Abstract
Antibodies to La/SSB are detected in sera of patients with primary Sjogren's syndrome (pSS) and systemic lupus erythematosus (SLE). The vast majority of anti-La/SSB positive sera contain antibodies directed towards a linear B-cell epitope of La/SSB spanning the sequence 349–364aa (pep349–364). The aim of this study was to evaluate the fluctuation of antibody levels to major B-cell epitopes of La/SSB over time and investigate for their possible crossreactions. Sequential sera from 15 SLE and 15 pSS patients, followed from 3 to 10 years were obtained. All patients with SLE were positive for anti-Ro/SSA, anti-La/SSB and anti-dsDNA antibodies and patients with pSS were positive for anti-Ro/SSA and anti-La/SSB antibodies. Sera from 30 patients with SLE without anti-La/SSB antibodies and 30 healthy individuals served as disease and negative control respectivelly. All sera tested for the presence of anti-pep349–364 antibodies, using a specific ELISA. Specific anti-pep349–364 IgG was purified from sera of SLE patients and evaluated for cross reactivity against dsDNA and histones. In all SLE sera the levels of anti-pep349–364 antibodies varied in time and fluctuated in parallel with anti dsDNA antibodies. Anti-pep349–364 IgG purified from 7 SLE patients. Five out of 7 were found to react with calf thymus DNA in ELISA. All purified (7/7) anti-pep349–364 IgG preparations reacted with histone H1 and failed to produce a positive immunofluorescence pattern in Crithidia luciliae anti-dsDNA assay which lacks histones. Competative inhibition experiments demonstrated that histone H1 could inhibit completely the binding of anti-pep349–364 IgG to pep349–364 while pep349–364 inhibited by 70% the binding of anti-pep349–364 IgG to histone H1. These findings indicate that a subgroup of SLE patients possess cross-reacting anti-histone H1 antibodies and anti-pep349–364 antibodies, which can be faulty considered as anti-dsDNA reactivity in regular ELISA techniques.
Keywords: anti-La/ SSB, histones, Sjogren's syndrome, systemic lupus erythematosus, B-cell epitopes
Introduction
La/SSB autoantigen is a 48 kD protein that is localized predominantly in the nucleus. It is a member of the ribonucleoprotein particle Ro/RNP consisting of at least three proteins (La, Ro60, Ro52 kD) and one small RNA molecule [1]. Autoantibodies targeting La/SSB protein are often detected in patients with primary Sjogren's Syndrome (pSS) and Systemic Lupus Erythematosus (SLE).
In our previous studies of B-cell epitope mapping of La/SSB autoantigen, we found 4 epitopes, spanning the sequences 147–154 aa, 291–302 aa, 301–318 aa, 349–364 aa of La/SSB [2]. One of these epitopes, the 349–364 a.a. (pep349–364) presented high sensitivity and specificity for the detection of anti-La/SSB antibodies [3]. Furthermore, antibodies targeting the pep349–364 epitope can be detected in the majority of ANA(+) – anti-La/SSB precipitin negative sera, after blocking the anti-idiotypic antibodies with a complementary to the epitope peptide [4].
One of the aims of this study was to determine the fluctuation of antibody levels to major B-cell epitopes of La/SSB over time, in sequential sera from patient with pSS and SLE. This study revealed that in the majority of SLE patients the autoantibody levels to pep349–364 of La/SSB varied in parallel with antibodies to dsDNA. This prompted us to investigate further this association. Using specific inhibition experiments with affinity purified human autoantibodies, we found that autoantibodies to pep349–364 react strongly with histone H1. This cross-reactivity is resposible for the anti-dsDNA reactivity observed in sera of a subgroup of SLE patients.
Materials and methods
Human sera
Thirty patients with either pSS or SLE and anti-La/SSB antibodies were selected on the basis of the availability of sequential serum samples, covering a period from three to 10 years. The temporal interval between two sequential serum samples was at least six months. Fifteen patients had SLE while 15 patients had pSS. All patients fulfilled the diagnostic criteria for SLE and pSS [5,6]. Thirty sera from healthy individuals and 30 sera from patients with SLE or pSS without anti-La/SSB antibodies served as normal and disease controls, respectively. All sera were taken for diagnostic and research purposes with the full consent of the patients.
Sera were separated from whole blood from selected patients after centrifugation at 3000 g for 10 min and stored at −30°C until testing.
