and UGT85B1 modeled with the donor molecule UDP-Glc and the acceptor p-hydroxymandelonitrile. Green star, position of removed loop A; yellow stars, beginning and ending of removed loop B; purple stars, beginning and ending of removed loop C; blue star, position of removed loop D.
Figure 3.
Multiple alignment of plant family 1 GTs. Only the region between α-helices 4 and 5 showing the hydrophobic cluster, loops B and C (red boxes), as well as β-strand 5 is shown. The UGT numbers correspond to the nomenclature at http://www.p450.kvl.dk//Arab_ugts/table.shtml. R201 is encircled.
After several cycles of energy minimization and consulting Ramachandran maps, the final UGT85B1 model adopted the expected GT-B fold without major overall constraints in the molecule. In the final model, all amino acids exhibited allowed conformations and only seven amino acid residues were in nearly allowed conformation. The resulting UGT85B1 model was validated by superimposing on the crystal structure of GtfA complexed with thymine diphosphate (TDP; Mulichak et al., 2003) and on T4 phage BGT complexed with UDP-Glc (protein data bank no. 1JG6; Morera et al., 2001). The UGT85B1 model displayed a distribution of α-helices and β-strands similar to the crystal structures of GtfA (Fig. 2) and GtfB. This indicated that the UGT85B1 model obtained displayed a level of accuracy that facilitated exploration of the geometry of the active site for identification of residues responsible for donor and acceptor binding as well as important residues driving catalysis.
Predicted Interactions with Aglycons (Acceptor Molecules)
p-Hydroxymandelonitrile, the endogenous substrate for UGT85B1, and the in vitro acceptor geraniol (Hansen et al., 2003) were docked into the substrate pocket of the UGT85B1 model. They were both positioned within hydrogen-bonding distance of E410 and packed in van der Waals distance against the H23 residue, which thereby served to stabilize the complex throughout catalysis (Fig. 4). The aromatic ring of p-hydroxymandelonitrile was distanced from H23 in the UGT85B1 model by only approximately 4 Å. The close proximity and the observation that both ring structures were in the same plane in the model indicated van der Waals interactions between p-hydroxymandelonitrile and H23 as parallel stacking arrangements. The E410 residue in UGT85B1, positioned in the crevice between the two Rossmann-type fold domains (Fig. 2), interacted with both the acceptor (p-hydroxymandelonitrile or geraniol) and the sugar moiety of UDP-Glc, indicating that E410 was catalytically important (Fig. 4).
Figure 4.
Predicted interactions in the catalytic pocket of UGT85B1 with the donor molecule UDP-Glc and the acceptor molecules p-hydroxymandelonitrile (A) and geraniol (B). Substrates are indicated by bold ball-and-stick presentations. Hydrogen bonds between individual amino acid residues (in gray) and substrates are indicated by dashed green lines.
Predicted Interactions with UDP Sugars (Donor Molecules)
In the UGT85B1 model, the nucleoside part of UDP-Glc was within hydrogen-bonding distance of C369 and E394, the pyrophosphate hydrogen bonded with N390 and S391, and the Glc part hydrogen bonded further to T152, H386, W389, E410, and Q411 (Fig. 4). Furthermore, additional amino acid residues in 4 Å binding distance to UDP-Glc in the active site of the UGT85B1 model were identified (data not shown). Analysis of the geometry of the active site predicted that UDP-Glc and UDP-Gal, but neither UDP-GlcUA nor UDP-Xyl, would serve as sugar donors. In the UGT85B1/UDP-Glc complex, the hydroxyl group on C-6 of the Glc moiety is predicted to hydrogen bond with E410 and thus to contribute to stabilization of this moiety in the active site (Fig. 4). UDP-Gal only differs from UDP-Glc with regard to the configuration of the C-4 carbon hydroxyl group on the sugar moiety (Fig. 5). UDP-Gal fit into the active site of the UGT85B1 model, interacted with E410, and, based on our model, may serve as an alternative sugar donor in UGT85B1-mediated sugar transfer. However, when the active site model was considered with the acceptor molecule (either p-hydroxymandelonitrile or geraniol) docked into the binding site, the axial O-4 hydroxyl group of Gal created a steric conflict with the acceptor oxygen. It was therefore predicted that UDP-Gal could be incorporated into the active site of UGT85B1 (albeit with a lower affinity than UDP-Glc), but that this would prevent simultaneous binding of the acceptor in proper position for catalysis to take place. UDP-GlcUA has a carboxyl group at the C-6 position instead of the hydroxyl group. This carboxylic group was predicted to create an unfavorable electrostatic contact with negatively charged E410. The pentose nucleotide sugar UDP-Xyl lacks the hydroxymethyl group (Fig. 5) and cannot hydrogen bond to E410. Accordingly, UDP-GlcUA and UDP-Xyl are less stabilized in the active site, as compared to UDP-Glc and UDP-Gal, and were not expected to serve as sugar donors.
Figure 5.
Structure of UDP-Glc, UDP-Gal, UDP-GlcUA, and UDP-Xyl.
