Abstract
The present study was performed to evaluate the ability of fosfomycin, a broad-spectrum antibiotic, to penetrate into abscess fluid. Twelve patients scheduled for surgical or computer tomography-guided abscess drainage received a single intravenous dose of 8 g of fosfomycin. The fosfomycin concentrations in plasma over time and in pus upon drainage were determined. A pharmacokinetic model was developed to estimate the concentration-time profile of fosfomycin in pus. Individual fosfomycin concentrations in abscess fluid at drainage varied substantially, ranging from below the limit of detection up to 168 mg/liter. The fosfomycin concentrations in pus of the study population correlated neither with plasma levels nor with the individual ratios of abscess surface area to volume. This finding was attributed to highly variable abscess permeability. The average concentration in pus was calculated to be 182 ± 64 mg/liter at steady state, exceeding the MIC50/90s of several bacterial species which are commonly involved in abscess formation, such as streptococci, staphylococci, and Escherichia coli. Hereby, the exceptionally long mean half-life of fosfomycin of 32 ± 39 h in abscess fluid may favor its antimicrobial effect because fosfomycin exerts time-dependent killing. After an initial loading dose of 10 to 12 g, fosfomycin should be administered at doses of 8 g three times per day to reach sufficient concentrations in abscess fluid and plasma. Applying this dosing regimen, fosfomycin levels in abscess fluid are expected to be effective after multiple doses in most patients.
Extensive research in the field of abscess treatment has established a claim for invasive drainage as the most efficient means of resolving suppurative lesions (7, 13, 30, 31). In particular, computer tomography-guided percutaneous abscess drainage has repeatedly been reported to be advantageous (31) compared to other invasive methods (13). However, application of the percutaneous interventional method is subject to some limitations. Coagulation abnormalities are considered a contraindication (7), and the absence of a safe anatomic access route, the presence of fistulas, severe inflammation of organs, or patients with advanced age have likewise been linked to low success rates of percutaneous abscess drainage (13, 30). From these considerations, it becomes evident that percutaneous and surgical abscess drainage alone will not entail satisfactory results in a number of patients suffering from abscess-related disease (30).
On the other hand, there is a substantial record of the benefit of antibiotic administration before (19), during (30), and after (32) drainage. Moreover, abscesses of different locations and origins have been efficiently cured with systemic anti-infective chemotherapy alone (12, 23, 29, 35). Factors challenging the antibiotic treatment of suppurative lesions include hard penetrability of the fibrous abscess capsule or degradation of the antibiotic by bacteria (5). Adverse physicochemical conditions, such as high protein binding, anaerobic environment, and low pH, as well as the high variety of pathogens in abscess fluid are claimed responsible for the failure of parenteral antibiotics to eradicate bacteria within suppurative lesions (3, 10, 16, 20). Therefore, it is eminent for the overall outcome of patients to select an appropriate antibiotic for abscess treatment. Substances considered suitable are characterized by high antibacterial activity against pathogens most commonly isolated from suppurative lesions and by high concentrations in abscess fluid.
Fosfomycin is a broad-spectrum antibiotic with a wide therapeutic range (14) and characteristic pharmacological properties. Fosfomycin penetrates excellently into various tissues (9, 14, 18, 21) and cerebrospinal fluid (14, 27) and is frequently administered in combination with other antimicrobial agents in Europe to combat severe bacterial infections. It exerts bactericidal activity under anaerobic conditions (17), such as is the case within encapsulated purulent lesions, and has a negligible protein binding. These characteristics constituted the rationale for choosing fosfomycin in the present study (6, 14).
Thus, the present explorative study was performed to evaluate whether fosfomycin penetrates into suppurative lesions reaching sufficient concentrations to eradicate clinically relevant bacteria in abscess fluid. In addition, we tested the hypothesis of a correlation between the Aa/Va and drug concentration in the abscess fluid (5).
MATERIALS AND METHODS
Abbreviations.
The following abbreviations are used in the text or tables; a subscript “a” or “p” indicates that an abbreviation refers to the abscess fluid or to the plasma compartment, respectively: Cp, Ca, fosfomycin concentration in plasma and in abscess fluid, respectively; Cav(ss), average concentration at steady state; PK, pharmacokinetic(s); LOD, limit of detection; V, volume of distribution; Vss, volume of distribution at steady state; AUC, total area under the concentration-time curve; AUC0-6, area under the concentration-time curve from 0 to 6 h from the start of infusion (6 h represents the study observation period); AUC0-8, area under the concentration-time curve from 0 to 8 h from the start of infusion (8 h represents the recommended dosing interval); AUC0-12, total area under the concentration-time curve from 0 to 12 h from the start of infusion (12 h represents the frequently applied dosing interval); t1/2β, terminal half-life; Tmax, time to maximum concentration (after start of infusion); Cmax, maximum concentration; CL, fosfomycin clearance; CLCR, creatinine clearance; ti, time point of abscess incision (after start of infusion); Ca(ti), fosfomycin concentration in abscess fluid at the time of incision; Va, abscess volume; Aa, abscess surface area; Aa/Va, ratio of the abscess surface area to volume; P, permeability coefficient of the abscess membrane for fosfomycin; p, P value used to describe statistical significance (used for the result of the runs test); τ, dosing interval.
