Abstract
A programmable device (Sterinis, Gloster Sante Europe) providing a dry fume of 5% hydrogen peroxide (H2O2) disinfectant was tested for decontamination of rooms, ambulances and different types of medical equipment. Pre-set concentrations were used according to the volumes of the rooms and garages. Three cycles were performed with increasing contact times. Repetitive experiments were performed using Bacillus atrophaeus (formerly Bacillus subtilis) Raven 1162282 spores to control the effect of decontamination; after a sampling plan, spore strips were placed in various positions in rooms, ambulances, and inside and outside the items of medical equipment. Decontamination was effective in 87% of 146 spore tests in closed test rooms and in 100% of 48 tests in a surgical department when using three cycles. One or two cycles had no effect. The sporicidal effect on internal parts of the medical equipment was only 62.3% (220 tests). When the devices were run and ventilated during decontamination, 100% (57/57) of spore strips placed inside were decontaminated. In the ambulances, the penetration of H2O2 into equipment, devices, glove boxes, under mattresses, and the drivers' cabins was 100% (60/60 tests) when using three cycles, but was less effective when using one or two cycles. In conclusion, an H2O2 dry fumigation system, run in three cycles, seemed to have a good sporicidal effect when used in rooms, ambulances, and external and internal parts of ventilated equipment. Further studies need to be performed concerning concentration, contact time and the number of cycles of H2O2. This is especially important for inner parts of medical equipment that cannot be ventilated during the decontamination process.
Keywords: Room decontamination, Ambulance decontamination, Medical equipment decontamination, Hydrogen peroxide fume decontamination, Spore test
Introduction
To control the spread of pathogens in hospital environments, good hygienic routines based on cleaning and disinfection of surfaces contaminated with biological materials are obligatory requirements. Chemicals such as chlorine and 5% chloramine have traditionally been used in Norway for surface disinfection during the terminal decontamination of isolation rooms and furniture after discharge of patients with infections.1, 2, 3, 4 The same procedure has been used for ambulances and for medical and other equipment after use by infectious patients.2, 3, 4 Manual chemical disinfection is both time and labour consuming, and there may be difficulties to ensure coverage. Internal parts of medical equipment such as ventilators, continuous positive airways pressure (CPAP) and other respiratory tract equipment, infusion pumps, suction equipment, etc. may be difficult to decontaminate. Most medical equipment has an air-cooled motor that takes air from the patient's room/environment, enabling internal parts to become contaminated.
Ambulances transporting patients with infectious diseases may often be contaminated because of the special construction and the narrow space inside, the ventilation system, and all the equipment and devices present.
In this study, a patented programmable device that provides a dry aerosol of hydrogen peroxide (H2O2) disinfectant was tested for surface and internal decontamination of rooms, ambulances and different types of medical equipment.
Materials and methods
H2O2 dry aerosol decontamination system
The robot technology system produces H2O2 as electrically charged particles of such a small size (approximately 10 μm in diameter) that they circulate freely in air as a dry aerosol or fume-making disinfectant accessible to all surfaces (Sterinis, Gloster Sante Europe, France www.sterinis.com). The H2O2 adheres to particles present in the atmosphere and on surfaces, and forms a dry film on these particles. The aerosol container (Sterusil) contains hydrogen peroxide 5%, phosphoric acid <50 ppm, silver cations <50 ppm, gum arabica <1 ppm and bi-osmotic water 95%. The system consists of a programmable robot that can be pre-programmed to the required concentration of H2O2 dry aerosol depending on the exact volume of the enclosed room. Consumption of disinfectant is 6 mL/m3 for full disinfection.
The aerosol-producing robot was placed to get the most effective concentration of dry aerosol, i.e. approximately 2 m in front and to the sides of the robot. The whole disinfection process, including the number of cycles (diffusion time) and the contact time, was pre-set on the robot. One or more pre-set cycles of H2O2 gas used varying diffusion times according to the volume of the room. Each cycle was followed by an increasing contact time of 30, 60 and 120 min after one, two and three cycles, respectively. Each cycle was immediately followed by the pre-set contact time, which was then immediately followed by the next cycle, etc. The test rooms (including garages for ambulances) were closed throughout the decontamination process, and no people were present. All ventilation openings in the rooms were taped over. The concentration of H2O2 was 2–17.4 parts per million (ppm) in the rooms and 36–60 ppm in the garages during the diffusion time. The Norwegian Directory of Labour Inspection has set exposure limits of 1 ppm of H2O2 for 8 h of exposure and less than 3 ppm for 15 min four times a day. The fume does not persist because of rapid decomposition. When the disinfection procedure was complete, the room or equipment could be used immediately.