Biochemical Assays with UDP Sugars
The sugar donor specificity of UGT85B1 has not been investigated previously. Accordingly, in order to assess the validity of the computer-based predictions, biochemical in vitro assays with radiolabeled UDP-Glc, UDP-Gal, UDP-Xyl, or UDP-GlcUA as sugar donors were performed. Mandelonitrile was utilized as an acceptor molecule instead of p-hydroxymandelonitrile because mandelonitrile is more stable than p-hydroxymandelonitrile. UGT85B1 has previously been shown to glucosylate the two compounds at comparable rates (Reay and Conn, 1974; Jones et al., 1999). Product formation was assessed by thin-layer chromatography (TLC) and liquid chromatography-mass spectrometry (LC-MS) and showed that UDP-Glc, but not UDP-Xyl or UDP-GlcUA, served as sugar donors for UGT85B1 (Fig. 6). When UDP-Gal was used as a sugar donor, TLC analysis revealed the presence of low amounts of a glycosidic product (Fig. 6). LC-MS analysis of the assay mixtures with UDP-Glc and UDP-Gal as sugar donors both revealed two products. In the assay using UDP-Glc, the product eluting at retention time (Rt) = 11.6 min is prunasin and the product eluting at Rt = 12.3 min is sambunigrin, with sambunigrin being the major component (Fig. 7). Sambunigrin (β-d-glucopyranosyloxy-(S)-mandelonitrile) and dhurrin (β-d-glucopyranosyloxy-(S)-p-hydroxymandelonitrile) possess the same conformation at the α-carbon, while prunasin (β-d-glucopyranosyloxy-(R)-mandelonitrile) is the epimer of sambunigrin. Two components with the same two Rts were also obtained in the assays with UDP-Gal (Fig. 7A). The two glycosides were isolated by LC, subjected to acid hydrolysis, and the structure of the liberated sugar residue identified by silylation and gas chromatography-MS. The sugar residue liberated from both components was Glc and not Gal. Analysis of the UDP-Gal preparation used as a sugar donor documented UDP-Glc as a minor contaminant, and it is this UDP-Glc contamination and not UDP-Gal that gave rise to product formation. In full agreement with the modeling studies, the biochemical studies show that UDP-Glc is the only sugar donor utilized by UGT85B1. The kinetic parameters determined for UDP-Glc were Km = 0.8 ± 0.3 mm and kcat = 8.9 s−1.
Figure 6.
Specificity of UGT85B1 toward different radiolabeled UDP sugars as monitored by TLC. A, UDP-Glc; B, UDP-GlcUA; C, UDP-Gal; D, UDP-Xyl. The arrow shows origin of application.
Figure 7.
The ability of UGT85B1 to catalyze mandelonitrile-glucoside formation as verified by LC-MS and LC-MS/MS. A, Total ion trace of a reaction mixture containing UGT85B1, UDP-Glc, and mandelonitrile and either UDP-Glc (solid line) or UDP-Gal (dashed line). The compound eluting at Rt = 11.6 min is prunasin and the compound eluting at Rt = 12.3 min is sambunigrin. B, Extracted ion monitoring (m/z 318) of a reaction mixture containing UGT85B1, UDP-Glc, and mandelonitrile. C, Fragmentation of the m/z ion 318 (♦) corresponding to the mandelonitrile-glucoside-Na+ adduct. m/z 290.9 corresponds to mandelonitrile-glucoside lacking hydrogen cyanide. m/z 184.9 corresponds to the sugar moiety.
Biochemical Analysis of UGT85B1 Mutant Proteins
The second approach taken to study the predictive power of the UGT85B1 model was generation of mutant proteins in which selected single amino acid residues predicted to facilitate substrate binding or catalytic activity were modified. Residue substitution known from the literature (Hans et al., 2004) to result in loss of function was avoided, as loss-of-function mutations do not necessarily indicate the role of the residue in catalysis. According to the UGT85B1 model, the mutation S391A would prevent hydrogen bonding from this residue to the pyrophosphate part of UDP-Glc and hence result in less stable binding of the sugar donor. Likewise, the mutant protein R201A would expectedly be less efficient in stabilizing the pyrophosphate part of the nucleotide sugar donor throughout the glucosylation reaction. R201 is located in between loops B and C, and in UGTs this position is generally occupied either by K, R, or H residues (Fig. 3). According to the UGT85B1 model, the Glu residue in position 410 is essential for catalysis because it forms hydrogen bonds with both the sugar donor and acceptor molecules and hence enables contact between the two molecules. This interaction would be predicted to be abolished in an E410A mutant protein.
The validity of these predictions was tested biochemically using extracts of Escherichia coli cells expressing either UGT85B1 or one of the mutant proteins R201A, S391A, and E410A. The protein extracts were subjected to immunoblotting and probed with a specific antibody against UGT85B1 (Jones et al., 1999). The immunoblotting analysis confirmed that each of the mutant proteins produced a discrete protein band with the expected Mr. This enabled quantification of the amount of UGT85B1 or mutant protein present in each extract and demonstrated that the mutant proteins were not proteolytically degraded (data not shown). The ability of the mutant enzymes R201A, S391A, and E410A to glucosylate mandelonitrile was reduced approximately 20-, 185-, and 225-fold as compared to wild-type UGT85B1.