Study protocol.
The study protocol was approved by the ethics committee of the Medical University of Vienna and was conducted in accordance with the Declaration of Helsinki (36) in its last revised version, the guidelines for Good Clinical Practice of the European Union (8), and the Austrian drug law. All patients were informed in detail about the purpose, procedures, and risks of this prospective study. Prior to inclusion, an informed consent was signed by all patients. Concomitant therapy was not changed due to study procedures.
Patients.
The study protocol designated the inclusion of 12 patients with documented abscess formation scheduled for surgical or computer tomography-guided drainage and a serum creatinine concentration below 1.5 mg/dl. This was based on the assumption that a number of 12 patients is sufficient to allow for an estimation of the extent of fosfomycin penetration into abscess fluid. The patients' demographic data are presented in Table 1.
TABLE 1.
Individual and mean demographic data of patients (n = 12)
| Sexb | Age (yr) | Wt (kg) | Ht (cm) | CLCR (ml · min−1) | Abscess
|
||
|---|---|---|---|---|---|---|---|
| Location | Aa (cm2) | Va (cm3) | |||||
| M | 43 | 60 | 172 | 106 | Abdominal | 543 | 1,165 |
| F | 70 | 80 | 162 | 57 | Abdominal | 98 | 91 |
| M | 37 | 80 | 186 | 151 | Perianal | 22 | 10 |
| M | 31 | 50 | 185 | 123 | Pancreas | 189 | 212 |
| M | 42 | 92 | 186 | 151 | Perianal | 66 | 44 |
| M | 39 | 117 | 192 | 170 | Perianal | 2.5 | 0.3 |
| F | 56 | 80 | 156 | 74 | Beside aortic prosthesis | 250 | 365 |
| F | 63 | 50 | 165 | 46 | Subcutaneously at the back | 329 | 295 |
| F | 57 | 69 | 160 | 128 | a, pancreas; b, abdominala | a, 34; b, 25 | a, 18; b, 10 |
| M | 81 | 90 | 187 | 64 | Psoas muscle | 217 | 228 |
| F | 46 | 80 | 167 | 90 | Abdominal | 233 | 189 |
| F | 34 | 76 | 164 | 77 | Mandibular | 8.7 | 2.1 |
| Mean ± SD | 50 ± 16 | 78 ± 18 | 174 ± 13 | 103 ± 41 | 155 ± 160c | 202 ± 315d | |
One patient had two abscesses, referred to as “a” and “b.”
M, male; F, female.
Median, 98 cm2.
Median, 91 cm3.
Study design.
The fosfomycin concentrations in abscess fluid can be measured only at one time point in each patient, i.e., upon abscess drainage. To estimate the concentration-versus-time profile of fosfomycin in abscess fluid, two approaches were initially taken into consideration for PK analysis. First, abscess drainage and pus sampling were performed at different and predefined time points in the study patients, each contributing to an overall PK profile of fosfomycin in pus and referred to below as “study population PK approach.”
More importantly, a second approach was developed which allows for the simulation of the concentration-versus-time profiles of fosfomycin in the abscess fluid from individual patients (see below) based on single-point measurements.
The study drug administration was started at predefined time points, i.e., 15, 45, 60, 90, 120, 240, and 360 min before the planned time of drainage. Eight grams of fosfomycin (Sandoz, Kundl, Austria) was dissolved in 200 ml Aqua bidestillata (Aqua bidest. Fresenius; Fresenius Pharma, Graz, Austria) and administered intravenously as a single dose over a period of approximately 30 min. Pus samples for the measurement of fosfomycin concentrations were collected upon drainage.
To determine concentrations of fosfomycin in plasma, blood samples were drawn via an intravenous catheter at defined intervals (baseline sample and 15, 30, 45, 60, 75, 105, 135, 180, 240, 300, and 360 min after the start of fosfomycin infusion) and at the time of abscess fluid sampling. Plasma was obtained by centrifugation (Rotanta; Hettich, Bäch, Switzerland) of blood at 1,360 × g per min for 10 min. Plasma and pus samples were stored at −80°C until analysis.
Determination of fosfomycin.
Fosfomycin concentrations in plasma and pus samples were determined by a capillary zone electrophoresis method recently described (25, 26). In brief, proteins and cell debris of both plasma and pus samples were removed by methanol precipitation. After centrifugation, an aliquot of the supernatant was analyzed with a 3DCE apparatus (Agilent Technologies, Palo Alto, CA). Separation was achieved with an extended light path fused silica capillary (length, 64 cm; inner diameter, 50 μm) at −25 kV. Analyses were performed in a background electrolyte consisting of 25 mM benzoic acid and 0.5 mM cetyltrimethylammonium bromide to reverse the electro-osmotic flow. The pH was adjusted with Tris to 6.95 for plasma samples and 7.25 for pus samples. Indirect UV detection was carried out with a photodiode array detector operating at 254 nm.