The rooms, equipment and ambulances were not cleaned prior to exposure to the disinfectant agent. No neutralization method was required after decontamination.
Spore control
Spores of Bacillus atrophaeus Raven 1162282, ATCC No. 9372 (Raven Biological Laboratories Inc., Nebraska 68127) were used to control the effect of decontamination. The mean strip recovery of spores was 2.5×106 colony-forming units/1.5×0.25 strip. Each spore strip's inner envelope was either opened or unopened during disinfection. The spore strips were marked by number and placed in various positions and surfaces in the rooms, in the ambulances, and on inner and outer parts of the medical equipment and devices in the rooms and the ambulances. During repetitive studies, the spore strips were placed in approximately the same place each time, according to a consistent sampling plan that was representative of all parts of the area. During the last test period of the experiments, the spore strips were removed using a pair of sterile tweezers and sterile gloves. The spore strips were removed after 18–20 h and sent to the Department of Sterility Control, Rikshospitalet, Oslo.
The spore strips were cultured blindly in a 10 mL broth of tryptone, glucose, yeast extract and disodiumhydrogenphosphate (Difco) for seven days at 37 °C. Growth or lack of growth was read each day, and microscopy of the broth was performed to look for bacteria and spores. A clear broth with no growth was defined as negative.
Room decontamination
Test rooms
Two single rooms of approximately 58 m3 (pilot study) and 40 m3 were chosen for the first experiments. Both were closed and all openings were taped over to control the aerosol during the experimental period. The fume-producing robot was placed in a corner and pre-programmed for three cycles, according to the volume of the room. The diffusion time for the smallest room was 19 min and that for the largest room was 26 min, resulting in concentrations of approximately 12 and 17.4 ppm of H2O2, respectively. The first, second and third cycles were followed by contact times of 30, 60 and 120 min, respectively. Spore strips were placed on and under a table, high up on the walls, on the ceiling, on the floor, and outside different pieces of medical equipment in the room. The procedures were repeated several times using a defined pre-set sampling plan.
Surgery department
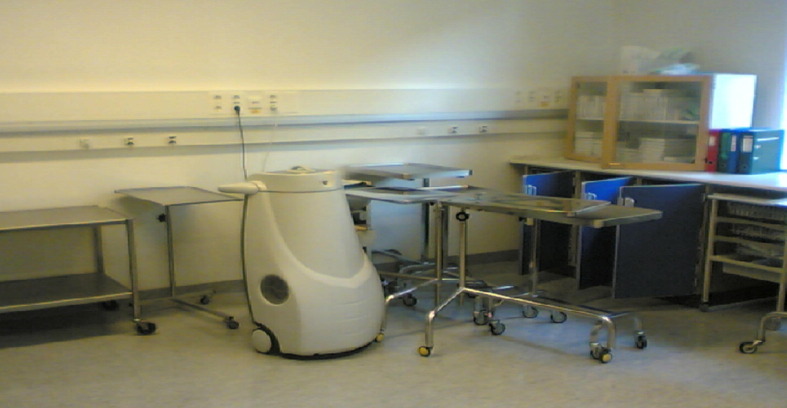
Decontamination was performed in 22 rooms in a surgery department during July 2004 (Figure 1 ). The rooms were of different sizes, varying between 4 and 50 m3, and were operating theatres, rooms for surgical hand hygiene, autoclaves, sterile packing rooms, decontamination rooms, store rooms for sterile devices, corridors, etc. In all rooms, spore strips were placed on walls, tables, floors, etc. The aerosol-producing robot was placed in a corner and pre-programmed as described above. Most rooms were treated with three diffusion cycles, followed by increasing contact times of 30, 60 and 120 min. A few rooms were only treated with two cycles, followed by increasing contact times of 30 and 60 min; or only one cycle, followed by a contact time of 30 min.
Figure 1.
Decontamination of rooms and equipment with a hydrogen peroxide aerosol, using a pre-programmed robot.
Decontamination of medical equipment
Test room
One test room of 40 m3 was closed and openings were taped over to control the concentration of the aerosol. The H2O2 concentration was programmed according to the volume of the room. Three pre-set cycles of H2O2 dry aerosol were performed. Each time, the diffusion time was 12 min, giving a concentration of 12 ppm, followed by increasing contact times of 30, 60 and 120 min.