DISCUSSION
No crystal structure of a plant UGT is currently available. Efforts to obtain well-diffracting crystals of UGT85B1 were not successful partly because of the formation of oligomers and because of enzyme lability (P. Jones, L. Lo Leggio, S. Larsen, and B.L. Møller, unpublished data). Accordingly, homology modeling is the best approach currently available to obtain more detailed information on the molecular structure of UGT85B1, including identification of amino acid residues involved in substrate docking and catalysis. The increasing number of crystal structures that has been solved renders structure prediction of proteins with known or even unknown functions feasible. In this study, UGT85B1 was modeled based on the crystal structures of the A. orientalis family 1 GT because these are the best available candidates with a position-specific scoring matrix E-value of 2.89 × 10−3 and 2.78 × 10−14 for GtfA and GtfB, respectively. Our results document that molecular modeling is a highly valuable tool for retrieving information on topology, for identifying important structural features, such as amino acid residues involved in catalysis, and for predicting alternative sugar donors. As verified experimentally in biochemical analysis of wild-type and mutated UGT85B1, accurate predictions were obtained despite a sequence identity between UGT85B1 and the template molecules GtfA and GtfB of only approximately 15%. In comparative modeling, the pivotal point is to assign the correct structural fold and to choose an optimal template to construct the best possible alignment. Current comparative modeling methods lack sufficient computer power to recover from an incorrect alignment (Imberty et al., 1999; Marti-Renom et al., 2000). It has been stated that, at approximately 30% amino acid sequence identity, the fraction of incorrectly aligned residues is about 20% and that this number rises dramatically with further decrease in sequence identity (Sanchez and Sali, 1997). Noticeably, models with 2 Å accuracy have been built, although the amino acid sequence identity between target and template was below 15% (Koehl and Levitt, 1999). Additionally, alignments based on HCA have produced optimal alignment of sequences displaying identity below 10% (Gaboriaud et al., 1987).
In the recent study of UGT73A5, the betanidin 5-O-glucosyltransferase from D. bellidiformis, a model was built that contained 17 α-helices, 6 β-strands, and a number of loops. Several of the loops could not be modeled (Hans et al., 2004). In our model, we used HCA and the template molecules GtfA and GtfB to build a model for UGT85B1 with no stereochemical defaults or steric conflicts. Our modeling resulted in a structure composed of 14 α-helices, 12 β-strands, and 11 loops. Only difficulties in modeling loops A, B, C, and D would discredit the conclusions drawn from the final UGT85B1 model. The four loops excluded from our model are all located in the N-terminal region (Figs. 1 and 2). It has been proposed that acceptor specificity resides in the n-terminal region between β-sheet 5 and α-helix 5 (residues 152–242; Figs. 1 and 3; Hu and Walker, 2002). This region covers the excluded loops B and C in the UGT85B1 model (Fig. 1). Removal of loops A and D (residues 59–71 and 288–300, respectively) should not hamper conclusions based on our UGT85B1 model since they are distant from the active site (Fig. 1). Loop C (residues 203–211; Fig. 1) of the UGT85B1 model corresponds to a hypervariable region of GtfB that has poor or no electron density, indicating conformational flexibility (Mulichak et al., 2001). Although located in the region believed to influence acceptor selectivity, the lack of a proper template impeded building of this loop in the UGT85B1 model. Loop B (residues 156–188) is the largest of all removed loops in the UGT85B1 model. Loop B is also located in the region assigned to procure for selectivity as proposed on the basis of the three GT-B-fold GTs: MurG, GtfB, and BGT encompassing very different acceptor molecules (Hu and Walker, 2002). None of the three GTs mentioned above contain loop B and, in multiple alignments, the region corresponding to loop B in UGT85B1 is hypervariable (Figs. 1 and 3; Paquette et al., 2003). A full alignment calculated with one representative from all UGT families is available at http://www.P450.kvl.dk/Figure1_alignment.pdf. The lack of sequence conservation may reflect that loop B is under no evolutionary constraints and that this loop has no distinct biological role. Alternatively, loop B may be highly evolutionarily constrained and the lack of sequence similarity could reflect specific biological differences between UGTs. Loop B is enriched in hydrophobic amino acid residues and contains a hydrophobic patch (Figs. 1 and 3). Hydrophobic patches often constitute membrane association or protein interaction sites, and loop B of UGT85B1 may well turn out to serve an important function by mediating attachment of UGT85B1 to CYP79A1 and CYP71E1 to facilitate channeling in the dhurrin pathway by metabolon formation (Jørgensen et al., 2005; Kristensen et al., 2005; Nielsen and Møller, 2005).