The reference specimens (blank pus and plasma) used for method validation were pooled from patients who were not on fosfomycin therapy. The accuracy and precision of the method was determined by analyzing plain plasma or pus samples spiked at three different fosfomycin concentrations (10, 100, and 300 mg/liter) in triplicate on three different days. Accuracy was between 90.6 and 101.2% for plasma and 75 to 102% for pus samples. Precision was better than 12% relative standard deviation. The calibration line was constructed by external calibration with the aid of the spiked samples. Based on a signal-to-noise ratio of 3, the achieved LODs were 0.86 and 4.5 mg/liter for plasma and pus samples, respectively. The higher LODs in pus than in plasma are attributed to considerably higher noise levels owing to the inhomogeneity of pus samples. However, the LODs were considered sufficiently sensitive for the present study because the MIC50/90s of clinically relevant bacterial species were reported to range between 12 and 128 mg/liter (14, 22, 24).
Determination of abscess surface area and volume.
The imaging of the abscesses was done either with computed tomography (n = 8) (Somatom Sensation 16; Siemens, Erlangen, Germany), magnetic resonance tomography (n = 2) (Magnetom Open Viva; Siemens, Erlangen, Germany), or ultrasound (n = 5) (HDI 5000; Koninklijke Philips Electronics, Eindhoven, The Netherlands). For abscesses of globular or ellipsoid configuration, the measurements were performed as follows. On computed and magnetic resonance tomography images, the largest diameter, a (x axis), of the abscess was determined by using multiplanar reconstructions. Then another set of multiplanar reconstructions was created perpendicular to the x axis, and the largest diameter, b, in the y axis was measured. The z axis was measured perpendicular to the y axis in the same plane, and the largest diameter, c, was determined.
Using the ultrasound imaging method, the largest diameter (x axis) was evaluated and measured directly during the examination. Then the abscess was imaged in the plane perpendicular to the x axis, and the largest diameters of the y axis and, consecutively, of the z axis were imaged and measured. The volume of an abscess was calculated with the ellipsoid volume formula (Va = 4/3 · π · a · b · c). The surface area was calculated with the ellipsoid surface area approximation formula derived from a scientific website (http://home.att.net/∼numericana/answer/ellipsoid.htm) with Aa ≈ 4π[(apbp + apcp + bpcp)/3]1/p, where p is 1.605 and the relative error is 1.061% for the worst case scenario.
For irregularly configured abscesses, the calculations were performed by using a commercially available tool (Leonardo Workstation; Siemens) for the analysis of computer tomography images.
Pharmacokinetic analysis.
Pharmacokinetic analysis was based on the following assumptions. (i) The PK of fosfomycin in plasma can be described by a two-compartment model (14). (ii) Abscess fluid represents a third, hardly accessible compartment which slowly equilibrates with plasma. (iii) Abscess fluid is regarded as a homogenous compartment, neglecting possible fosfomycin concentration gradients within pus; the degradation of fosfomycin in abscess fluid is not taken into consideration. (iv) Equilibration and elimination processes between compartments follow first-order kinetics, and there are no active transport mechanisms for fosfomycin between compartments. (v) Drug flow from plasma to abscess fluid or vice versa is directly proportional to (a) the surface area of the abscess, (b) the drug concentration gradient between these compartments, and (c) the permeability of the abscess capsule for the drug (4). (vi) The PK of fosfomycin in plasma is not significantly influenced by the drug amounts penetrating to or from abscess fluid.
To describe abscess fluid pharmacokinetics of fosfomycin, the differential equation established by Barza and Cuchural (5) was applied:
 |
(1) |
Cp(t) was obtained by fitting a linear two-compartment pharmacokinetic model for intravenous administration to the measured plasma concentration data of fosfomycin by using
 |
(2) |
and
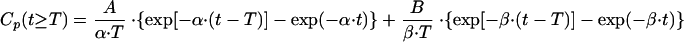 |
(3) |
where T is the duration of infusion and A, B, α, and β are the corresponding macro constants. The parameters A, B, α, and β were estimated by fitting the pharmacokinetic model to the plasma concentration data of fosfomycin. The weighted residual sum of squares (WRSS) was calculated as
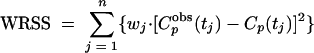 |
(4) |
and was minimized (method of nonlinear least squares) using the lsqnonlin function of the Optimization Toolbox of MATLAB (Mathworks, Natick, MA). Cpobs(tj) is the observed plasma concentration of fosfomycin at time point tj, and Cp(tj) is the predicted plasma concentration of fosfomycin at time point tj. n is the number of observed concentrations per patient. The weighting factor, wj, was set to 1/[Cpobs(tj)]2. Goodness-of-fit was assessed by visual inspection of plots of observed and predicted plasma concentrations, by looking at the correlation between observed and predicted concentrations, and finally, by the randomness of the residuals (runs test). By use of the macro constants A, B, α, and β, the values of the parameters V, Vss, AUCp, t1/2β,p, and CL were calculated by standard equations.