Synthetic peptides
The La/SSB epitopes 349–364 a.a. (GSGKGKVQFQGKK TKF) and 289–308 a.a. (ANNGNLQLRNKEVTWEVLEG) were purchased, as peptides in their N-acetylated/C-amide form, from Biosynthesis Co, Lewisville, USA. The peptides were purified by High Performance Liquid Chromatography (HPLC) and subjected to amino acid analysis and mass spectroscopy (MS) that confirmed their purity and identity. As control peptide the 250–257 a.a.region (IASRYDQL) from Leismania glycoprotein gp63 was used.
Recombinant La/SSB protein
La/SSB recombinant protein prepared from a La/SSB cDNA as previously described [7] and purified by poly(U)-Sepharose affinity chromatography [8].
Assays for the detection of anti-peptide antibodies
COSTAR high binding microtitre plates were coated overnight at 4 °C with 100 µl of the peptide solution at a concentration 5 µg/ml in phosphate buffer pH = 7·2.
The remaining binding sites were blocked with blocking buffer (BB) (BB: 2% bovine serum albumin, 0.1% Tween 20 in PBS) for 1 h at room temperature and were washed with PBS −0.05% Tween 20. Subsequently, sera of patients were added in dilution (1 : 100 in BB) and the plates were incubated overnight at 4 °C. This dilution was selected after the initial optimization experiments.
After 5 washes, goat anti-human IgG conjugated to alkaline phosphatase (1 : 3000 in BB) was added. The plates were incubated for 1 h at room temperature followed by washing and addition of 100 µl p-nitrophenol substrate at 37 °C. The optical density was evaluated at 405 nm after 20 min.
In order to normalize our OD readings between different ELISA plates, 3 common positive sera and 3 common normal sera were used in each plate. Experiments with OD coefficient variation more than 10% were repeated. All ODs were transformed and expressed as binding units according the formula:
where SampleOD is the OD reading of the current sample and PosCtrlOD is the mean OD of the 3 positive controls in the current ELISA plate. The cutoff value for anti-peptide ELISA was calculated as the mean normal sera Binding Units +3 standard deviations.
Assay for the detection of anti-dsDNA reactivity
Anti-dsDNA ELISA performed according to the method described by Tzioufas et al. [9]. COSTAR high binding microtitre plates were coated with 50 µl of Poly–L–lysine (50 µg/ml) (Sigma, St. Louis, Mo, USA) for 1 h at 37 °C. After washing with PBS, the plates were coated overnight at 4 °C with 50 µl of DNA (50 µg/ml) (Sigma). Then the plates were washed with PBS and incubated with 50 µl of S1-nuclease (50 IU/ml) (Promega, Madison, WI, USA) for 1 h at 37°C. After washing and blocking the remaining binding sites with 10% Bovine Serum (BS) for 1 h at room temperature, the plates were incubated overnight at 4 °C with patients’ sera (1 : 50 in BS) or with purified anti-pep349–364 IgG (5 µg/ml in BS). Afterwards, goat anti-human IgG conjugated to alkaline phosphatase (1 : 3000 in BB) was added. The plates were incubated for 1 h at room temperature followed by washing and addition of 100 µl p-nitrophenol substrate at 37°C. Absorbance of the colour was measured at 405 nm after 20 min.
Isolation and purification of IgG specific for pep349–364 of La/SSB
Specific immunoaffinity column of CNBr activated sepharose 4B was generated by standard methods, using 20 mg of the synthetic pep349–364. IgG from seven sera with high titre of anti-pep349–364 antibodies was purified by protein A Sepharose, concentrated and passed through the peptide immunoaffinity column. The column was washed with PBS and eluted with urea 8 M. The eluate was dialysed against PBS, concentrated at about 1 mg/ml and redialysed. Purified IgGs were stored at −30 °C, until testing. The initial serum volume was about 5 ml and the amount of purified anti-pep349–364 IgG was ranging from 0·5 to 0·7 mg/serum, as evaluated by Lowry assay (DC Protein Assay, Biorad, Hercules, CA, USA). The purification procedure led to approximately 24× increase in specific antibody activity per µg IgG.