GtfA and GtfB serve to catalyze decoration steps in the synthesis of the large heptapeptide vancomycin molecule (Mulichak et al., 2001), whereas UGT85B1 glucosylates a range of relatively small aglycones (Jones et al., 1999; Hansen et al., 2003). Despite the difference in the nature of the sugar acceptors utilized, geraniol and p-hydroxymandelonitrile could be fit into the substrate pocket of the UGT85B1 model (Fig. 3). However, the inaccuracy of the UGT85B1 model regarding the geometry of the active site around the aglycone (sugar acceptor) did not allow for discrimination of all the established nonsubstrates (e.g. farnesol, linalool, and α-terpineol; Hansen et al., 2003), demonstrating the problems in using structural information of GtfA and GtfB to comprehend the aglycone substrate specificity of plant family 1 UGTs like UGT85B1. This illustrates the general limitation of homology models based on highly divergent sequences and emphasizes the demand for plant family 1 GT crystal structures in order to obtain more knowledge on aglycone binding in the active site. Sugar donor specificity was predicted from the final UGT85B1 model and biochemical in vitro data correlated well with the model (Fig. 6). This emphasizes the fact that, despite only approximately 15% amino acid sequence identity between UGT85B1 and the template molecules, computer-based methods can create accurate models of the geometry of the active site and indicates that the UGT85B1 model reflects true properties of the enzyme. The high accuracy may reflect that GtfA, GtfB, and UGT85B1 all utilize nucleotide diphosphate sugars as donor molecules and utilize the same strategy for coordination of the donor sugar.
In this article, the main emphasis is on understanding sugar binding in the active site of UGT85B1 and subsequent UGT85B1-mediated sugar transfer. Plant GTs contain a highly conserved consensus sequence denoted as a putative secondary plant GT (PSPG) motif, which is an expansion of the PROSITE UGT consensus sequence (Hughes and Hughes, 1994; Paquette et al., 2003). The PROSITE UGT consensus and the PSPG motif are generally considered to represent the nucleotide diphosphate sugar-binding domain. The UGT85B1 model presented here enabled identification of amino acid residues important in catalysis and binding of sugar donors and acceptors in the active site. Three key amino acid residues, R201, S391, and E410, were predicted to interact primarily with the sugar donor in the substrate pocket of UGT85B1. S391 and E410 reside in the PSPG motif, whereas R201 is located between loops B and C (Figs. 1 and 3). These three residues were mutated to test how these point mutations would affect the activity of UGT85B1 to glucosylate p-hydroxymandelonitrile. The three mutant proteins, R201A, S391A, and E410A, showed approximately 20-, 185-, and 225-fold activity decreases, respectively, compared to wild-type UGT85B1. As shown experimentally, the E410A mutation resulted in the largest decrease in catalytic activity. This is in agreement with the model that predicts E410 to coordinate both the donor and the acceptor molecules. Interestingly, in a multiple alignment including one UGT of each subfamily from all phyla, the position occupied by E410 in UGT85B1 is consistently occupied by either E or D (Paquette et al., 2003), two amino acids that share similar physicochemical properties (full alignment available at http://www.p450.kvl.dk/UGT.shtml). Similarly, the position occupied by S391 in UGT85B1 is preferentially occupied with either Ser or Thr across phyla (Paquette et al., 2003). Mutagenesis of the conserved D residue to A in GtfB resulted in a 250-fold reduction in catalytic activity (Mulichak et al., 2001), underpinning the important role of E410 as predicted from the UGT85B1 model and as supported by the mutagenesis studies. In the modeling study of betanidin 5-O-glucosyltransferase (UGT73A5), residue E378 corresponding to E410 in UGT85B1 was similarly shown to interact with both sugar donors and acceptors and the corresponding mutant enzyme E378A was fully inactivated (Hans et al., 2004).
More and more crystal structures and protein folds are becoming available. Likewise, software modeling programs are being steadily improved. Hence, modeling accuracy will improve and molecular modeling will gain increased importance as a means to guide biologists in rational experimental design to elucidate biological questions and to engineer desired metabolic pathways. One such implication of this modeling study of UGT85B1 is the ability to predict and understand sugar donor specificity. Understanding of sugar donor specificity based on the geometry of the active site may pave the way for a rational design of mutant GTs with altered sugar donor specificity to engineer novel glycosides. Furthermore, the model implies a putative involvement of loop B in protein-protein interaction as a prerequisite for metabolon formation (Jørgensen et al., 2005). Future studies will test this hypothesis. Knowledge of the geometry of the active site and identification of key amino acid residues of UGT85B1 important for catalysis should facilitate identification of the corresponding residues in other plant UGTs and pave the way for design of mutant enzymes with new or altered catalytic activities with respect to aglycone and sugar donor specificity.
MATERIALS AND METHODS
Comparative Modeling of UGT85B1
α-Helix and β-strand distribution in the UGT85B1 sequence (GenBank accession no. AF199453) was predicted with several secondary-structure prediction servers (www.sbg.bio.ic.ac.uk/approximately 3dpssm; www.infobiogen.fr, www.npsa-pbil.ibcp.fr/cgi-bin). Information provided by HCA (Lemesle-Varloot et al., 1990) of UGT85B1 was compared to distribution of α-helices and β-strands in the crystal structures of GtfA and GtfB (Mulichak et al., 2001, 2003; protein data bank nos. 1PN3 and 1IIR, respectively; Berman et al., 2000). Alignments of GtfA, GtfB, and UGT85B1 to define structurally conserved regions were constructed manually and evaluated against a multiple alignment of GtfA, GtfB, GtfC, GtfD, and GtfE involved in vancomycin biosynthesis (Mulichak et al., 2001). Regions between two structurally conserved regions were defined as loops. UGT85B1 models were built with the homology-modeling program COMPOSER (Blundell et al., 1988) of the Sybyl software (SYBYL). High-resolution crystal structures of GtfA and GtfB were employed in building structurally conserved regions of the UGT85B1 model. Loops were subsequently modeled from a library of GT-B-fold GTs and, from a general nonredundant protein-fold database in COMPOSER. Four loops (A–D) could not be modeled and were not considered in the final model. The resulting models were analyzed with PROCHECK (Laskowski et al., 1993) to reveal backbone conformations that fell outside the allowed areas of the Ramachandran plot and demanded further optimization. Hydrogen atoms were added on all atoms of the UGT85B1 model and partial atomic charges were derived using the Pullman procedure.