Medical equipment tested
A Freedom nebulizer (Profile Therapeutics, Bognor Regis, UK), Micro Air U22 (Omron Medizintechnik Handelsgesellschaft GmbH, Mannheim, Germany), compressor CR 60 (Profile Respriatory Systems, Bognor Regis, UK), Walk boy Pari (PARI GmbH, Starnberg, Germany), pulse oximeter (SN P 1020601402-N-550; Tyco Healthcare Group, Pleasanton, CA, USA), CPAP (Autoset Sprint, ResMed Ltd., North Ryde, NSW, Australia), Medela suction apparatus (Medela AG, Medical Technology, Baar, Switzerland), ventilator (Lifecare PVL-100; Lifecare, Lafayette, CO, USA), and 571 infusion pump (IVAC Medical Systems Inc., San Diego, CA, USA) were tested. The effect on the inner parts of the medical equipment was tested with and without the motors running during disinfection.
Spore strips were placed inside and outside different types of medical equipment. The spore strips were placed in the same places inside the equipment in patient-associated, ventilated inner areas where air was blowing through (Freedom Freeway Nebulizer, ventilator, CPAP, etc.), or in inner parts of the equipment where air from the room was blowing through to cool the machinery or used as a compressor (pulse oximeter, Medela suction apparatus, etc.). The spore strips were placed in the middle, in dead ends, and at the inlet and outlet of air of the equipment. The inner parts of the items of medical equipment were accessed by medical technicians as these parts could not be reached by ordinary procedures. The positions of strips within the items of medical equipment were chosen to reflect parts that were not reached by ordinary disinfection procedures. Spore strip envelopes were either opened or unopened before the disinfection test was performed. Spore strips were removed after 18–20 h.
Decontamination of ambulances
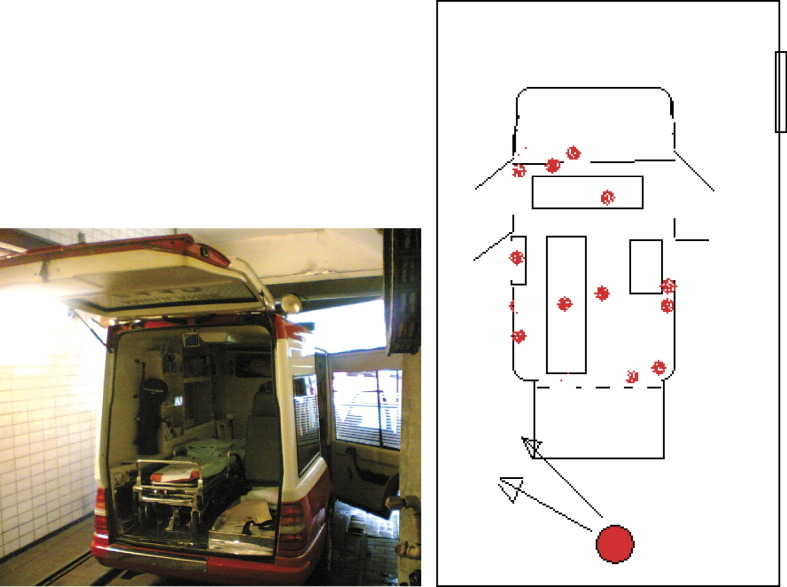
Ambulances were parked in closed garages A and B, all openings to the garages were taped over to control the concentration of the aerosol, and the ventilation systems were shut down. The doors of the ambulances were open during the decontamination period and were closed afterwards. The concentration of H2O2 was programmed according to the volume of the garages. The robot was placed in a corner so that the most effective aerosol concentration was found approximately 2 m in front of the robot. Garages A and B were of different sizes. A single robot was used in garage A (120 m3), and one and two robots were used in garage B (200 m3). Spore strips were placed inside and outside the ambulances, and inside devices, glove boxes, equipment, 15 cm under mattresses, and in the drivers' cabins (Figure 2 ).
Figure 2.
Decontamination of ambulances with a hydrogen peroxide aerosol, using a robot in a garage (round large spot), and control of effect using spore tests (small dots).
Garage A—three cycles
The diffusion time was 29 min for each cycle, reaching a concentration of 36 ppm, followed by increasing contact times of 30, 60 and 120 min. Two separate studies were performed in June and September 2004. The spore strips were placed according to a sampling plan.
Garage B—one and two cycles, using one robot
The diffusion time was 27 min for each cycle, reaching a concentration of approximately 42 ppm, followed by increasing contact times of 30 min in the first cycle and 60 min in the second cycle. The spore strips were placed according to a sampling plan.
Garage B—three cycles, using two robots
The diffusion time was 27 min for each cycle, reaching a concentration of approximately 60 ppm, followed by increasing contact times of 30, 60 and 120 min. The spores were placed according to a sampling plan.