From each patient, only one pus sample was available for the measurement of the fosfomycin concentration. The differential equation of the model for fosfomycin with an initial concentration of zero Ca(0) = 0 (equation 1) was solved by means of the Symbolic Math Toolbox function of MATLAB. The P of each abscess was estimated by adjusting the symbolic solution of equation 1 by direct search to the fosfomycin concentration Ca(ti) measured in the abscess fluid at the time of abscess drainage. For one patient, the individual plasma PK was not available. For this particular patient, the mean plasma PK data derived from the remaining patients were applied for solving equation 1.
The AUC of fosfomycin for abscess fluid was estimated by numerical integration of Ca(t) using the quad function of MATLAB (adaptive Simpson method), and the t1/2β in abscess fluid was evaluated by fitting a monoexponential function, C · e(−β · t), at the terminal slope of Ca(t). The time of the maximum fosfomycin concentration in abscess fluid was calculated by determining the zeros of dCa(t)/dt by means of the fzero function of MATLAB. The mean concentration in abscess fluid at steady state [Cav(ss),a] was calculated as
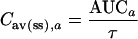 |
(5) |
Simulation of multiple dosing.
To assess the fosfomycin concentrations that may be achieved in abscess fluid after multiple dosing of fosfomycin, equation 1 was solved symbolically for Ca(t) for each τ of 8 h. Hereby, Cp(t) was set to the time course of the drug concentrations in plasma in a two-compartment, linear pharmacokinetic model with short-time infusion (30 min) and multiple dosing, using the PK parameters estimated from fosfomycin plasma data. The respective initial condition for equation 1 at the start of each dosing interval, Ca(t = nτ), was set to the already calculated abscess concentration at the end of the preceding dosing interval. PK profiles in plasma and abscess fluid are depicted for the patients with the lowest, median, and highest maximum concentrations of fosfomycin in abscess fluid (Cmax,a). Hereby, only patients with a Cmax,a above the LOD were considered.
Creatinine clearance.
The CLCR for male patients was estimated by the formula of Cockcroft and Gault [(140 − age) × weight]/[72 × Ccreatinine in plasma] (reviewed in reference 34). For female patients, this value was multiplied by 0.85.
Statistics.
The Spearman coefficient of correlation was applied for analysis of the relationship between PK parameters. Statistical calculations were performed using the SPSS 11.5.1 software, a p value of <0.05 was considered significant. Values are presented as means ± standard deviations.
RESULTS
The present study was carried out between February 2003 and May 2004 to determine fosfomycin concentrations in abscess fluid from 12 patients. Six study patients had to be replaced, owing to inadequate availability of purulent material due to admixture of blood to pus or small quantity or absence of pus. One of the patients had two abscesses which were handled separately. Fosfomycin was well tolerated, and no serious adverse event occurred.
After a single intravenous dose of 8 g of fosfomycin, interventional abscess drainage was performed at time points ranging from 17 to 220 min after the start of infusion. For most patients, the time period between fosfomycin infusion and abscess drainage deviated from those defined in the study protocol because the performance of surgical or computer tomography-guided drainage was brought forward or delayed in dependence on the working capacities available or other unexpected events that occur in routine clinical patient care.
The fitted plasma PK profiles are shown in Fig. 1. The correlation between observed and predicted plasma concentrations was high (r2 > 0.98). Plots of the residuals versus time showed a random scatter around the predicted concentrations. Randomness was confirmed by a runs test (p = 0.69 ± 0.21).
FIG. 1.
Individual fosfomycin concentrations in plasma (open circles) and abscess fluid (solid circles) after a single dose of 8 g. Solid lines indicate fitted concentration-time curves for plasma. Dotted lines show simulated abscess fluid concentration-time curves obtained from the solutions to equation 1 after adjustment to the respective data. Plasma PK results were available for 11 patients. For abscesses, results for 10 abscesses were used because the Ca(ti)s were below the LOD for 3 abscesses.
The observed fosfomycin concentrations in abscess fluid varied substantially between individuals (Fig. 1), ranging from below the LOD up to 168 mg/liter. When presenting all individual abscess concentrations of fosfomycin in a single plot, a sawtooth time-versus-concentration profile is obtained (Fig. 1), even if accounting for individual Aa, Va, and plasma PK (data not shown). Therefore, this study population PK approach was considered inadequate to obtain useful information, and the concentration-versus-time curves of fosfomycin in abscess fluid were calculated and simulated for the individual patients by use of the single-point measurements (Fig. 1). The PK parameters calculated for plasma and abscess fluid are presented in Tables 2 and 3, respectively. Hereby, it turned out that the values computed for P had, by far, the highest variability among the presented parameters (see ranges in Table 3).
TABLE 2.