Modeling UDP-Glc and Acceptor Molecules into the Active Site of UGT85B1
UDP-Glc was docked into the proposed active site of UGT85B1 in a conformation and location similar to that of TDP in the GtfA crystal structure (Mulichak et al., 2003). The acceptor molecules p-hydroxymandelonitrile and geraniol were docked manually into the predicted active site of UGT85B1. The primary criterion for acceptor molecule docking was to position the reactive hydroxyl group close to the reactive C-1 of UDP-Glc. Additionally, the position of the acceptor in the active site of the GtfA crystal structure was taken into consideration (Mulichak et al., 2003). Models for two enzyme-substrate complexes were generated: UGT85B1/UDP-Glc/p-hydroxymandelonitrile and UGT85B1/UDP-Glc/geraniol. In these complexes, several cycles of energy minimization were performed to optimize both the geometry of the ligands and the interacting protein side chains in the binding site and its vicinity. Energy calculations were performed using the TRIPOS force field (Clark et al., 1989) in the Sybyl package together with energy parameters derived for carbohydrates (Imberty et al., 1999) and for nucleotide sugars (Petrova et al., 1999).
Modeling Different UDP Sugars into the Substrate Pocket of UGT85B1
In addition to UDP-Glc, UDP-Gal, UDP-GlcUA, and UDP-Xyl were docked into the proposed binding site of the UGT85B1 model. These sugars were built in Sybyl and placed in the substrate pocket without energy minimization. The energy of the complexes was not minimized since sugars are often constrained in the active site; hence, minimization could result in nonfavorable conformations.
Determination of Kinetic Parameters for UDP-Glc and UGT85B1
Km and Vmax values for UDP-Glc were determined in assay mixtures (total volume, 20 μL) containing 100 mm Tris-HCl (pH 7.5), 5 mm mandelonitrile, 10 μg bovine serum albumin, approximately 14 ng purified heterologously expressed UGT85B1 protein (5), and UDP-Glc ranging from 0.1 to 1.5 mm [14C]-UDP-Glc. After incubation (10 min, 30°C, 400 rpm), the reaction was stopped (2 μL 10% acetic acid) and product formation determined by TLC (silica gel 60 F254 plates; Merck) developed in ethyl acetate:acetone:dichloromethane:methanol:water (20:15:6:5:4, v/v/v/v/v). Radiolabeled products were visualized using a STORM 840 PhosphorImager (Molecular Dynamics). Kinetic parameters were calculated with SigmaPlot (SPSS).
UDP Sugar Assay
Donor specificity of UGT85B1 was assessed using assay mixtures (total volume, 20 μL) containing 100 mm Tris HCl (pH 7.5), 5 mm mandelonitrile, 10 μg bovine serum albumin, approximately 14 ng isolated UGT85B1 protein (5), and 3.7 μm of either [14C]-UDP-Glc, [14C]-UDP-Gal, [14C]-UDP-GlcUA, or [14C]-UDP-Xyl. Product formation was analyzed as described above, except that the incubation period was prolonged (30 min, 30°C, 450 rpm).
LC-MS Analyses
Assays (total volume, 20 μL) were performed as outlined for the quantitative assays, except that 1.25 mm unlabeled UDP-Glc, UDP-Gal, UDP-GlcUA, or UDP-Xyl was included in each assay. Enzyme reaction was stopped by addition of 40 μL 85% MeOH and the supernatant obtained after centrifugation (10 min, 4°C, 10,000g) was subjected to LC-MS. Analytical LC-MS was carried out using an Agilent 1100 Series LC (Agilent Technologies) coupled to a Bruker Esquire 3000+ ion trap mass spectrometer (Bruker Daltonics). An XTerra MS C18 column (Waters; 3.5 μm, 2.1 × 100 mm) was used at a flow rate of 0.2 mL min−1. The mobile phases were as follows: A, 0.1% (v/v) formic acid and 50 mm NaCl; B, methanol. The gradient program was as follows: 0 to 2 min, isocratic 15% (v/v) methanol; 2 to 30 min, linear gradient 15% to 22% methanol; 30 to 35 min, linear gradient 22% to 100% (v/v) methanol; 35 to 40 min, isocratic 100% methanol. The mass spectrometer was run in positive ion mode. Total ion current and ion traces for specific [M + Na]+ adduct ions were used for locating compounds.
