Abstract
Tyrosine phosphorylation of FAK (focal adhesion kinase) regulates signalling that results from the interaction of integrins with extracellular matrix and growth factor receptors. A critical step in this process is the phosphorylation of Tyr397 of FAK, which creates a binding site for Src family kinases, PI3K (phosphoinositide 3-kinase) and Shc (Src homology and collagen homology). An intact Tyr397 site is required for FAK-mediated regulation of cell migration, survival signals and full responsiveness to soluble growth factors. We showed previously that the adaptor protein paxillin is required for the overall tyrosine phosphorylation of FAK in embryonic stem cells [Wade, Bohl and Vande Pol (2002) Oncogene 21, 96–107]. In the present paper, we identify the minimal structural features of paxillin that are required to support overall FAK tyrosine phosphorylation and Tyr397 phosphorylation. Paxillin contains N-terminal leucine-rich LD motifs that bind directly to FAK and four LIM (Lin-11, Isl-1 and Mec-3) domains in the C-terminus. We show that paxillin LIM domains 1, 2 and 3 are each required for FAK tyrosine phosphorylation, while LIM4 is dispensable. In addition to paxillin LIM domains 1, 2 and 3, a single LD motif on paxillin is required to support FAK tyrosine phosphorylation in embryonic stem cells. Both sequence and spatial requirements exist for LD motifs to support FAK tyrosine phosphorylation. Interestingly, synthetic LD motifs that fail to bind FAK in vitro are able to fully support FAK tyrosine phosphorylation, indicating that minimal interactions of LD motifs with FAK suffice. Our results demonstrate at least four distinct structural domains of paxillin support at least three distinct functions that are each required for FAK tyrosine phosphorylation.
Keywords: cytoskeleton, focal adhesion kinase (FAK), growth factor receptor, LD motif and LIM domain, paxillin, tyrosine phosphorylation
Abbreviations: ARF-GAP, ADP-ribosylation factor GTPase-activating protein; ECM, extracellular matrix; ES, embryonic stem; FAK, focal adhesion kinase; FAT, focal adhesion targeting; FBS, foetal bovine serum; GST, glutathione S-transferase; LIM, Lin-11, Isl-1 and Mec-3; MEF, mouse embryonic fibroblast; NP40, Nonidet P40; PI3K, phosphoinositide 3-kinase; PTP, protein tyrosine phosphatase; SH2, Src homology 2; Shc, Src homology and collagen homology
INTRODUCTION
Integrins are heterodimeric cell surface receptors for ECM (extracellular matrix) proteins. Signalling from integrins is required for cell attachment, spreading and migration on ECM-coated surfaces, as well as normal organism development [1,2]. Since the intracellular domain of most integrin subunits is generally less than 50 amino acids in length and lacks known enzymatic activity, the integrins initiate signalling cascades through association with intracellular proteins [3]. ECM-bound integrins cluster together in the plane of the membrane and recruit actin-structuring proteins and signalling molecules to form a connection between the extracellular matrix and a complex of intracellular cytoskeletal components called a focal adhesion [4]. The focal adhesion provides a site where the cell contacts the ECM and actin can be anchored to the plasma membrane. Focal adhesions allow a cell to spread on to a surface, and the co-ordinated formation and disruption of the focal adhesions is required for cell motility [5].
Regulation of signalling from the focal adhesions involves many different molecules including adaptors, kinases, phosphatases and G-proteins [6,7]. FAK (focal adhesion kinase) was first described as a prominently tyrosine-phosphorylated band in v-src transformed cells [8]. FAK localizes to focal adhesions and is tyrosine-phosphorylated upon integrin attachment to ECM-coated surfaces and growth factor receptor stimulation (reviewed in [9]). FAK is composed of three domains: an N-terminal FERM (protein 4.1/ezrin/radixin/moesin) domain, a central catalytic tyrosine kinase domain and a C-domain which includes the FAT (focal adhesion targeting) domain that is necessary for FAK localization and interaction with paxillin and talin [9,10]. In addition to FAK's tyrosine kinase activity, FAK can function as a scaffolding protein by associating with many cellular proteins including Src, p130Cas (Crk-associated substrate), PI3K (phosphoinositide 3-kinase), Shc (Src homology and collagen homology), paxillin and talin [9,10]. FAK binds to paxillin's leucine-rich peptides LD2 and LD4 using the PBS1 and PBS2 (paxillin-binding sequences 1 and 2) located within the FAT domain of FAK [11,12]. The FAT domain of FAK is composed of four α-helix regions that create hydrophobic pockets between helices 1 and 4 as well as helices 2 and 3, where LD motifs 2 and 4 of paxillin bind [11–13]. Mutation of both of the paxillin-binding sequences of FAK is necessary to disrupt paxillin association with FAK [11,12]. Mutational analysis of paxillin indicates that FAK associates with LD2 and LD4 with similar affinity, with co-operative binding if both the LD2 and LD4 motifs are intact; mutation of both LD2 and LD4 is required to abrogate FAK binding to paxillin in vitro [11,12].
FAK contains six tyrosine residues (Tyr397, Tyr407, Tyr576, Tyr577, Tyr861 and Tyr925) that are phosphorylated in response to diverse stimuli [9,14]. The tyrosine at position 397 is an autophosphorylation site that, when phosphorylated, creates a binding site for the Src SH2 (Src homology 2) domain [14,15]. It has been postulated that recruitment of c-src to phosphorylated Tyr397 of FAK results in the tyrosine phosphorylation of the remaining sites on FAK [16]. In addition to Src, other known binding partners for phosphorylated Tyr397 of FAK include the adaptor protein Shc and the p85 subunit of PI3K [16,17]. Reconstitution of FAK−/− fibroblasts with mutants of FAK showed that an intact Tyr397 of FAK was required to restore FAK phenotypes of motility, migration and cell spreading [18,19]. In the C-terminus of FAK, Tyr861 and Tyr925 can be phosphorylated by Src, creating a binding site for the small adaptor protein Grb2 (growth-factor-receptor-bound protein 2) and thereby coupling FAK signalling to the Ras-MAPK (mitogen-activated protein kinase) pathway [9,20,21].
Paxillin is an adaptor protein that binds to FAK, Src, vinculin and actopaxin, as well as other proteins [22,23]. Paxillin is the prototype of a small family of structurally similar proteins, including the family members Hic-5 and leupaxin [24–26]. As noted above, paxillin contains five small α-helical leucine-rich peptide motifs termed LD motifs that provide binding sites for many paxillin-associated proteins, including FAK. The C-terminus consists of four double zinc finger LIM (Lin-11, Isl-1 and Mec-3) domains that are necessary for paxillin localization to focal adhesions as well as protein interactions [27,28].