Fosfomycin in plasma after a single intravenous dose of 8 g (n = 11a)
| Parameter | Mean ± SD | Median | Minimum | Maximum |
|---|---|---|---|---|
| Tmax,p (h) | 0.47 ± 0.12 | 0.47 | 0.3 | 0.75 |
| Cmax,p (mg · liter−1) | 446 ± 128 | 425 | 252 | 692 |
| t1/2β,p (h) | 3.7 ± 2.2b | 3.1 | 1.0 | 8.4 |
| CL (ml · min−1) | 126 ± 68b | 95 | 23 | 245 |
| V (l) | 15.5 ± 4.5b | 15.9 | 8.9 | 23.6 |
| Vss (l) | 28.6 ± 9.9b | 25.1 | 19.0 | 46.4 |
| AUCp (mg · h · liter−1) | 1,330 ± 609b | 1,428 | 544 | 2,188 |
| AUC0-6,p (mg · h · liter−1) | 918 ± 333b | 943 | 533 | 1,476 |
| AUC0-8,p (mg · h · liter−1) | 1,029 ± 398b | 1,057 | 542 | 1,687 |
| AUC0-12,p (mg · h · liter−1) | 1,162 ± 484b | 1,209 | 544 | 1,940 |
For one patient, the plasma PK data were incomplete.
Parameter presented only for patients whose terminal slope included at least three data points (n = 8).
TABLE 3.
Fosfomycin in abscess fluid after a single intravenous dose of 8 g (n = 13)a
| Parameter | Mean ± SD | Median | Minimum | Maximum |
|---|---|---|---|---|
| Aa/Va ratio (cm−1) | 2.1 ± 2.1 | 1.2 | 0.5 | 8.3 |
| ti (h) | 1.9 ± 1.1 | 1.7 | 0.3 | 3.7 |
| Ca (ti) (mg · liter−1) | 43 ± 57 | 12 | <LOD | 168 |
| P (cm · s−1) | 2.1 × 10−4 ± 3.6 × 10−4 | 7.0 × 10−5 | 2.0 × 10−9 | 1.3 × 10−3 |
| Tmax,a (h) | 10.5 ± 7.8b | 9.3 | 2.1 | 51.2 |
| Cmax,a (mg · liter−1) | 64 ± 67b | 27 | 12 | 171 |
| t1/2β,a (h) | 31.7 ± 39.4b | 21.8 | 2.8 | 108.2 |
| AUCa (mg · h · liter−1) | 1,458 ± 515b | 1,425 | 686 | 5,736 |
| AUC0-6,a (mg · h · liter−1) | 284 ± 330b | 98 | 18 | 787 |
| AUC0-8,a (mg · h · liter−1) | 368 ± 400b | 148 | 30 | 993 |
| AUC0-12,a (mg · h · liter−1) | 493 ± 464b | 248 | 60 | 1,359 |
| Cav(ss),a (mg · liter−1) | 182 ± 64b | 178 | 86 | 273 |
One patient had two abscesses.
In three pus samples, the Ca(ti) was less than the LOD. Parameters are presented only for patients for whom the Ca(ti) was greater than the LOD and whose terminal slope included at least three data points (n = 6).
In the study population, there was no evidence of a relationship between Cmax,a and the ratio of Aa/Va, nor between AUC0-6,a and the ratio of Aa/Va (data not shown). Further analysis, including a correction of PK data according to individual body weight, did not show any relationship between relevant plasma and abscess PK parameters (data not shown). In contrast, there was a good correlation between the values computed for P and Cmax,a (r = 0.96, p < 0.01) and for P and AUC0-6/8/12,a(r > 0.9; p < 0.01).
To evaluate how fosfomycin concentrations in abscess fluid may change after administration of multiple doses, a computer simulation of abscess PK was performed using a τ of 8 h. Figure 2 shows these simulations for the three patients with the lowest, the median and the highest Cmax,a.
FIG. 2.
Simulated drug concentrations in plasma (solid lines) and abscess fluid (dotted lines) for three patients after multiple intravenous administrations of 8 g fosfomycin over 30 min and a dosing interval of 8 h. The patients with the minimum (a), median (b), and the maximum (c) Cmax,a were chosen. The three patients with a Ca(ti) below the LOD were not considered. The horizontal dotted line at 32 mg/liter represents a common MIC50 of relevant bacterial pathogens.
DISCUSSION
Risks linked to interventional abscess drainage have increased the importance of anti-infective chemotherapeutics in bridging patients to more stable conditions. Fosfomycin was chosen for this study due to its excellent tissue penetration properties, low plasma protein binding, and increased bactericidal activity under anaerobic conditions (9, 14, 15, 18, 21). The study was performed in a representative patient population, with high variability in body weight and abscess size and origin, to evaluate the ability of fosfomycin to penetrate into abscess fluid.