Construction and Analysis of Site-Directed Mutants
The mutants R201A, R201K, S391A, S391T, and E410A were constructed according to the manufacturer's instructions (Quick-Change; Stratagene). UGT85B1 cDNA inserted into the EcoRI site of pKK223-3 (Amersham Biosciences) served as template for PCR amplification. PCR for site-directed mutagenesis was performed with primers R201A-forward, 5′-ccgggatgagccacatgGCgctcagggacatgccg-3′; R201A-reverse, 5′-ggccctactgcgtgtacCGcgagtccctgtacggc-3′; R201K-forward, 5′-ccgggatgagccacatgAAgctcagggacatgccg-3′; R201K-reverse, 5′-ggccctactgcgtgtacTTcgagtccctgtacggc-3′; S391A-forward, 5′-cgcactgcggatggaacGccctgctggaggcgacggc-3′; S391A-reverse, 5′-gcgtgacgcctaccttgCgggacgacctccgctgccg-3′; S391T-forward, 5′-cgcactgcggatggaacAccctgctggaggcgacggc-3′; S391T-reverse, 5′-gcgtgacgcctaccttgTgggacgacctccgctgccg-3′; E410A-forward, 5′-ggccctgccacggggCacagaccaccaactgcaggc-3′; E410A-reverse, 5′-ccgggacggtgccccGtgtctggtggttgacgtccg-3′; E410D-forward, 5′-ggccctgccacggggaCcagaccaccaactgcaggc-3′; and E410D-reverse, 5′-ccgggacggtgcccctGgtctggtggttgacgtccg-3′. Thermocycling parameters were 95°C for 30 s, 16 cycles (95°C for 30 s, 55°C for 1 min, 68°C for 8 min). The PCR reaction products were digested with DpnI prior to transformation of competent Escherichia coli (XLIBlue or SURE) to remove templates. Plasmids were extracted from overnight cultures of transformed E. coli cells and verified by DNA sequencing (MWG Biotech). The density (OD600) of an overnight culture of E. coli transformed with the mutated plasmids was adjusted to 0.1, and protein synthesis induced by addition of 100 μm isopropylthio-β-galactoside followed by incubation for 3 h (37°C, 200 rpm). Cells were harvested by centrifugation and resuspended in ice-cold buffer (100 mm Tris-HCl, pH 7.5, 500 μm EDTA, 2.5 mm dithiothreitol, and 0.05 mg/mL lysozyme). After incubation (4°C, 45 min), cells were disrupted by three freeze-thaw cycles, 100 units of DNase, and 1 mm phenylmethylsulfonyl fluoride were added before centrifugation (15 min, 4°C, 15,000g). The soluble E. coli proteins present in the supernatant were used directly in subsequent enzyme activity experiments.
Immunoblot Analysis
Extracts of soluble E. coli wild-type and mutant proteins were subjected to immunoblot analysis to quantify and correlate the amount of UGT85B1 in the different preparations. Proteins separated by SDS-PAGE were transferred to nitrocellulose membranes and incubated with a UGT85B1 antibody (concentration 1:5,000) raised in rabbits (Jones et al., 1999). The intensity of cross-reaction between the primary antibody and different protein extracts was determined using a chemiluminescent detection system (ECL; BioRad). Quantitative estimation of the ability of the different mutant proteins to glucosylate mandelonitrile was carried out using assay mixtures (total volume, 20 μL) containing 1 mm UDP-Glc and E. coli-soluble protein extracts corresponding to approximately 20 ng of isolated UGT85B1 as estimated by immunoblotting. After incubation (30 min), TLC and product monitoring were carried out as described above.
Acknowledgments
We are grateful to Professor Søren Balling Engelsen, Department of Food Technology/Plant Food Science, Royal Veterinary and Agricultural University (RVAU) for efficient matchmaking between our research groups, to Professor Peter Ulvskov, Danish Institute of Agricultural Sciences, and to Professor Henrik Vibe Scheller, Department of Plant Biology, RVAU, for helpful discussions. We also thank Anne Vinther Rasmussen, Department of Plant Biology, RVAU, for critically reading the manuscript and Dr. P.R. Jones, Australian Wine Research Institute, Adelaide, for providing the UGT85B1 antibody.
This work was supported by a grant from the Danish National Research Foundation, by the Danish Veterinary and Agricultural Research Council, and by the Danish Research Council for Technology and Production.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.063842.