FAK, Src and paxillin have all been implicated in the regulation of focal adhesion turnover and cell motility [29]. Studies using mutants of FAK and Src in FAK−/− or SYF (Src, Yes and Fyn−/−) cells respectively indicate that these kinases are intimately involved in the regulation of cell spreading and migration [19,30,31]. Specifically, the FAK kinase domain and phosphorylation of Tyr397 have been shown to be required for cell motility [19,32]. The mechanism by which FAK, Src and paxillin can control focal adhesion turnover is not yet fully elucidated.
It is likely that the activation of Src and FAK is tightly regulated to control cell spreading and migration on ECM-coated surfaces. Src kinase activity can be inhibited by phosphorylation of Src at Tyr527 by the negative regulatory kinase CSK (C-terminal Src kinase). Likewise, a number of tyrosine phosphatases have been shown to associate with focal adhesion proteins including Shp2 (SH2-containing tyrosine phosphatase 2), PTPα (protein tyrosine phosphatase α), PTEN (phosphatase and tensin homologue deleted on chromosome 10) and PTP–PEST (Pro-Glu-Ser-Thr) [33–36]. These proteins may have a role in co-ordinating the activation of FAK, paxillin, Src and other focal adhesion proteins. The activation of FAK, Src and paxillin may also be regulated by localization within the cell. Several members of the ARF-GAP (ADP-ribosylation factor GTPase-activating domain) family of proteins that regulate vesicular trafficking have been shown to bind to paxillin and FAK [37–39].
In order to understand the role of paxillin, we created ES (embryonic stem) cells where both alleles of paxillin have been disrupted [40]. These knockout ES cells do not express paxillin or related family members Hic-5 and leupaxin. In paxillin−/− ES cells, re-expression of paxillin is required for the tyrosine phosphorylation of FAK [40]. In the present paper, we extend our analysis to show that paxillin LIM domains 1, 2 and 3 and at least one LD motif are required for the phosphorylation of FAK at Tyr397 and Tyr861. Surprisingly, a minimal paxillin molecule that fully supported FAK tyrosine phosphorylation showed no in vitro or in vivo association with FAK. Although such paxillin molecules required an intact LD motif, extensive mutagenesis revealed that such motifs need not form stable associations with FAK to be able to fully support FAK tyrosine phosphorylation. FAK tyrosine phosphorylation was associated with cell spreading, as there was complete correspondence between paxillin mutants that supported FAK tyrosine phosphorylation and restored cell spreading. We show further that neither paxillin localization nor direct binding of FAK with paxillin is sufficient for FAK tyrosine phosphorylation, implying that additional paxillin-associated functions are required for the tyrosine phosphorylation of FAK in ES cells.
MATERIALS AND METHODS
Cell culture
ES cells were derived from the R1 line [41]. Paxillin heterozygous (+/−) clone 43 cells and the paxillin−/− clone 17 cells have been described previously [40]. All ES cells were grown in Iscove medium supplemented with 15% ES KnockOut SR (Life Technologies), 1 mM non-essential amino acids (Life Technologies), 10 mM 2-mercaptoethanol (Sigma) and penicillin/streptomycin (Life Technologies) as described in [42]. LIF (leukemia inhibitory factor) was grown as a GST (glutathione S-transferase) fusion protein in Escherichia coli and was purified as described in [40]. ES cells were grown on tissue culture plates coated in 0.1% gelatin (Sigma). The paxillin−/− T17 cell line was generated by injecting athymic nude mice with 106 ES clone 17 cells. The mice were killed at 6 weeks, and the benign teratoma was removed, minced and digested in trypsin. The cells were selected in DMEM (Dulbecco's modified Eagle's medium) with 10% (v/v) FBS (foetal bovine serum) (Life Technologies) and 400 μg/ml G418 (Life Technologies) for 2 weeks. T17 cells are paxillin−/−; however, they do express Hic-5 (results not shown).
DNA vectors and transfections
The expression vector pCX-EGFP was modified as described in [40,43] and was used as the expression plasmid in all experiments. This plasmid was modified further with the addition of a FLAG epitope tag insertion into the multiple cloning site so that FLAG fusions to the N-terminus of proteins could be expressed in cells. Truncation and deletion mutants were generated by oligonucleotide-directed mutagenesis. Individual LIM domain mutants are deleted as follows: ΔLIM1 (deletion of amino acids 326–376), ΔLIM2 (deletion of amino acids 385–435), ΔLIM3 (deletion of amino acids 444–494) and ΔLIM4 (deletion of amino acids 502–559). The LD swap mutants consist of LD1 (amino acids 4–10) or LD4 (amino acids 267–273) fused in frame to amino acids 310–502. The LD2/LD4-deleted paxillin mutant was described previously [40]. ES cells were co-transfected with Lipofectamine™ 2000 (Life Technologies) with paxillin or paxillin mutants and a PGK-Puro plasmid [44], and transfected cells were selected with 2 μg/ml puromycin (Life Technologies). All stable cell lines were screened for expression of the appropriate protein by Western blotting, frozen at passage two and used in experiments at early passage. The pTM1 plasmid [45] was modified by inserting the GST protein into the multiple cloning site so that fusion proteins of GST to the N-terminus of other proteins could be generated in vitro or in cells using a vaccinia virus expression system. GST–FAK was generated by infecting confluent CV1 cells with the vTF7 strain of vaccinia virus (53). The pTM1 GST–FAK plasmid was transfected into vaccinia-infected CV1 cells, and protein was recovered 24 h after infection. Cells were lysed in NP40 (Nonidet P40) lysis buffer (150 mM NaCl, 50 mM Tris/HCl, pH 7.5, 50 mM NaF, 5 mM sodium phosphate, 1% NP40, 0.01% PMSF, 1 mM sodium vanadate and 1 μg/ml leupeptin/aprotinin) and GST fusion proteins were recovered by binding to GSH–agarose beads at 4 °C for 1 h, followed by washing the beads three times with binding buffer. Soluble lysates from paxillin−/− cells transfected with FLAG-tagged paxillin and paxillin mutants were incubated with 1 μg of GSH-bound fusion protein, rocked at 4 °C for 1 h, washed three times with binding buffer, and bound proteins were eluted with 1× SDS sample buffer [2% (w/v) SDS, 60 mM Tris/HCl, pH 6.8, 100 mM dithiothreitol, 2.5% (v/v) glycerol and 0.01% (w/v) Bromophenol Blue].
Cell-spreading assays were performed as described in [40]. Briefly, mouse paxillin−/− ES cells were grown in 20% FBS and transfected transiently for 24 h. The cells were co-transfected with GFP (green fluorescent protein) and the indicated paxillin mutant. The cells were then trypsinized and plated on to fibronectin-coated coverslips for 7 h before fixing and staining.