Crithidia luciliae anti-dsDNA assay
Commercial Crithidia luciliae anti-dsDNA assay was used according to manufacture's instructions (INOVA Diagnonstics Inc, San Diego, CA, USA). Briefly, 50 µl of diluted sera (1 : 150 in PBS) or purified anti-pep349–364 antibodies (7·5 µg/ml in 2% bovine serum albumin/PBS) was added to Crithidia luciliae slides. After incubation for 30 min, the slides were washed and 50 µl of FITC/anti-IgG conjugate (INOVA Diagnonstics Inc) was added. Subsequently, after 30 min incubation, the slides were washed again and examined through a fluorescent microscope.
Assays for the detection of anti-histone H1 and anti-La/SSB antibodies
COSTAR high binding microtitre plates were coated overnight at 4 °C with 100 µl of histone H1 (10 µg/ml, SIGMA, St. Louis, USA) or recombinant La/SSB (2 µg/ml) in carbonate bicarbonate buffer pH = 9·3. In case of anti-histone H1 ELISA, the plates were incubated for 1 h at 37 °C with micrococcal nuclease (100 U/ml) (Amersham Biosciences Inc, New Jersey, USA) prior to the addition of blocking buffer, in order to remove any RNA or DNA contaminants.
The same experimental procedure was followed as previously described using anti-pep349–364 IgG (5 µg/ml in BB).
Inhibition assays
Inhibition of binding of anti-dsDNA positive sera to DNA using serial concentration of DNA or pep349–364 as inhibitors.
Increasing concentrations ranging from 0 to 10 µg/ml of S1 nuclease pretreated DNA or pep349–364 were mixed with human anti-dsDNA serial positive sera dilution. The mixtures were incubated for 3 h at room temperature before its application in wells coated with DNA. The procedure continued as for the anti-dsDNA ELISA.
Inhibition of binding of anti-dsDNA positive sera to pep349–364 using serial concentration of DNA as inhibitor
Increasing concentrations ranging from 0 to 10 µg/ml of S1 nuclease pretreated DNA preparation were mixed with human anti-dsDNA positive sera and the mixtures incubated for 3 h at room temperature before its application in ELISA plates coated with pep349–364. The procedure continued as for the anti-pep349–364 assay.
Inhibition of binding of anti-349–364 IgG to DNA using serial concentration of DNA as inhibitor
Several concentrations ranging from 0 to 10 µg/ml of S1 nuclease pretreated DNA were mixed with anti-pep349–364 IgG and the mixtures incubated to 3 h at room temperature before its application in wells coated with calf thymus DNA. The procedure continued as for anti-dsDNA ELISA.
Inhibition of binding of anti-pep349–364 IgG to histone H1 using histone H1 or pep349–364 as inhibitor
Serial concentrations of histone H1 or pep349–364 ranging from 0 to 10 µg/ml were mixed with anti-pep349–364 IgG and the mixtures were incubated for 3 h at room temperature, before its application in ELISA wells coated with histone H1. The procedure continued as for histones H1 antiantibody ELISA.
Inhibition of binding of anti-pep349–364 IgG to pep349–364 using histone H1 as inhibitor
Increasing concentrations of histone H1 ranging from 0 to 10 µg/ml were mixted with anti-pep349–364 IgG and the mixtures were incubated for 3 h at room temperature before its application in ELISA plates coated with pep349–364.The procedure continued as for anti-pep349–364 ELISA.
Results
Antibody response to epitope pep349–364 of La autoantigen
One hundred and fifty serial samples from 30 anti-La/SSB positive patients (15 with SS and 15 with SLE) were tested against pep349–364. Analysis of the data showed that: 85% of patients sera had antibodies against the peptide pep349–364(data not shown) and that the antibodies to pep349–364 appeared early in the disease course, exhibiting variations which did not correlated with the clinical picture of SLE patients, the clinical picture of SS patients, or the duration of the disease (Fig. 1).
Fig. 1.
Antibody responses against pep349–364 for 12 representative patients. The x-axis holds the sequential patient sera sorted by the collection time (e.g. D1 the first serum collected, D2 the second serum collected, etc.), while the z-axis represents the 12 different patients (P1 to P12). Patients P1-P4, P5-P8 and P9-P12 participate with 5 (D1-D5), 6 (D1-D6) and 7 (D1-D7) sequential sera, respectively. The antibody responses, appear early in the disease course, vary in time and could not be correlated with the duration of the disease.