References
- Blundell T, Carney D, Gardner S, Hayes F, Howlin B, Hubbard T, Overington J, Singh DA, Sibanda BL, Sutcliffe M (1988) 18th Sir Hans Krebs lecture. Knowledge-based protein modelling and design. Eur J Biochem 172: 513–520 [DOI] [PubMed] [Google Scholar]
- Breton C, Heissigerova H, Jeanneau C, Moravcova J, Imberty A (2002) Comparative aspects of glycosyltransferases. Biochem Soc Symp 69: 23–32 [DOI] [PubMed] [Google Scholar]
- Clark M, Cramer RDI, van den Opdenbosch N (1989) Validation of the general purpose Tripos 5.2 force field. J Comput Chem 10: 982–1012 [Google Scholar]
- Combet C, Blanchet C, Geourjon C, Deleage G (2000) NPS@: network protein sequence analysis. Trends Biochem Sci 25: 147–150 [DOI] [PubMed] [Google Scholar]
- Coutinho PM, Deleury E, Davies GJ, Henrissat B (2003) An evolving hierarchical family classification for glycosyltransferases. J Mol Biol 328: 307–317 [DOI] [PubMed] [Google Scholar]
- Gaboriaud C, Bissery V, Benchetrit T, Mornon JP (1987) Hydrophobic cluster analysis: an efficient new way to compare and analyze amino acid sequences. FEBS Lett 224: 149–155 [DOI] [PubMed] [Google Scholar]
- Hans J, Brandt W, Vogt T (2004) Site-directed mutagenesis and protein 3D-homology modelling suggest a catalytic mechanism for UDP-glucose-dependent betanidin 5-O-glucosyltransferase from Dorotheanthus bellidiformis. Plant J 39: 9–33 [DOI] [PubMed] [Google Scholar]
- Hansen KS, Kristensen C, Tattersall DB, Jones PR, Bak S, Møller BL (2003) The in vitro substrate regiospecificity of recombinant UGT85B1, the cyanohydrin glucosyltransferase from Sorghum bicolor. Phytochemistry 64: 143–151 [DOI] [PubMed] [Google Scholar]
- Hu Y, Walker S (2002) Remarkable structural similarities between diverse glycosyltransferases. Chem Biol 9: 1287–1296 [DOI] [PubMed] [Google Scholar]
- Hughes J, Hughes MA (1994) Multiple secondary plant product UDP-glucose glucosyltransferase genes expressed in cassava (Manihot esculenta Crantz) cotyledons. DNA Seq 5: 41–49 [DOI] [PubMed] [Google Scholar]
- Imberty A, Bettler E, Karababa M, Mazeau K, Petrova P, Pérez S (1999) In M Vijayan, N Yathindra, and AS Kolaskar, eds, Perspectives in Structural Biology. Indian Academy of Sciences and Universities Press, Hyderabad, India, pp 392–409
- Jackson RG, Lim EK, Li Y, Kowalczyk M, Sandberg G, Hoggett J, Ashford DA, Bowles DJ (2001) Identification and biochemical characterization of an Arabidopsis indole-3-acetic acid glucosyltransferase. J Biol Chem 276: 4350–4356 [DOI] [PubMed] [Google Scholar]
- Jones P, Messner B, Nakajima J-I, Schäffner AR, Saito K (2003) UGT73C6 and UGT78D1, glycosyltransferases involved in flavonol glycoside biosynthesis in Arabidopsis thaliana. J Biol Chem 278: 43910–43918 [DOI] [PubMed] [Google Scholar]
- Jones PR, Møller BL, Høj PB (1999) The UDP-glucose:p-hydroxymandelonitrile-O-glucosyltransferase that catalyzes the last step in synthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor. Isolation, cloning, heterologous expression, and substrate specificity. J Biol Chem 274: 35483–35491 [DOI] [PubMed] [Google Scholar]
- Jørgensen K, Rasmussen AV, Morant M, Nielsen AH, Bjarnholt N, Zagrobelny M, Bak S, Møller BL (2005) Metabolon formation and metabolic channelling in the biosynthesis of plant natural products. Curr Opin Plant Biol 8: 280–291 [DOI] [PubMed] [Google Scholar]
- Koehl P, Levitt M (1999) A brighter future for protein structure prediction. Nat Struct Biol 6: 108–111 [DOI] [PubMed] [Google Scholar]
- Kristensen C, Morant M, Olsen CE, Ekstrom CT, Galbraith DW, Lindberg Møller B, Bak S (2005) Metabolic engineering of dhurrin in transgenic Arabidopsis plants with marginal inadvertent effects on the metabolome and transcriptome. Proc Natl Acad Sci USA 102: 1779–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26: 283–291 [Google Scholar]
- Leloir LF (1964) Nucleoside diphosphate sugars and saccharide synthesis. Biochem J 91: 1–8 [PubMed] [Google Scholar]
- Lemesle-Varloot L, Henrissat B, Gaboriaud C, Bissery V, Morgat A, Mornon JP (1990) Hydrophobic cluster analysis: procedures to derive structural and functional information from 2-D-representation of protein sequences. Biochimie 72: 555–574 [DOI] [PubMed] [Google Scholar]
- Lim EK, Baldauf S, Li Y, Elias L, Worrall D, Spencer SP, Jackson RG, Taguchi G, Ross J, Bowles DJ (2003. a) Evolution of substrate recognition across a multigene family of glycosyltransferases in Arabidopsis. Glycobiology 13: 139–145 [DOI] [PubMed] [Google Scholar]
- Lim EK, Doucet CJ, Li Y, Elias L, Worrall D, Spencer SP, Ross J, Bowles DJ (2001. a) The activity of Arabidopsis glycosyltransferases toward salicylic acid, 4-hydroxybenzoic acid, and other benzoates. J Biol Chem 277: 586–592 [DOI] [PubMed] [Google Scholar]