Cell lysis and immunoprecipitation
Cells were lysed on ice in 0.5× NP40 lysis buffer. Lysates were clarified by centrifugation at 14000 g for 20 min at 4 °C. Clarified cell lysates were equalized for protein content using Coomassie Plus protein reagent (Pierce) before immunoprecipitation or Western blot analysis. Immunoprecipitations were carried out using 0.5 mg of protein lysate and 1 μg of purified antibody. The complex that formed on ice after 1 h was precipitated using rabbit anti-(mouse IgG) and Protein A–Sepharose beads (Repligen) and rocked at 4 °C for 1 h. Immunoprecipitations with mouse monoclonal antibodies were precipitated with goat anti-mouse magnetic beads (Pierce) or Protein A–Sepharose and a rabbit anti-mouse bridging antibody. All precipitations were washed three times with 1.5 ml of 4 °C lysis buffer, and the complex was eluted with SDS sample buffer.
Antibodies
Antibodies against paxillin, and PY20 antibodies, were from Transduction Laboratories. Anti-FAK antibodies for immunoprecipitation (clone A7) and Western blot (clone 77) were from Upstate Biotechnology and Tranduction Laboratories respectively. The rabbit polyclonal phosphotyrosine-specific antibodies against FAK Tyr397 and Tyr861 were from BioSource International. The monoclonal anti-phosphotyrosine antibody 4G10 was from Upstate Biotechnology. Primary antibodies on Western blots were detected using horseradish-peroxidase-conjugated secondary antibody and Supersignal substrate solution (Pierce). Rabbit anti-paxillin antibody CU4 was made by injecting 1 mg of a GST fusion of paxillin amino acids 1–331 (amino acids 45–54 deleted) into New Zealand white rabbits. CU4 recognizes mouse, chicken and bovine paxillin by Western blotting and immunofluorescence. CU4 was adsorbed against paxillin−/− T17 cells to reduce non-specific binding.
Immunofluorescence
T17 cells were transiently transfected for 16 h on glass coverslips, washed twice with PBS and fixed for 6 min in 3% (w/v) formaldehyde in PBS. Fixed coverslips were treated with 0.2% (w/v) SDS for 10 min blocked in TTBS [0.15 M NaCl/20 mM Tris (pH 7.2)/0.05% Tween-20] for 1 h, and incubated with primary antibody for 1 h at room temperature, with three 10 min washes after all antibody incubations. Primary antibodies were detected with secondary fluorochrome-conjugated antibodies.
RESULTS
Paxillin is required for FAK phosphorylation on Tyr397 and Tyr861
Paxillin−/− ES cells fail to express any member of the paxillin family (paxillin, Hic-5 or leupaxin), whereas differentiated fibroblasts derived from paxillin−/− ES cells or paxillin−/− mouse embryos express Hic-5 [40,46]. Therefore, in order to determine directly the role of paxillin in the tyrosine phosphorylation of FAK, paxillin−/− ES cells were analysed for FAK tyrosine phosphorylation. FAK was immunoprecipitated from clone 43 (paxillin+/−) and clone 17 (paxillin−/−) ES cells, and Western-blotted with the phosphotyrosine-specific antibodies PY20 and 4G10 (Figure 1A). Equal amounts of FAK were present in each cell line; however, FAK in paxillin−/− clone 17 cells was not appreciably tyrosine-phosphorylated (Figure 1A). Equalized total cell lysates from clone 43 (+/−) and 17 (−/−) cells were separated by SDS/10%-(w/v)-PAGE, and the blots were probed with phosphospecific antibodies against Tyr397 and Tyr861 of FAK (Figure 1B). In clone 43 cells, FAK was prominently phosphorylated at Tyr397 and Tyr861, while phosphorylation of FAK at these sites was barely detectable in paxillin−/− clone 17 cells (Figure 1B).
Figure 1. Paxillin−/− clone 17 cells are deficient in FAK phosphorylation at Tyr397 and Tyr861.
(A) FAK was immunoprecipitated (Ip) from clone 43 (+/−) or 17 (−/−) cells and Western-blotted (Wb) with antibodies against phosphotyrosine (pY20 and 4G10) and FAK as indicated. (B) Whole-cell lysates from clone 43 or 17 cells were probed with the indicated antibodies. FAK is minimally phosphorylated at Tyr397 and Tyr861 in clone 17 (−/−) cells.
Paxillin structural domains required to support FAK tyrosine phosphorylation
While individual paxillin LD motifs (1, 3, 4 and 5), in the context of the full-length paxillin molecule are expendable for FAK tyrosine phosphorylation, deletion of the full amino terminus of paxillin or the four LIM domains abrogates FAK tyrosine phosphorylation [40]. This indicates a requirement for features of both the amino terminus and the LIM domains of paxillin to support FAK tyrosine phosphorylation. In order to determine the minimal structural features of paxillin required to support FAK tyrosine phosphorylation, we created additional paxillin deletion and truncation mutants that lacked one or more domains (illustrated in Figure 2A).
Figure 2. Diagram and localization of paxillin mutants.
(A) Paxillin mutants used in the present study showing the relative location of LD motifs, tyrosine phosphorylation sites (Tyr31 and Tyr118) and the LIM domains. (B) Localization of wild-type and mutant paxillin molecules expressed transiently in paxillin−/− T17 cells. Localization of the expressed paxillin mutants was visualized with the rabbit anti-paxillin CU4 antibody (Pax.). Endogenous vinculin (Vin.) localization is also shown corresponding to the same cell as for paxillin.
One function of paxillin is localization to focal adhesions. Focal adhesions are very difficult to visualize in ES cells, so paxillin−/− differentiated T17 fibroblast cells (see the Materials and methods section) were transiently transfected with paxillin and paxillin mutants to ascertain the ability of these mutants to localize to focal adhesions. T17 cells express the paxillin family member Hic-5 and spread on coverslips. T17 cells that were transfected with empty vector did not show any staining with the CU4 anti-paxillin antibody; however, these cells displayed vinculin containing focal adhesions (Figure 2B). When wild-type paxillin was expressed in T17 cells, paxillin co-localized with vinculin in focal adhesions (Figure 2B). Paxillin mutants deleted of individual LIM domains were tested for localization in the paxillin−/− T17 cell line. Paxillin molecules with LIM1 or LIM4 deletions localized to focal adhesions; however, mutants of paxillin with LIM2 or LIM3 deletions did not localize with vinculin-stained focal adhesions (Figure 2B). This is consistent with results published previously on the role of LIM3 in paxillin localization [27].