A key finding of the present study was that no relationship was detected between relevant plasma and abscess PK parameters (i.e., AUC0-6/8/12, Cmax). However, significant correlations were observed between calculated P and Cmax,a as well as between P and AUC0-6/8/12,a in abscess fluid. This is not surprising considering that fosfomycin concentrations in pus are dependent on P, the ratio of Aa/Va, and Cp(t) in the applied model. While, Ca(ti), Aa/Va, and Cp(t) were directly measured, P was estimated from these parameters. As P turned out to be the parameter with the highest variability, ranging from 2.0 × 10−9 to 1.3 × 10−3 cm · s−1, it surpassed the other variables regarding impact on fosfomycin concentrations in pus. It needs to be mentioned here that the PK profiles of fosfomycin in abscess fluid were simulated on the basis of single-point measurements. Thus, the data calculated by use of the present simulation need to be verified in subsequent clinical studies.
Nevertheless, the present study proved that there is a high interindividual variability in the penetrability of abscesses and showed that permeability can be considered the parameter most strongly influencing the PK of fosfomycin in abscess fluid. Morphological or anatomical barriers of the abscess can be considered particularly important in the drug penetration process. Since abscess morphology is best described as consisting of an outer collagen wall, an inner layer of leukocytes, and a central area of necrotic debris (3), the thickness of the abscess wall, the viscosity of the bodily fluids inside and outside the capsule, and the coefficient of diffusion are considered highly eminent (5). Also, the constitution of the abscess wall and, consequently, its permeability are believed to vary dependent on the duration of infection and the stage of encapsulation (11). Finally, the degree of vascularization of the surrounding tissue interferes with the penetration of a drug from plasma into abscess fluid and vice versa. Considering these numerous factors, it becomes evident that there is little chance of predicting fosfomycin abscess penetration from the information which is available to the physician in a medical routine setting.
To assess the clinical impact of the highly variable levels of fosfomycin in abscess fluid, the concentrations in pus should be related to the MIC50/90s, which have been reported to range from 12 to 128 mg/liter for relevant bacterial species (14, 22, 24). Given an 8-g dosing regimen and a dosing interval of 8 h, the Cav(ss),a was calculated to be 182 ± 64 mg/liter (Table 3). Thus, there is circumstantial evidence that concentrations above relevant MICs can be achieved in abscess fluid at steady state. However, antimicrobially effective levels of fosfomycin in pus may be reached with a significant or critical delay in abscesses with low P. On the other hand, the extensively prolonged half-life of fosfomycin with sustained concentrations in pus will be favorable in some patients, as fosfomycin exerts time-dependent bacterial killing (15).
In an attempt to overcome the penetration barriers of abscesses, high levels in plasma should be attained because the gradient from Cp to Ca is the driving force and the only influenceable factor in increasing absolute drug transport from plasma to pus. According to standard PK equations, a Cav(ss) of fosfomycin of 150 mg · liter−1 may be reached in plasma and most likely also in abscess fluid by administration of an initial loading dose of 10 to 12 g and a maintaining dose of about 8 g at intervals of 8 h. However, in patients with a CLCR below 40 ml · min−1, the maintenance dose should be reduced in accordance with the manufacturer's recommendations while keeping the loading dose unaffected. The exceptionally long half-life of fosfomycin in abscess fluid of 32 ± 39 h implicates minimal fluctuations of fosfomycin concentrations in pus, suggesting that continuous infusion would not be advantageous compared to intermittent dosing regimens from the microbiologic point of view and with respect to the patient's comfort.
Finally, it is of interest to note that abscess development usually involves distinct bacteria such as staphylococci, streptococci, and Escherichia coli. These bacteria frequently cause purulent infections and are largely susceptible to fosfomycin (24). In contrast, Bacteroides fragilis, casually causative for intra-abdominal infections, and other anaerobic bacteria display natural resistance to fosfomycin (24). Thus, it may be advisable to combine fosfomycin with other antimicrobial agents (24) active against anaerobic bacteria. Since therapeutic levels of fluoroquinolones and beta-lactams have also been detected in abscess fluid (1, 2, 28, 33, 37) and synergistic effects of these drugs with fosfomycin (14) were reported, they also appear eligible for the combined administration.
In summary, there is a very high interindividual variability in the PK of fosfomycin in pus due to highly variable abscess permeability. In most patients, the concentration of fosfomycin in abscess fluid is expected to exceed the MIC50/90s of several relevant bacteria after multiple doses. An initial loading dose of 10 to 12 g and a maintenance dose of approximately 8 g fosfomycin three times per day appear advisable to achieve antimicrobially active concentrations in plasma and abscess fluid. However, the validity and robustness of the applied model to estimate the concentration-versus-time profiles in abscess fluid should be verified by additional clinical studies using multiple doses.
Acknowledgments
We are indebted to our study nurse, Petra Zeleny, and to Heimo Lagler and Thomas Scheuba, all from the Medical University of Vienna, for their contributions to this study.