The anti-pep349–364 antibody titre varies in parallel to anti-dsDNA titre over time
We evaluated whether the titre of antibodies against pep349–364 correlated with anti-dsDNA antibodies.
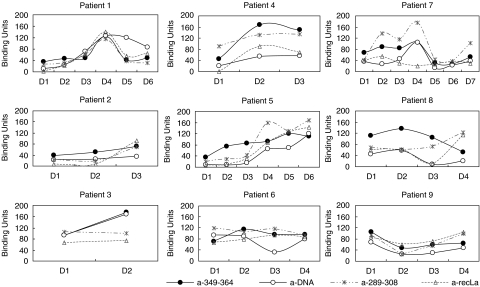
It was found that the titre of antibodies against pep349–364 of La/SSB varied in parallel with anti-dsDNA antibody titre in all 15 SLE sera tested, indicating an association between the two autoantibody specificities (Fig. 2). The titre of antibodies against epitope pep289–308 of La/SSB, however, followed the anti-recLa/SSB pattern but not the anti-dsDNA pattern, in the majority of sera tested (Fig. 2).
Fig. 2.
Combined antibody reactivity against pep349–364, pep289–308, recLa/SSB and dsDNA for 9 representative patients. The titre of antibodies against pep349–364 of La/SSB, varied in parallel with the titre of anti-dsDNA antibodies, in serial sera from 15 SLE patients. Data for 9 representative series of sera are presented in the Figure.
Cross-reactivity between anti-dsDNA and anti-pep349–364 antibodies
To clarify whether the apparent correlation between the titres of anti-pep349–364 and anti-dsDNA antibodies is due to cross recognition of the two autoantigens, specific inhibition experiments were performed.
These experiments revealed that anti-dsDNA serum activity could be inhibited by either dsDNA or pep349–364 peptide at percentages as high as 90% and 95%, respectively (at an inhibitor concentration of 5 µg/ml) (Fig. 3).
Fig. 3.
Inhibition experiments using SLE patient sera. Anti-dsDNA serum reactivity could be inhibited using (a) DNA as an inhibitor 90% and (b) pep349–364 as an inhibitor at 93%. Similarly, anti-pep349–364 serum reactivity could be inhibited using (a) DNA as an inhibitor at 68% and (b) pep349–364 as an inhibitor at 65%. The ODs without the inhibitor were in the range 1·7–2·0.
Similarly, the binding of autoantibodies to pep349–364 could be inhibited by either dsDNA or pep349–364 peptide with a maximum inhibition rate of 70% and 72%, respectively (at an inhibitor concentration of 5 µg/ml) (Fig. 3).
Cross-reactivity between anti-pep349–364 and anti-dsDNA is probably due to interactions with histones which are present in the dsDNA preparations
In order to study further the interaction of autoantibodies with pep349–364 and dsDNA antigens, we performed experiments, using affinity purified anti-pep349–364 autoantibodies.
All purified anti-pep349–364 antibodies exhibited high reactivity against pep349–364, as expected. On the contrary, purified anti-pep349–364 did not react with poly L-lysine used to coat DNA ELISA plates (data not shown).
Five of seven purified anti-pep349–364 IgG preparations, obtained from SLE patients, also recognized dsDNA, further supporting the cross-reaction hypothesis. None of the 3 normal IgG, used as control, reacted with either dsDNA or pep349–364 (Fig. 4).
Fig. 4.
Anti-dsDNA and anti-histone H1 reactivity of purified anti-pep349–364 antibodies. All purified anti-pep349–364 IgGs recognized histone H1 while five of seven IgGs recognized also the dsDNA preparation.
Inhibition experiments revealed that the binding of affinity purified anti-pep349–364 antibodies to dsDNA could be inhibited almost completely by calf thymus dsDNA (Fig. 5). The observed cross-recognition of dsDNA and pep349–364 of La/SSB is apparently peculiar since: (i) The two antigens are different in nature (peptide versus nucleic acid) and (ii) their physicochemical properties differ substantially; dsDNA is negatively charged while the peptide pep349–364 is positively charged. Taking into consideration that commercial dsDNA preparations contain approximately 3% histones and that histone H1 resembles physicochemically the pep349–364 (similar pI: pIpep349−364 = 10·60 versus pIhistoneH1 10·99, lysine content: Lyspep349−364 = 31·2%versus Lys histoneH1 = 26·6% and percentages of positively/negatively charged amino acids: pos/negpep349−364 = 31%/0%versus pos/neghistoneH1 = 28%/3%), we investigated whether histone H1 is indeed responsible for the observed cross-reactivity.