Paxillin LIM domains are required for FAK tyrosine phosphorylation
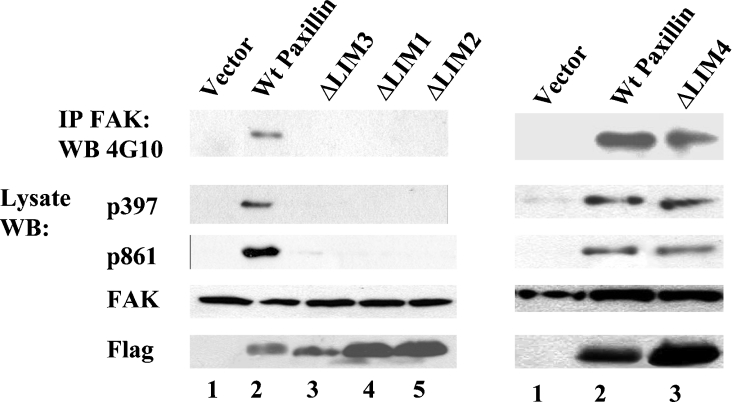
The paxillin mutants illustrated in Figure 2(A) were transfected into clone 17 (−/−) ES cells to generate stably expressing cell lines that were tested for the tyrosine phosphorylation of FAK. FAK immunoprecipitates separated by SDS/10%-(w/v)-PAGE were probed with anti-phosphotyrosine antibody 4G10 and whole-cell lysates were probed with phosphospecific antibodies against Tyr397 and Tyr861 of FAK. As observed previously [40], expression of exogenous paxillin was able to restore the tyrosine phosphorylation of FAK in clone 17 (−/−) cells (Figure 3, left-hand panel, lane 2). Wild-type paxillin and ΔLIM4 both restored overall FAK tyrosine phosphorylation, as well as phosphorylation at Tyr397 and Tyr861 (Figure 3, left-hand panel, lane 2, and right-hand panel, lanes 2 and 3). However, paxillin molecules deleted of LIM 1, LIM2 or LIM3 were all unable to support FAK tyrosine phosphorylation (Figure 3, left-hand panel, compare lane 2 with lanes 3–5). The inability of paxillin mutants with LIM2 or LIM3 deletions to support FAK tyrosine phosphorylation correlated with the failure of these mutants to localize to focal adhesions (Figure 2B). However, the ΔLIM1 paxillin molecule localized to focal adhesions (Figure 2B) yet failed to support FAK tyrosine phosphorylation (Figure 3, left-hand panel, compare lane 2 with lane 4).
Figure 3. Paxillin LIM domains 1–3 are required for FAK tyrosine phosphorylation.
In the top row, FAK immunoprecipitates (IP) from clone 17 cell lines stably expressing the indicated paxillin molecules were analysed by Western blotting (WB) with the anti-phosphotyrosine antibody 4G10. The remaining strips show the same whole-cell lysates analysed by separate Western blots with the indicated antibodies. ΔLIM4 is able to support total FAK phosphorylation as well as phosphorylation at Tyr397 and Tyr861, while loss of LIM1, LIM2 or LIM3 abrogates FAK tyrosine phosphorylation. All paxillin constructs were FLAG-tagged at the N-terminus. Wt, wild-type.
Paxillin LD motifs 2 and 4 are not required for FAK tyrosine phosphorylation
In order to expand upon our findings, a series of paxillin mutants that were truncated at both the N- and C-terminal ends were created (illustrated in Figure 2A). These mutants were transfected into ES cells, and stable cell lines were generated to test for their ability to restore overall FAK tyrosine phosphorylation, as well as phosphorylation at Tyr397 and Tyr861 (Figure 4). A paxillin molecule starting immediately after LD3 and ending just after LIM3 (mutant 225–502), which contains the FAK-binding site LD4, was able to support FAK tyrosine phosphorylation (Figure 4, lane 3). Further deletion of the N-terminus after the LD4 motif (mutant 274–502) did not reduce FAK tyrosine phosphorylation (Figure 4, compare lane 3 with lane 5). However, deletion of LD5 in either the 225–502 or the 274–502 constructs eliminated FAK tyrosine phosphorylation (Figure 4, compare lane 3 with lane 4, and lane 5 with lane 6). Further deletion of the paxillin N-terminus past LD5 (mutant 310–502) failed to support FAK tyrosine phosphorylation (Figure 4, lane 7).
Figure 4. FAK tyrosine phosphorylation does not require LD motifs 2 and 4.
The truncation mutants of paxillin shown in Figure 2(A) were used to define the minimal domain for FAK tyrosine phosphorylation. Mutants listed as ΔLD5 have amino acids 302–309 deleted. Clone 17 cells stably expressing the indicated paxillin mutant were grown and lysed, and FAK was recovered by immunoprecipitation (IP). The immunoprecipitated FAK was split, and SDS/10% (w/v) polyacrylamide gels were Western-blotted (WB) and probed with the 4G10, p397 and p861 antibodies. The p397 blot was then stripped and reprobed with the FAK antibody. The bottom panel shows the expression of the paxillin mutants by Western blotting whole-cell lysates with anti-FLAG antibody. The paxillin N-terminus is almost completely dispensable for FAK tyrosine phosphorylation.
LIM domains 1–3 and at least one LD motif are required for FAK tyrosine phosphorylation in ES cells
Interestingly, the N-terminus of paxillin was almost completely dispensable for FAK tyrosine phosphorylation, as mutants missing all sequences upstream of LD5 still promoted FAK tyrosine phosphorylation (Figure 5, lane 2). These results, taken together with the results of Figure 4, indicate that the minimal paxillin molecule structure that is able to support FAK tyrosine phosphorylation contains LD5 and LIM1, LIM2 and LIM3. The finding that LD5 was required for FAK tyrosine phosphorylation prompted us to determine whether other LD motifs could substitute for LD5 when placed at the position of LD5. Both LD1 and LD4 could promote FAK tyrosine phosphorylation when fused to amino acids 310–502 (Figure 5, lanes 3 and 4). However, fusions of LD2 and LD3 to amino acids 310–502 resulted in unstable proteins (results not shown).
Figure 5. FAK tyrosine phosphorylation requires one LD motif and LIM domains 1, 2 and 3.
The experiments were carried out as in Figure 4. The LD1 and LD4 swap mutants contain amino acids 4–10 and 267–273 respectively fused in-frame to LIM domains 1–3 (310–502).
FAK phosphorylation requires the hydrophobic core of an LD motif
In order to understand the structural requirements of an LD motif that could support FAK tyrosine phosphorylation, amino acid substitutions in the LD5 motif were created in the paxillin 302–502 fragment and stably expressed in paxillin−/− ES cells. Mutation of individual leucines at positions 302, 306 and 309 (L302V, L306A and L309A), as well as the double mutation of Met305 and Leu306 to alanine (ML-AA) in the paxillin 302–502 construct impaired FAK tyrosine phosphorylation (Figures 6A, compare lane 2 with lanes 3, 6 and 9, and 6B, compare lane 2 with lane 3). Mutation of most of the other amino acids of LD5 (D303A, T304V, G307A and S308A) had no effect upon FAK tyrosine phosphorylation (Figure 6A, lanes 4, 5, 7 and 8). This analysis of the LD5 sequence (LDTMLGSL) showed sensitivity to mutation of the core hydrophobic sequence of an LD motif (LXXLLXXL).
Figure 6. LD motifs that support FAK tyrosine phosphorylation contain the hydrophobic LD core leucines.
(A, B) Experiments were carried out as in Figure 4. All mutants listed are point mutations in the 302–502 construct. Mutations of the leucines of the LD motif abrogated FAK tyrosine phosphorylation.