REFERENCES
- 1.Akimoto, Y., Y. Mochizuki, A. Uda, H. Omata, J. Shibutani, H. Nishimura, M. Komiya, K. Kaneko, and A. Fujii. 1994. Amoxicillin concentration in pus from abscess caused by odontogenic infection. Gen. Pharmacol. 25:111-113. [DOI] [PubMed] [Google Scholar]
- 2.Akimoto, Y., H. Nishimura, H. Omata, J. Shibutani, K. Kaneko, T. Kawana, T. Kaneda, H. Yamamoto, and A. Fujii. 1996. Cefaclor concentration in pus from abscess caused by odontogenic infection after a single oral administration. Gen. Pharmacol. 27:177-179. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett, J. G. 1984. Experimental aspects of intraabdominal abscess. Am. J. Med. 76:91-98. [DOI] [PubMed] [Google Scholar]
- 4.Barza, M., J. Brusch, W. G. Bergeron, and L. Weinstein. 1974. Penetration of antibiotics into fibrin loci in vivo. 3. Intermittent vs. continuous infusion and the effect of probenecid. J. Infect. Dis. 129:73-78. [DOI] [PubMed] [Google Scholar]
- 5.Barza, M., and G. Cuchural. 1985. General principles of antibiotic tissue penetration. J. Antimicrob. Chemother. 15:59-75. [DOI] [PubMed] [Google Scholar]
- 6.Bothe, H. W., and W. Paschen. 1986. Regional morphology and biochemistry in experimental brain abscesses. Acta Neuropathol. 69:17-22. [DOI] [PubMed] [Google Scholar]
- 7.Drolsum, A., and A. Skjennald. 1994. Percutaneous drainage of abdominal abscesses. Tidsskr. Nor. Laegeforen. 114:3327-3330. [PubMed] [Google Scholar]
- 8.European Agency for the Evaluation of Medicinal Products. 2002. Guideline for good clinical practice. ICH topic E6. [Online.] http://www.emea.eu.int/pdfs/human/ich/013595en.pdf.
- 9.Frossard, M., C. Joukhadar, B. M. Erovic, P. Dittrich, P. E. Mrass, M. Van Houte, H. Burgmann, A. Georgopoulos, and M. Muller. 2000. Distribution and antimicrobial activity of fosfomycin in the interstitial fluid of human soft tissues. Antimicrob. Agents Chemother. 44:2728-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galandiuk, S., J. Lamos, W. Montgomery, S. Young, and H. C. Polk, Jr. 1995. Antibiotic penetration of experimental intra-abdominal abscesses. Am. Surg. 61:521-525. [PubMed] [Google Scholar]
- 11.Gerding, D. N., A. J. Kozak, L. R. Peterson, and W. H. Hall. 1980. Failure of single doses of cefazolin and cefamandole to penetrate experimental chronic Escherichia coli abdominal abscesses. Antimicrob. Agents Chemother. 17:1023-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geronimo-Pardo, M., F. Mateos-Rodriguez, J. J. Blanch-Sancho, and E. Martinez-Alfaro. 2002. Dual dosage individualization of vancomycin therapy to treat Staphylococcus epidermidis intraperitoneal vascular paraprosthetic abscesses. Int. J. Antimicrob. Agents 19:435-437. [DOI] [PubMed] [Google Scholar]
- 13.Giangreco, L., S. Di Palo, M. Castrucci, E. Angeli, and C. Staudacher. 1997. Abdominal abscesses: their treatment and the study of prognostic factors. Minerva Chir. 52:369-376. [PubMed] [Google Scholar]
- 14.Gobernado, M. 2003. Fosfomycin. Rev. Esp. Quimioter. 16:15-40. [PubMed] [Google Scholar]
- 15.Grif, K., M. P. Dierich, K. Pfaller, P. A. Miglioli, and F. Allerberger. 2001. In vitro activity of fosfomycin in combination with various antistaphylococcal substances. J. Antimicrob. Chemother. 48:209-217. [DOI] [PubMed] [Google Scholar]
- 16.Hays, R. C., and G. L. Mandell. 1974. PO2, pH, and redox potential of experimental abscesses. Proc. Soc. Exp. Biol. Med. 147:29-30. [DOI] [PubMed] [Google Scholar]
- 17.Inouye, S., T. Watanabe, T. Tsuruoka, and I. Kitasato. 1989. An increase in the antimicrobial activity in vitro of fosfomycin under anaerobic conditions. J. Antimicrob. Chemother. 24:657-666. [DOI] [PubMed] [Google Scholar]
- 18.Joukhadar, C., N. Klein, P. Dittrich, M. Zeitlinger, A. Geppert, K. Skhirtladze, M. Frossard, G. Heinz, and M. Muller. 2003. Target site penetration of fosfomycin in critically ill patients. J. Antimicrob. Chemother. 51:1247-1252. [DOI] [PubMed] [Google Scholar]
- 19.Kaushik, S. P., R. Vohra, G. R. Verma, S. Kaushik, and A. Sabharwal. 1984. Pancreatic abscess: a review of seventeen cases. Br. J. Surg. 71:141-143. [DOI] [PubMed] [Google Scholar]