Fig. 5.
Homologous inhibition of the anti-dsDNA reactivity of purified anti-pep349–364 antibodies. Inhibition of binding of anti-349–364 IgG to dsDNA using dsDNA as inhibitor (0–10 µg/ml) (ODs without the inhibitor: 1·4–1·6).
All purified pep349–364 antibody preparations were tested and found to bind histone H1 on the ELISA plate (Fig. 4). Even the two anti-pep349–364 antibody preparations which did not recognized dsDNA in ELISA, reacted strongly with histone H1. Moreover, all purified anti-pep349–364 IgGs demonstrated higher reactivity against H1 histone, compared to dsDNA.
Purified anti-pep349–364 antibodies fail to react with dsDNA in a Crithidia luciliace immunofluorescence assay
Crithidia luciliace protozoa contain exclusively double-stranded kinetoplast DNA and lack histone molecules. Although some investigators claim that Crithidia actually possess some kinetoplast associated proteins with common to histone features, this microorganism is a good substrate to study the proposed histone-H1 mediated cross-reactions. Purified anti-pep349–364 antibodies failed to produce a positive immunofluorence pattern in Crithidia luciliace dsDNA assay, contrary to anti-dsDNA reference sera (Fig. 6). These results further support the suggested histone-contaminant recognition by anti-pep349–364 antibodies.
Fig. 6.
Immunofluerence pattern on Crithidia luciliace anti-dsDNA assay. (a, b) anti-dsDNA laboratory reference sera; (c) Normal IgG; (d–f) purified anti-pep349–364 antibodies.
Purified anti-pep349–364 antibodies specifically recognize histone H1
As all purified pep349–364 antibody preparations were found to bind histone H1 in ELISA, we sought to study the specificity of this reaction with inhibition experiments. Thus, the binding of purified anti-pep349–364 to histone H1 was inhibited up to 93% by histone H1 in homologous inhibition experiments (Fig. 7b). The cross reactivity between pep349–364 and histone H1 was apparently confirmed by heterologous inhibition experiments. In this regard, H1 histone was able to block the binding of anti-pep349–364 antibodies to pep349–364 at 70–90% in a concentration as low as 0·5 µg/ml (Fig. 7a).
Fig. 7.
Homologous and heterologous inhibition experiments using histone H1 as inhibitor. (a) the binding of anti-349–364 IgG to histone H1 almost completely inhibited (ODs without the inhibitor: 1·7–2·0) and (b) the binding of anti-pep349–364 IgG to pep349–364 inhibited at 70%– 90% (ODs without the inhibitor: 1·5–1·8).
The reaction between anti-pep346–364 antibodies and histone H1 was also inhibited by 30–70%, with soluble pep349–364 as inhibitor, at a concentration of 5 µg/ml (Fig. 8). Thus, a 10 fold higher concentration of soluble pep349–364 (as compared with histone H1) is required to achieve lower heterologous inhibition values in anti-pep349–364-histone H1 reaction. Therefore, purified anti-pep349–364 antibodies demonstrate a preferential interaction with histone H1 than the pep349–364.
Fig. 8.
Heterologous inhibition experiments using pep349–364 as inhibitor. The recognition of histone H1 by anti-pep349–364 IgG was inhibited at 30%-70% by soluble pep349–364 (ODs without the inhibitor: 1·7–2·0).
Discussion
One of the aims of this study was to investigate the relationship of the antibodies titres against B-cell epitope pep349–364 a.a. of the La/SSB autoantigen with the serological picture of patients, during the course of the disease. It was demonstrated that the antibody response against the epitope 349–364 a.a. of La/SSB varied over time and did not correlate with the clinical picture of patients. Both anti-pep349–364 and anti-pep289–308 antibodies were found to appear early in the disease course, which is in agreement with recent findings suggesting that in Lupus patients, anti-La antibodies develop up to nine years before the disease onset (mean = 3·3 years before) [10]. However, it was unexpectedly found that in Lupus sera anti-pep349–364 antibody titres fluctuated in parallel with anti-dsDNA antibodies, over the time. Specific inhibition experiments suggested that a true cross reactivity between anti-pep349–364 and anti-dsDNA antibodies indeed occur.