Stable association of FAK with paxillin is not required for the paxillin-dependent tyrosine phosphorylation of FAK
Our initial experiments failed to demonstrate an association between FAK and the 302–502 fragment of paxillin by co-immunoprecipitation in ES cells (results not shown). To determine whether the single LD motif required for FAK tyrosine phosphorylation reflected a requirement of that LD motif to interact with FAK, the paxillin molecules were expressed in ES cells and were tested for their ability to associate with full-length GST–FAK in a more sensitive in vitro binding assay. While wild-type paxillin was able to associate with FAK in vitro and in vivo, a paxillin molecule with LD2/LD4 and LIM4 deleted was unable to associate with FAK (Figures 7A, compare lane 1 with lane 2, and 7B, compare lane 1 with lane 3). Likewise, none of the paxillin 302–502 molecules, including the LD swap mutants, were able to associate with FAK in vitro or in vivo (Figures 7A, compare lane 1 with lanes 3–6, and 7B, compare lane 3 with lane 4). This is consistent with previous experiments in other laboratories demonstrating the requirement of paxillin LD motifs 2 and 4 for the association with FAK [12,27].
Figure 7. Stable association of FAK with paxillin is not required for FAK tyrosine phosphorylation.
(A) Paxillin mutants were tested for their ability to associate with FAK in vitro as described in the Materials and methods section. Although wild-type (Wt) paxillin associates with GST–FAK, none of the 302–502 mutants of paxillin associate with FAK, irrespective of their ability to promote FAK tyrosine phosphorylation. ‘PD’ refers to pull-down with GST–FAK. (B) HEK-293 (human embryonic kidney) cells were transfected with the indicated paxillin construct and were lysed and subjected to immunoprecipitation (IP) with the anti-FLAG antibody, and Western blots (WB) were probed with an antibody against FAK. Paxillin LD2 and LD4 are required for direct association of FAK and paxillin in vivo. M.W., molecular mass (sizes are given in kDa).
FAK tyrosine phosphorylation correlates with cell spreading
Our previous report identified a spreading defect in paxillin−/− ES cells after attachment to an ECM-coated surface [40]. In order to determine whether the minimal paxillin fragment required for FAK tyrosine phosphorylation could also support other paxillin-mediated events, cell-spreading assays were performed. ES cells transfected with wild-type paxillin spread on a fibronectin-coated coverslip after 7 h, while the cells transfected with empty vector did not spread (Figure 8A). Those paxillin mutants that failed to support FAK tyrosine phosphorylation failed to support cell spreading on fibronectin (Figure 8B, compare lane 2 with lanes 3, 4, 5, 8, 10, 11, 15, 18, 21 and 22). Paxillin mutants that supported FAK tyrosine phosphorylation were able to support cell spreading (Figure 8B, lanes 2, 6, 7, 9, 12–14, 16, 17, 19 and 20). The expression of the paxillin mutants used in Figure 8(B) is shown in Figure 8(C).
Figure 8. Paxillin mutants that support FAK tyrosine phosphorylation support cell spreading on fibronectin.
(A) ES clone 17 (−/−) cells spreading on fibronectin. Clone 17 cells were transiently co-transfected with GFP (green fluorescent protein) and the indicated plasmid for 24 h and then plated on to a fibronectin-coated coverslip for 7 h. Clone 17 cells transfected with vector had not appreciably spread at 7 h when compared with cells expressing wild-type paxillin. (B) Cell spreading assays of clone 17 (−/−) cells transfected with paxillin or paxillin mutants were performed as described (see the Materials and methods section). GFP-positive cells were counted, and spreading was determined. A cell was counted as spread if the diameter of the cell was at least twice the diameter of the DAPI (4,6-diamidino-2-phenylindole)-stained nucleus. Results are the means±S.D. for three independent experiments. FAK tyrosine phosphorylation and cell spreading are closely related. (C) Expression of the paxillin mutants used in (B) was determined by probing Western blots (WB) of cell lysates with anti-FLAG antibody.
DISCUSSION
In order to better understand the role of paxillin in the regulation of FAK, we used paxillin−/− mouse ES cells reconstituted with paxillin molecules and analysed FAK tyrosine phosphorylation. Expression of the paxillin family members Hic-5 or leupaxin may complicate the analysis of phenotypes of paxillin−/− fibroblasts. ES cells express only paxillin and not other paxillin family members, making analysis less complex; the T17 fibroblasts cells used in the present study, as well as paxillin−/− MEFs (mouse embryonic fibroblasts) express Hic-5. In other experiments (R. Wade and S. Vande Pol, unpublished work) we found that exogenous expression of Hic-5 in paxillin−/− clone 17 ES cells can support FAK tyrosine phosphorylation and cell spreading similar to paxillin. This is presumed to be one reason why paxillin−/− MEFs and our clone T17 cells spread readily on coverslips. ES cells grown on gelatin form two populations of cells: those that contact the surface directly and those clusters of cells that attach to the surface-associated cells, therefore we investigated the state of FAK tyrosine phosphorylation under these circumstances. All of the studies on FAK tyrosine phosphorylation were performed under stable expression conditions. In the present study, we found that paxillin was required for the phosphorylation of FAK at Tyr397 and Tyr861. We show that a minimal molecule of paxillin that is composed of an LD motif containing the core hydrophobic sequence LXXLLXXL, followed by LIM domains 1, 2 and 3 is necessary for FAK tyrosine phosphorylation. Strong direct binding of FAK to the paxillin fragment is not required for FAK tyrosine phosphorylation. In addition, FAK tyrosine phosphorylation is strongly correlated with early cell spreading.
Our previous study demonstrated that many of the features of paxillin including Tyr31 and Tyr118, the polyproline tract and the individual LD motifs 1, 3, 4 and 5 were each individually dispensable for FAK tyrosine phosphorylation; however, the minimal features of paxillin that are necessary and sufficient for FAK tyrosine phosphorylation remained undefined. Previous reports have suggested that FAK mutants that do not bind to paxillin are tyrosine-phosphorylated in differentiated cells; however, these experiments may be complicated by the expression of PYK2 (protein tyrosine kinase 2), endogenous FAK or the disruption of the FAT domain structure by the mutations [47]. Our results suggest that direct association of paxillin with FAK, as determined by in vitro binding, is not required for paxillin to support the tyrosine phosphorylation of FAK, as mutants of paxillin that have LD2 and LD4 deleted still support FAK tyrosine phosphorylation, but fail to associate with FAK (Figures 5 and 7).