- 20.Kunin, C. M. 1970. Binding of antibiotics to tissue homogenates. J. Infect. Dis. 121:55-64. [DOI] [PubMed] [Google Scholar]
- 21.Legat, F. J., A. Maier, P. Dittrich, P. Zenahlik, T. Kern, S. Nuhsbaumer, M. Frossard, W. Salmhofer, H. Kerl, and M. Muller. 2003. Penetration of fosfomycin into inflammatory lesions in patients with cellulitis or diabetic foot syndrome. Antimicrob. Agents Chemother. 47:371-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchese, A., L. Gualco, E. A. Debbia, G. C. Schito, and A. M. Schito. 2003. In vitro activity of fosfomycin against gram-negative urinary pathogens and the biological cost of fosfomycin resistance. Int. J. Antimicrob. Agents 22(Suppl. 2):53-59. [DOI] [PubMed] [Google Scholar]
- 23.Matoba, M., H. Tonami, M. Kuginuki, I. Yamamoto, and S. Takashima. 2004. Intermittent hepatic artery antibiotic infusion therapy for pyogenic hepatic abscess. Acta Radiol. 45:13-17. [DOI] [PubMed] [Google Scholar]
- 24.Patel, S. S., J. A. Balfour, and H. M. Bryson. 1997. Fosfomycin tromethamine. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy as a single-dose oral treatment for acute uncomplicated lower urinary tract infections. Drugs 53:637-656. [DOI] [PubMed] [Google Scholar]
- 25.Petsch, M., B. X. Mayer-Helm, R. Sauermann, C. Joukhadar, and E. Kenndler. 2004. Capillary electrophoresis analysis of fosfomycin in biological fluids for clinical pharmacokinetic studies. Electrophoresis 25:2292-2298. [DOI] [PubMed] [Google Scholar]
- 26.Petsch, M., B. X. Mayer-Helm, R. Sauermann, C. Joukhadar, and E. Kenndler. 2005. Determination of fosfomycin in pus by capillary zone electrophoresis. J. Chromatogr. A 1081:55-59. [DOI] [PubMed] [Google Scholar]
- 27.Pfausler, B., H. Spiss, R. Beer, A. Kampl, K. Engelhardt, M. Schober, and E. Schmutzhard. 2003. Treatment of staphylococcal ventriculitis associated with external cerebrospinal fluid drains: a prospective randomized trial of intravenous compared with intraventricular vancomycin therapy. J. Neurosurg. 98:1040-1044. [DOI] [PubMed] [Google Scholar]
- 28.Sawyer, R. G., R. B. Adams, and T. L. Pruett. 1994. Aztreonam vs. gentamicin in experimental peritonitis and intra-abdominal abscess formation. Am. Surg. 60:849-853. [PubMed] [Google Scholar]
- 29.Siddiq, F., A. Chowfin, R. Tight, A. E. Sahmoun, and R. A. Smego, Jr. 2004. Medical vs. surgical management of spinal epidural abscess. Arch. Intern. Med. 164:2409-2412. [DOI] [PubMed] [Google Scholar]
- 30.Sirinek, K. R. 2000. Diagnosis and treatment of intra-abdominal abscesses. Surg. Infect. (Larchmont) 1:31-38. [DOI] [PubMed] [Google Scholar]
- 31.Snyder, S. K., and H. H. Hahn. 1982. Diagnosis and treatment of intra-abdominal abscess in critically ill patients. Surg. Clin. N. Am. 62:229-239. [DOI] [PubMed] [Google Scholar]
- 32.Stearne, L. E., S. L. Buijk, J. W. Mouton, and I. C. Gyssens. 2002. Effect of a single percutaneous abscess drainage puncture and imipenem therapy, alone or in combination, in treatment of mixed-infection abscesses in mice. Antimicrob. Agents Chemother. 46:3712-3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tudor, R. G., D. J. Youngs, K. Yoshioka, D. W. Burdon, and M. R. Keighley. 1988. A comparison of the penetration of two quinolones into intra-abdominal abscess. Arch. Surg. 123:1487-1490. [DOI] [PubMed] [Google Scholar]
- 34.Verhave, J. C., C. P. Balje-Volkers, H. L. Hillege, D. de Zeeuw, and P. E. de Jong. 2003. The reliability of different formulae to predict creatinine clearance. J. Intern. Med. 253:563-573. [DOI] [PubMed] [Google Scholar]
- 35.Wessling, H., and P. de las Heras. 2003. Cervicothoracolumbar spinal epidural abscess with tetraparesis. Good recovery after non-surgical treatment with antibiotics and dexamethasone. Case report and review of the literature. Neurocirugia (Asturias) 14:529-533. [DOI] [PubMed] [Google Scholar]
- 36.World Medical Association. 6 October 2002, posting date. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. [Online.] http://www.wma.net/e/policy/b3.htm.
- 37.Youngs, D. J., D. W. Burdon, and M. R. Keighley. 1989. The penetration of aztreonam, a monobactam antibiotic, into intra-abdominal abscesses. J. Antimicrob. Chemother. 24:425-429. [DOI] [PubMed] [Google Scholar]