The supposed cross reaction between anti-pep349–364 and anti-dsDNA antibodies seems peculiar for at least two reasons: First, the physicochemical properties of pep349–364 a.a and dsDNA are extremely different, pep349–364 is highly positively charged, while dsDNA is highly negatively charged. Second, the synthetic peptide antigen lacks post-translational modifications, such as phosphorylation that could explain the observed cross-reaction (e.g. through the negatively charged phosphate groups). Calf thymus dsDNA preparations that widely used in dsDNA ELISA, contains approximately 3% histones. Purcell et al.[11] have demonstrated that even such low level contaminants might be responsible for the antigenic recognition of a given sample. Studying the physicochemical properties of histones, we found certain similarities between pep349–364 a.a, and H1 histone. All purified anti-pep349–364 IgGs recognized histone H1 and this recognition could be specifically inhibited by both pep349–364 and histone H1. On the other hand, the same antibodies gave almost no-reaction in a dsDNA immunofluorescence assay based on the protozoa Crithidia luciliace that lacks histone molecules, further supporting our contaminant (histone H1) recognition hypothesis.
Structurally, the histone core consists of H2A, H2B, H3 and H4 histone, while histone H1 is located near the DNA linker region, bridging neighbouring nucleosomes [12]. Because histone H1 is localized at the outer face of the nucleosome, it may be more accessible to antibodies than the remaining histones. Actually in SLE, histone H1 has been considered as an autoantigen and has been implicated as the major target protein accounting for the Lupus Erythematosus Cell (L.E.C) phenomenon [12–14]. Although anti-histone H1 antibodies are very common in SLE, they are also found in other systemic autoimmune diseases, including SS and RA [15]. The presence of high antibody levels to histone H1 in SLE patients with a positive LEC phenomenon indicates serologically and clinically active disease with major organ involvement, particularly glomerulonephritis.
Despite the fact that anti-La/SSB and anti-histone antibodies cross recognize common antigenic structures, it is inquisitive that anti-La/SSB antibodies are not commonly found in SLE sera. Thus, our observation is valid only for a limited proportion of SLE sera, which contain anti-La/SSB antibodies. On the other hand, it is possible that the presence of anti-La/SSB antibodies in SLE is underestimated. This assumption is supported by two observations. First, it is has been observed that the detection of anti-La/SSB antibodies with CIE underestimates their prevalence, since a significant proportion of anti-La/SSB antibodies are nonprecipitating in CIE [16]. Secondly, the vast majority of ANA positive–anti-La negative SLE sera (that most likely possess also anti-dsDNA or/and anti-histone antibodies) may contain antibodies to pep349–364 of La/SSB, which cannot be detected by conventional CIE, as they are masked by anti-idiotypic antibodies [4].
Our observation that the dsDNA used in ELISA technique may lead to false positive results, emphasize the need for re-evaluation the assays used for anti-dsDNA antibody detection. In this regard, sera containing multiple anti-nuclear antibody specificities should be evaluated by immunofluorescence, using as substrate the protozoa Crithidia luciliae[17]. This method displays high specificity in the identification of anti-dsDNA antibodies. Most importantly, the kinetoplast DNA (kDNA) of Crithidia luciliae does not contain histones nor single stranded DNA [17,18]. In our experiments Crithidia luciliae kDNA gave almost no reaction with the purified anti-pep349–364 antibodies unlike the reaction observed in the classical dsDNA ELISA.
Taken together, our findings show that antibodies to the La/SSB B-cell major autoepitope react with histone H1. This cross-reaction may account for the anti-dsDNA reactivity observed in a subgroup of SLE patients, when commercially available dsDNA is used in the ELISA technique.
Acknowledgments
The authors would like to thank Professor Haralampos M. Moutsopoulos for a thorough review of the manuscript and helpful suggestions and Katerina Theofillopoulou for performing the Crithidia luciliae immunofluorescence assays.