To elucidate the regions of paxillin's C-terminus that are necessary for the tyrosine phosphorylation of FAK, we stably expressed paxillin mutants with LIM domain deletions in clone 17 paxillin−/− cells. The ΔLIM1, ΔLIM2 and ΔLIM3 mutants failed to restore the tyrosine phosphorylation of FAK. The paxillin mutant ΔLIM4 localized to focal adhesions and permitted the tyrosine phosphorylation of FAK (Figures 2B and 3). These results indicate a necessary role for paxillin LIM1, LIM2 and LIM3 in FAK tyrosine phosphorylation. The LIM3 domain has been implicated in paxillin focal adhesion localization and interactions with tubulin and PTP–PEST, although the functional consequences of these interactions are not fully defined [48,49]. Since LIM3 and LIM4 are both required for PTP–PEST association with paxillin [48], this would indicate that the PTP–PEST interaction with paxillin is not required for the tyrosine phosphorylation of FAK in ES cells. Our results also demonstrate that paxillin localization is not the only feature of the LIM domains that is required for FAK tyrosine phosphorylation, as the paxillin ΔLIM1 mutant localizes to vinculin-stained focal adhesions, yet fails to support FAK tyrosine phosphorylation (Figures 2B and 3). Taken together, these results indicate that neither stable association of FAK with paxillin nor localization of paxillin to focal adhesions is sufficient for paxillin to enable FAK tyrosine phosphorylation.
The necessity for LIM1, LIM2 and LIM3 of paxillin to support FAK tyrosine phosphorylation suggests multiple roles for the LIM domains of paxillin. LIM2 and LIM3 of paxillin have been shown to be required for focal adhesion localization. To date, no evidence proves that the requirement of LIM domains 2 and 3 for both paxillin localization and FAK tyrosine phosphorylation represent different manifestations of the same function. LIM1 of paxillin has not been shown to be required in paxillin localization; however, LIM domains 1, 2 and 3 are each required for FAK tyrosine phosphorylation, indicating that another function of the LIM1 domain, independent of paxillin localization, is required for FAK tyrosine phosphorylation. Therefore it is likely that the LIM domains play a primary role in assembly of an as-yet-unidentified protein complex that permits the accumulation of FAK phosphorylated at Tyr397 and subsequent phosphorylation of other tyrosine residues on FAK. Interestingly, a paxillin homologue in Drosophila that contains only LIM1, LIM2 and LIM3 without an LD motif has been described; however, no function has been attributed to this molecule [50].
Paxillin molecules that consist of only LIM domains 1–3 are unable to support FAK tyrosine phosphorylation, demonstrating a requirement for some N-terminal function of paxillin; if an LD motif is fused to the LIM1–LIM3 fragment, FAK tyrosine phosphorylation is restored. Interestingly, there are both spatial and sequence specific requirements for the LD motif. While there is an absolute requirement for a single LD motif, there was a surprising lack of sequence specificity or strength of interaction with FAK in the LD motifs that supported FAK tyrosine phosphorylation. Many of the paxillin mutants that were permissive for FAK tyrosine phosphorylation interacted so weakly with FAK that we were unable to demonstrate binding in vitro or in vivo (Figure 7). In the case of the LD4 swap mutant, this may reflect the use of a minimal LD motif without flanking amino acids. It is unclear what minimal affinity for FAK an LD motif must have to support FAK tyrosine phosphorylation, or whether the requirement for an LD motif represents a requirement for interaction with a different cellular factor or interaction with another region of the paxillin molecule. Our previous report showed that LD5 could be deleted in the context of full-length paxillin and that the mutant still supports FAK tyrosine phosphorylation, suggesting that the LD5 sequence and its position near the LIM domains may not be the features of the LD motif that are absolutely required for FAK tyrosine phosphorylation [40]. A spatial requirement for the position of the LD motif with respect to the LIMS was also evident. Mutants such as 225–502 (ΔLD5) contain an intact LD4, yet this mutant is unable to promote FAK tyrosine phosphorylation; however, LD4 was able to support FAK tyrosine phosphorylation in the LD4 swap mutant (Figure 5) where LD4 is inserted at the position of LD5, despite our inability to detect FAK binding in vitro (Figure 7). There are two possibilities for the LD requirement. First, it may be that the LD must be present to structure the LIM domains properly and that in order for this to occur the LD motif must be either close enough to the LIM domains (LD5) or far enough away so that the LD motif can wrap around (the full-length ΔLD5 molecule) and contact the LIM domains. Intermediate distances of the LD motif from the LIM domains (225–502 ΔLD5) may not have the required flexibility in order to contact the LIM domains. Alternatively, the required LD motif may need to bind one specific paxillin-binding sequence of FAK, and that a very short distance from the LIMS (302–502) or a longer more flexible LD (FL paxillin ΔLD5) is required to contact the paxillin-binding sequence of FAK and that the intermediate distance (225–502 ΔLD5) cannot access the paxillin-binding sequence. Taken together, these results indicate both spatial and sequence-specific roles for the LD motifs of paxillin that are independent of stable binding with FAK.
There was a clear association between early cell spreading and the tyrosine phosphorylation of FAK. Mutants of paxillin that fail to support FAK phosphorylation fail to support cell spreading, although mutants that support FAK tyrosine phosphorylation were able to enhance cell spreading (Figure 8B). These results indicate that FAK tyrosine phosphorylation and cell spreading in ES cells are closely related events.
Our results show that four separate structural motifs of paxillin (an LD motif and LIM domains 1–3) are required for three distinct functions that support of FAK tyrosine phosphorylation (an LD interaction, an unknown interaction at LIM1 and a function that correlates with focal adhesion localization that requires LIM2 and LIM3). When associated with proteins that bind LIM domains 1–3, an LD motif that is not an ideal FAK-interacting LD motif may be sufficient to transiently associate with the FAT domain, promoting an ‘unlocking’ effect that permits either FAK tyrosine autophosphorylation or phosphorylation of Tyr397 by another kinase. While it is possible that some other as-yet-unidentified protein binds the single necessary LD motif to promote FAK tyrosine phosphorylation, this seems less likely given the ability to swap such a variety of mutants, including other LD motifs of paxillin in place of LD5 in the 302–502 mutant. Future work will focus on determining the proteins that interact with the paxillin LIM domains and the role these complexes have in the tyrosine phosphorylation of FAK in ES cells.
Our analysis demonstrates a surprising simplicity in the requirements of the N-terminus of paxillin, as only a single LD motif is required to support FAK tyrosine phosphorylation. This is surprising, given the considerable literature noted in the Introduction that defines roles for numerous motifs in the N-terminus of paxillin to regulate cytoskeletal dynamics in differentiated cells, such as interactions with kinases, ARF-GAPs, Crk adapter proteins and phosphorylation by extracellular-signal-regulated kinases. Clearly, the use of ES cells has simplified the analysis of the minimal paxillin features required for FAK tyrosine phosphorylation. Thus our analysis does not exclude the possibility that alternative mechanisms, independent of paxillin family members, may exist in differentiated cell types that could support the tyrosine phosphorylation of FAK.
Acknowledgments
We thank all members of the Vande Pol lab for helpful assistance during the course of this study. We thank Dr Tom Parsons for helpful comments and Michael Schaller for critical reading of this manuscript. This work was supported by NIH (National Institutes of Health) grants CA-69292 and CA-80931 (S.V.P.).