- Lim EK, Higgins GS, Li Y, Bowles DJ (2003. b) Regioselectivity of glucosylation of caffeic acid by a UDP-glucose:glucosyltransferase is maintained in planta. Biochem J 373: 987–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim EK, Li Y, Parr A, Jackson R, Ashford DA, Bowles DJ (2001. b) Identification of glucosyltransferase genes involved in sinapate metabolism and lignin synthesis in Arabidopsis. J Biol Chem 276: 4344–4349 [DOI] [PubMed] [Google Scholar]
- Mackenzie PI, Owens IS, Burchell B, Bock KW, Bairoch A, Belanger A, Fournel-Gigleux S, Green M, Hum DW, Iyanagi T, et al (1997) The UDP glycosyltransferase gene superfamily: recommended nomenclature update based on evolutionary divergence. Pharmacogenetics 7: 255–269 [DOI] [PubMed] [Google Scholar]
- Marti-Renom MA, Stuart AC, Fiser A, Sanchez R, Melo F, Sali A (2000) Comparative protein structure modeling of genes and genomes. Annu Rev Biophys Biomol Struct 29: 291–325 [DOI] [PubMed] [Google Scholar]
- Møller BL, Conn EE (1980) The biosynthesis of cyanogenic glucosides in higher plants. Channeling of intermediates in dhurrin biosynthesis by a microsomal system from Sorghum bicolor (linn) Moench. J Biol Chem 255: 3049–3056 [PubMed] [Google Scholar]
- Morera S, Lariviere L, Kurzeck J, Aschke-Sonnenborn U, Freemont PS, Janin J, Ruger W (2001) High resolution crystal structures of T4 phage beta-glucosyltransferase: induced fit and effect of substrate and metal binding. J Mol Biol 311: 569–577 [DOI] [PubMed] [Google Scholar]
- Mulichak AM, Losey HC, Lu W, Wawrzak Z, Walsh CT, Garavito RM (2003) Structure of the TDP-epi-vancosaminyltransferase GtfA from the chloroeremomycin biosynthetic pathway. Proc Natl Acad Sci USA 100: 9238–9243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulichak AM, Losey HC, Walsh CT, Garavito RM (2001) Structure of the UDP-glucosyltransferase GtfB that modifies the heptapeptide aglycone in the biosynthesis of vancomycin group antibiotics. Structure 9: 547–557 [DOI] [PubMed] [Google Scholar]
- Mulichak AM, Lu W, Losey H, Walsh CT, Garavito M (2004) Crystal structure of vancosaminyltransferase GtfD from the vancomycin biosynthetic pathway: interactions with acceptor and nucleotide ligands. Biochemistry 43: 5170–5180 [DOI] [PubMed] [Google Scholar]
- Nielsen KA, Møller BL (2005) Cytochrome P450s in plants. In PR Ortiz de Montellano, Cytochrome P450: Structure, Mechanism and Biochemistry, Ed 3. Kluwer Academic/Plenum Publishers, New York, pp 553–583
- Paquette S, Møller BL, Bak S (2003) On the origin of family 1 plant glycosyltransferases. Phytochemistry 62: 399–413 [DOI] [PubMed] [Google Scholar]
- Petrova P, Koca J, Imberty A (1999) Potential energy hypersurfaces of nucleotide sugars: ab initio calculations, force-field parametrization, and exploration of the flexibility. J Am Chem Soc 121: 5535–5547 [Google Scholar]
- Reay PF, Conn EE (1974) The purification and properties of a uridine diphosphate glucose: aldehyde cyanohydrin β-glucosyltransferase from sorghum seedlings. J Biol Chem 249: 5826–5830 [PubMed] [Google Scholar]
- Sanchez R, Sali A (1997) Advances in comparative protein-structure modelling. Curr Opin Struct Biol 7: 206–214 [DOI] [PubMed] [Google Scholar]
- Sawada S, Suzuki H, Ichimaida F, Yamaguchi M, Iwashita T, Fukui Y, Hemmi H, Nishino T, Nakayama T (2005) UDP-glucuronic acid: anthocyanin glucuronosyltransferase from red daisy (Bellis perennis) flowers: enzymology and phylogenetics of a novel glucuronosyltransferase involved in flower pigment biosynthesis. J Biol Chem 280: 899–906 [DOI] [PubMed] [Google Scholar]
- Taguchi G, Imura H, Maeda Y, Kodaira R, Hayashida N, Shimosaka M, Okazaki M (2000) Purification and characterization of UDP-glucose: hydroxycoumarin 7-O-glucosyltransferase, with broad substrate specificity from tobacco cultured cells. Plant Sci 157: 105–112 [DOI] [PubMed] [Google Scholar]
- Tattersall DB, Bak S, Jones PR, Olsen CE, Nielsen JK, Hansen ML, Høj PB, Møller BL (2001) Resistance to an herbivore through engineered cyanogenic glucoside synthesis. Science 293: 1826–1828 [DOI] [PubMed] [Google Scholar]
- Vogt T (2002) Substrate specificity and sequence analysis define a polyphyletic origin of betanidin 5- and 6-O-glucosyltransferase from Dorotheanthus bellidiformis. Planta 214: 492–495 [DOI] [PubMed] [Google Scholar]
- Vrielink A, Rüger W, Driessen HPC, Freemont PS (1994) Crystal structure of the DNA modifying enzyme beta-glucosyltransferase in the presence and absence of the substrate uridine diphosphoglucose. EMBO J 13: 3413–3422 [DOI] [PMC free article] [PubMed] [Google Scholar]