References
- 1.Pruijn GJ, Simons FH, van Venrooij WJ. Intracellular localization and nucleocytoplasmic transport of Ro RNP components. Eur J Cell Biol. 1997;74:123–32. [PubMed] [Google Scholar]
- 2.Tzioufas AG, Yiannaki E, Sakarellos-Daitsiotis M, Routsias JG, Sakarellos C, Moutsopoulos HM. Fine specificity of autoantibodies to La/SSB. epitope mapping, and characterization. Clin Exp Immunol. 1997;108:191–8. doi: 10.1046/j.1365-2249.1997.d01-1003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yiannaki EE, Tzioufas AG, Bachmann M, et al. The value of synthetic linear epitope analogues of La/SSB for the detection of autoantibodies to La/SSB; specificity, sensitivity and comparison of methods. Clin Exp Immunol. 1998;112:152–8. doi: 10.1046/j.1365-2249.1998.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Routsias JG, Touloupi E, Dotsika E, et al. Unmasking the anti-La/SSB response in sera from patients with Sjogren's syndrome by specific blocking of anti-idiotypic antibodies to La/SSB antigenic determinants. Mol Med. 2002;8:293–305. [PMC free article] [PubMed] [Google Scholar]
- 5.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 6.Vitali C, Bombardieri S, Moutsopoulos HM, et al. Preliminary criteria for the classification of Sjogren's syndrome. Results of a prospective concerted action supported by the European Community. Arthritis Rheum. 1993;36:340–7. doi: 10.1002/art.1780360309. [DOI] [PubMed] [Google Scholar]
- 7.Troster H, Metzger TE, Semsei I, et al. One gene, two transcripts: isolation of an alternative transcript encoding for the autoantigen La/SS-B from a cDNA library of a patient with primary Sjogrens' syndrome. J Exp Med. 1994;180:2059–67. doi: 10.1084/jem.180.6.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stefano JE. Purified lupus antigen La recognizes an oligouridylate stretch common to the 3′ termini of RNA polymerase III transcripts. Cell. 1984;36:145–54. doi: 10.1016/0092-8674(84)90083-7. [DOI] [PubMed] [Google Scholar]
- 9.Tzioufas AG, Manoussakis MN, Drosos AA, Silis G, Gharavi AE, Moutsopoulos HM. Enzyme immunoassays for the detection of IgG and IgM anti-dsDNA antibodies: clinical significance and specificity. Clin Exp Rheumatol. 1987;5:247–53. [PubMed] [Google Scholar]
- 10.Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Medical. 2003;349:1526–33. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 11.Purcell AW, Chen W, Ede NJ, et al. Avoidance of self-reactivity results in skewed CTL responses to rare components of synthetic immunogens. J Immunol. 1998;160:1085–90. [PubMed] [Google Scholar]
- 12.Darnel J, editor. Molecular Cell Biology. 3. New York: Scientific American Books Inc.; 1998. [Google Scholar]
- 13.Schett G, Rubin RL, Steiner G, Hiesberger H, Muller S, Smolen J. The lupus erythematosus cell phenomenon: comparative analysis of antichromatin antibody specificity in lupus erythematosus cell-positive and – negative sera. Arthritis Rheum. 2000;43:420–8. doi: 10.1002/1529-0131(200002)43:2<420::AID-ANR24>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 14.Schett G, Steiner G, Smolen JS. Nuclear antigen histone H1 is primarily involved in lupus erythematosus cell formation. Arthritis Rheum. 1998;41:1446–55. doi: 10.1002/1529-0131(199808)41:8<1446::AID-ART15>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 15.Gripenberg M, Helve T, Kurki P. Profiles of antibodies to histones, DNA and IgG in patients with systemic rheumatic diseases determined by ELISA. J Rheumatol. 1985;12:934–9. [PubMed] [Google Scholar]
- 16.Beer RG, Rischmueller M, Coates T, et al. Nonprecipitating anti-La (SS-B) autoantibodies in primary Sjogren's syndrome. Clin Immunol Immunopathol. 1996;79:314–8. doi: 10.1006/clin.1996.0084. [DOI] [PubMed] [Google Scholar]
- 17.Aarden LA, de Groot ER, Feltkamp TE. Immunology of DNA. III Crithidia luciliae, a simple substrate for the determination of anti-dsDNA with the immunofluorescence techniquE. Ann NY Acad Sci. 1975;254:505–15. doi: 10.1111/j.1749-6632.1975.tb29197.x. [DOI] [PubMed] [Google Scholar]
- 18.Crowe W, Kushner I. An immunofluorescent method using Crithidia luciliae to detect antibodies to double-stranded DNA. Arthritis Rheum. 1977;20:811–4. doi: 10.1002/art.1780200308. [DOI] [PubMed] [Google Scholar]