References
- 1.Brown N. H. Cell-cell adhesion via the ECM: integrin genetics in fly and worm. Matrix Biol. 2000;19:191–201. doi: 10.1016/s0945-053x(00)00064-0. [DOI] [PubMed] [Google Scholar]
- 2.Bokel C., Brown N. H. Integrins in development: moving on, responding to, and sticking to the extracellular matrix. Dev. Cell. 2002;3:311–321. doi: 10.1016/s1534-5807(02)00265-4. [DOI] [PubMed] [Google Scholar]
- 3.Juliano R. L. Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu. Rev. Pharmacol. Toxicol. 2002;42:283–323. doi: 10.1146/annurev.pharmtox.42.090401.151133. [DOI] [PubMed] [Google Scholar]
- 4.Danen E. H., Yamada K. M. Fibronectin, integrins, and growth control. J. Cell. Physiol. 2001;189:1–13. doi: 10.1002/jcp.1137. [DOI] [PubMed] [Google Scholar]
- 5.Martin K. H., Slack J. K., Boerner S. A., Martin C. C., Parsons J. T. Integrin connections map: to infinity and beyond. Science. 2002;296:1652–1653. doi: 10.1126/science.296.5573.1652. [DOI] [PubMed] [Google Scholar]
- 6.Zamir E., Geiger B. Molecular complexity and dynamics of cell–matrix adhesions. J. Cell Sci. 2001;114:3583–3590. doi: 10.1242/jcs.114.20.3583. [DOI] [PubMed] [Google Scholar]
- 7.van der Flier A., Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305:285–298. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- 8.Kanner S. B., Reynolds A. B., Vines R. R., Parsons J. T. Monoclonal antibodies to individual tyrosine-phosphorylated protein substrates of oncogene-encoded tyrosine kinases. Proc. Natl. Acad. Sci. U.S.A. 1990;87:3328–3332. doi: 10.1073/pnas.87.9.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlaepfer D. D., Hauck C. R., Sieg D. J. Signaling through focal adhesion kinase. Prog. Biophys. Mol. Biol. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- 10.Schaller M. D. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim. Biophys. Acta. 2001;1540:1–21. doi: 10.1016/s0167-4889(01)00123-9. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi I., Vuori K., Liddington R. C. The focal adhesion targeting (FAT) region of focal adhesion kinase is a four-helix bundle that binds paxillin. Nat. Struct. Biol. 2002;9:101–106. doi: 10.1038/nsb755. [DOI] [PubMed] [Google Scholar]
- 12.Thomas J. W., Cooley M. A., Broome J. M., Salgia R., Griffin J. D., Lombardo C. R., Schaller M. D. The role of focal adhesion kinase binding in the regulation of tyrosine phosphorylation of paxillin. J. Biol. Chem. 1999;274:36684–36692. doi: 10.1074/jbc.274.51.36684. [DOI] [PubMed] [Google Scholar]
- 13.Gao G., Prutzman K. C., King M. L., Scheswohl D. M., DeRose E. F., London R. E., Schaller M. D., Campbell S. L. NMR solution structure of the focal adhesion targeting domain of focal adhesion kinase in complex with a paxillin LD peptide: evidence for a two-site binding model. J. Biol. Chem. 2004;279:8441–8451. doi: 10.1074/jbc.M309808200. [DOI] [PubMed] [Google Scholar]
- 14.Schlaepfer D. D., Hunter T. Evidence for in vivo phosphorylation of the Grb2 SH2-domain binding site on focal adhesion kinase by Src-family protein-tyrosine kinases. Mol. Cell. Biol. 1996;16:5623–5633. doi: 10.1128/mcb.16.10.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J., Avraham H., Rogers R. A., Raja S., Avraham S. Characterization of RAFTK, a novel focal adhesion kinase, and its integrin-dependent phosphorylation and activation in megakaryocytes. Blood. 1996;88:417–428. [PubMed] [Google Scholar]
- 16.Schlaepfer D. D., Hunter T. Signal transduction from the extracellular matrix – a role for the focal adhesion protein-tyrosine kinase FAK. Cell Struct. Funct. 1996;21:445–450. doi: 10.1247/csf.21.445. [DOI] [PubMed] [Google Scholar]
- 17.Schaller M. D., Parsons J. T. Focal adhesion kinase and associated proteins. Curr. Opin. Cell Biol. 1994;6:705–710. doi: 10.1016/0955-0674(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 18.Owen J. D., Ruest P. J., Fry D. W., Hanks S. K. Induced focal adhesion kinase (FAK) expression in FAK−/− cells enhances cell spreading and migration requiring both auto- and activation loop phosphorylation sites and inhibits adhesion-dependent tyrosine phosphorylation of Pyk2. Mol. Cell. Biol. 1999;19:4806–4818. doi: 10.1128/mcb.19.7.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sieg D. J., Hauck C. R., Schlaepfer D. D. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J. Cell Sci. 1999;112:2677–2691. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- 20.Calalb M. B., Zhang X., Polte T. R., Hanks S. K. Focal adhesion kinase tyrosine-861 is a major site of phosphorylation by Src. Biochem. Biophys. Res. Commun. 1996;228:662–668. doi: 10.1006/bbrc.1996.1714. [DOI] [PubMed] [Google Scholar]
- 21.Schlaepfer D. D., Hanks S. K., Hunter T., van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature (London) 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 22.Turner C. E. Paxillin. Int. J. Biochem. Cell Biol. 1998;30:955–959. doi: 10.1016/s1357-2725(98)00062-4. [DOI] [PubMed] [Google Scholar]
- 23.Turner C. E. Paxillin: a cytoskeletal target for tyrosine kinases. BioEssays. 1994;16:47–52. doi: 10.1002/bies.950160107. [DOI] [PubMed] [Google Scholar]
- 24.Thomas S. M., Hagel M., Turner C. E. Characterization of a focal adhesion protein, Hic-5, that shares extensive homology with paxillin. J. Cell Sci. 1999;112:181–190. doi: 10.1242/jcs.112.2.181. [DOI] [PubMed] [Google Scholar]
- 25.Ishino K., Kaneyama J. K., Shibanuma M., Nose K. Specific decrease in the level of Hic-5, a focal adhesion protein, during immortalization of mouse embryonic fibroblasts, and its association with focal adhesion kinase. J. Cell. Biochem. 2000;76:411–419. doi: 10.1002/(sici)1097-4644(20000301)76:3<411::aid-jcb9>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 26.Lipsky B. P., Beals C. R., Staunton D. E. Leupaxin is a novel LIM domain protein that forms a complex with PYK2. J. Biol. Chem. 1998;273:11709–11713. doi: 10.1074/jbc.273.19.11709. [DOI] [PubMed] [Google Scholar]
- 27.Brown M. C., Perotta J. A., Turner C. E. Identification of LIM3 as the principal determinant of paxillin focal adhesion localization and characterization of a novel motif on paxillin directing vinculin and focal adhesion kinase binding. J. Cell Biol. 1996;135:1109–1123. doi: 10.1083/jcb.135.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaller M. D. Paxillin: a focal adhesion-associated adaptor protein. Oncogene. 2001;20:6459–6472. doi: 10.1038/sj.onc.1204786. [DOI] [PubMed] [Google Scholar]
- 29.Webb D. J., Donais K., Whitmore L. A., Thomas S. M., Turner C. E., Parsons J. T., Horwitz A. F. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- 30.Illc D., Furuta Y., Kanazawa S., Takeda N., Sobue K., Nakatsuji N., Nomura S., Fujimoto J., Okada M., Yamamoto T., et al. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature (London) 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan K. B., Bibbins K. B., Swedlow J. R., Arnaud M., Morgan D. O., Varmus H. E. Association of the amino-terminal half of c-Src with focal adhesions alters their properties and is regulated by phosphorylation of tyrosine 527. EMBO J. 1994;13:4745–4756. doi: 10.1002/j.1460-2075.1994.tb06800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parsons J. T., Martin K. H., Slack J. K., Taylor J. M., Weed S. A. Focal adhesion kinase: a regulator of focal adhesion dynamics and cell movement. Oncogene. 2000;19:5606–5613. doi: 10.1038/sj.onc.1203877. [DOI] [PubMed] [Google Scholar]
- 33.Manes S., Mira E., Gomez-Mouton C., Zhao Z. J., Lacalle R. A., Martinez A. C. Concerted activity of tyrosine phosphatase SHP-2 and focal adhesion kinase in regulation of cell motility. Mol. Cell. Biol. 1999;19:3125–3135. doi: 10.1128/mcb.19.4.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng X. M., Wang Y., Pallen C. J. Cell transformation and activation of pp60c-src by overexpression of a protein tyrosine phosphatase. Nature (London) 1992;359:336–339. doi: 10.1038/359336a0. [DOI] [PubMed] [Google Scholar]
- 35.Yu D. H., Qu C. K., Henegariu O., Lu X., Feng G. S. Protein-tyrosine phosphatase Shp-2 regulates cell spreading, migration, and focal adhesion. J. Biol. Chem. 1998;273:21125–21131. doi: 10.1074/jbc.273.33.21125. [DOI] [PubMed] [Google Scholar]
- 36.Tsuda M., Matozaki T., Fukunaga K., Fujioka Y., Imamoto A., Noguchi T., Takada T., Yamao T., Takeda H., Ochi F., et al. Integrin-mediated tyrosine phosphorylation of SHPS-1 and its association with SHP-2: roles of Fak and Src family kinases. J. Biol. Chem. 1998;273:13223–13229. doi: 10.1074/jbc.273.21.13223. [DOI] [PubMed] [Google Scholar]
- 37.Turner C. E., Brown M. C., Perrotta J. A., Riedy M. C., Nikolopoulos S. N., McDonald A. R., Bagrodia S., Thomas S., Leventhal P. S. Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: a role in cytoskeletal remodeling. J. Cell Biol. 1999;145:851–863. doi: 10.1083/jcb.145.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.West K. A., Zhang H., Brown M. C., Nikolopoulos S. N., Riedy M. C., Horwitz A. F., Turner C. E. The LD4 motif of paxillin regulates cell spreading and motility through an interaction with paxillin kinase linker (PKL) J. Cell Biol. 2001;154:161–176. doi: 10.1083/jcb.200101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norman J. C., Jones D., Barry S. T., Holt M. R., Cockcroft S., Critchley D. R. ARF1 mediates paxillin recruitment to focal adhesions and potentiates Rho-stimulated stress fiber formation in intact and permeabilized Swiss 3T3 fibroblasts. J. Cell Biol. 1998;143:1981–1995. doi: 10.1083/jcb.143.7.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wade R., Bohl J., Vande Pol S. Paxillin null embryonic stem cells are impaired in cell spreading and tyrosine phosphorylation of focal adhesion kinase. Oncogene. 2002;21:96–107. doi: 10.1038/sj.onc.1205013. [DOI] [PubMed] [Google Scholar]
- 41.Nagy A., Rossant J., Nagy R., Abramow N. W., Roder J. C. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc. Natl. Acad. Sci. U.S.A. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matise M. P., Auerbach W., Joyner A. L. New York: Oxford University Press; 2000. Production of targeted embryonic stem cell clones. [Google Scholar]
- 43.Okabe M., Ikawa M., Kominami K., Nakanishi T., Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- 44.Tucker K. L., Beard C., Dausmann J., Jackson-Grusby L., Laird P. W., Lei H., Li E., Jaenisch R. Germ-line passage is required for establishment of methylation and expression patterns of imprinted but not of nonimprinted genes. Genes Dev. 1996;10:1008–1020. doi: 10.1101/gad.10.8.1008. [DOI] [PubMed] [Google Scholar]
- 45.Elroy-Stein O., Fuerst T. R., Moss B. Cap-independent translation of mRNA conferred by encephalomyocarditis virus 5′ sequence improves the performance of the vaccinia virus/bacteriophage T7 hybrid expression system. Proc. Natl. Acad. Sci. U.S.A. 1989;86:6126–6130. doi: 10.1073/pnas.86.16.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hagel M., George E. L., Kim A., Tamimi R., Opitz S. L., Turner C. E., Imamoto A., Thomas S. M. The adaptor protein paxillin is essential for normal development in the mouse and is a critical transducer of fibronectin signaling. Mol. Cell. Biol. 2002;22:901–915. doi: 10.1128/MCB.22.3.901-915.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cooley M. A., Broome J. M., Ohngemach C., Romer L. H., Schaller M. D. Paxillin binding is not the sole determinant of focal adhesion localization or dominant-negative activity of focal adhesion kinase/focal adhesion kinase-related nonkinase. Mol. Biol. Cell. 2000;11:3247–3263. doi: 10.1091/mbc.11.9.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cote J. F., Turner C. E., Tremblay M. L. Intact LIM 3 and LIM 4 domains of paxillin are required for the association to a novel polyproline region (Pro 2) of protein-tyrosine phosphatase-PEST. J. Biol. Chem. 1999;274:20550–20560. doi: 10.1074/jbc.274.29.20550. [DOI] [PubMed] [Google Scholar]
- 49.Brown M. C., Turner C. E. Roles for the tubulin- and PTP-PEST-binding paxillin LIM domains in cell adhesion and motility. Int. J. Biochem. Cell Biol. 2002;34:855–863. doi: 10.1016/s1357-2725(01)00154-6. [DOI] [PubMed] [Google Scholar]
- 50.Yagi R., Ishimaru S., Yano H., Gaul U., Hanafusa H., Sabe H. A novel muscle LIM-only protein is generated from the paxillin gene locus in Drosophila. EMBO Rep. 2001;2:814–820. doi: 10.1093/embo-reports/kve178. [DOI] [PMC free article] [PubMed] [Google Scholar]