Results
Decontamination of rooms
Test rooms
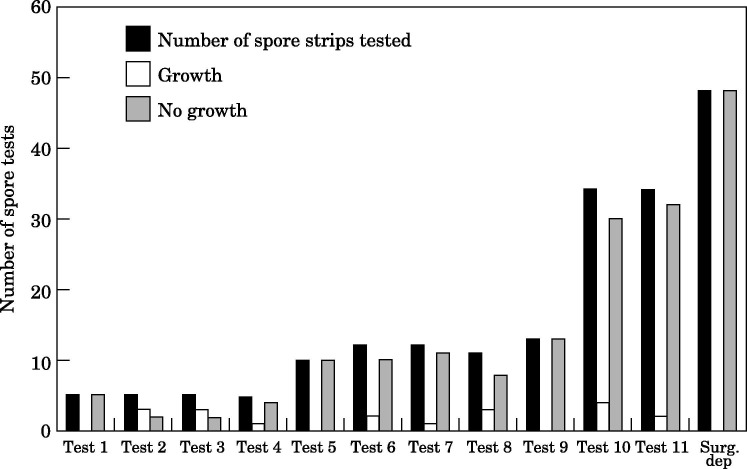
Preliminary results from disinfection of the enclosed rooms showed no growth in 6/6 spore tests. Repetitive studies were performed for 11 days (Figure 3 ). The H2O2 treatment disinfected 33/42 spore tests (78.6%) in opened envelopes, and 94/104 spore tests (90.4%) in closed envelopes. The treatment was effective in 127/146 (87%) tests.
Figure 3.
Decontamination of test rooms and 22 rooms in a surgical department with three cycles of a hydrogen peroxide aerosol.
Use in an operating department
When three cycles of H2O2 aerosol were used in 17 rooms, 48/48 (100%) spore tests showed no growth (Figure 3). When two cycles were used, 6/6 spore tests were positive, and when only one cycle was used, 12/12 spore tests were positive (not shown).
Decontamination of inner parts of medical equipment
H2O2 treatment decontaminated spores placed inside medical equipment in 37/82 (45.1%) cases when the spore strip envelope was open, and in 77/118 (65.2%) cases when the envelope was closed. The treatment was effective in 137/220 (62.3%) tests. However, decontamination was 100% effective (57/57 tests) when the device was run and ventilated during the period of decontamination.
Decontamination of ambulances
Garage A—three cycles
No growth occurred in any of the 24 spore tests during two separate experiments in June and September 2004.
Garage B—one and two cycles
There was growth after one cycle in 6/6 samples and after two cycles in 12/12 spore tests.
Garage B—three cycles
There was no growth in 36/36 spore tests, even after placement of spore strips inside equipment and in the driver's cabin. Therefore, the penetration of H2O2 into equipment, devices and the driver's cabin was effective when using three cycles of disinfection.
Discussion
Safe fumigation processes are important due to the need for infection control of areas, rooms, buildings and equipment contaminated with anthrax spores, Legionella spp., Mycobacterium tuberculosis, viruses such as the severe acute respiratory syndrome virus, Marburg, Lassa, Ebola and other high-risk micro-organisms. After the intentional release of Bacillus anthracis in Autumn 2001 in the USA, there was a need for published materials about inactivating bacillus spores. A survey of the literature showed that boiling water for >10 min can reduce B. anthracis spores by at least 106, that sodium hypochlorite is sporicidal (depending on the concentration of free available chlorine and pH), and that gaseous ‘sterilization’ of residual spores is dependent on gas type, concentration, humidity, temperature and carrier material.5
Many vapours used for decontamination are not easily eliminated by brief aeration. They may be toxic or bind to (and change) the materials in technical equipment and devices. In addition, the presence of organic material may reduce the effectiveness of most chemical disinfectants.5, 6
‘Decontamination’ is the irreversible inactivation of most, but not all, infectious agents, while ‘sterilization’ is the destruction of all microbial viability, including spores (probability of one living microbe present is less than 10−6). Chemical liquids and vapours are mainly used as decontaminating agents.1, 2, 5, 6, 7
Some chemicals, such as H2O2 gas plasma, may kill all micro-organisms including spores after prolonged exposure times (6–10 h) and are called ‘chemical sterilants’.6 H2O2 in liquid form (3–6%) is an effective disinfectant for most microbes, and a concentration of 6% or higher even has an effect on Cryptosporidium parvum with a contact time of 20 min.7 H2O2 liquid (0.88 mol/L) has been found to kill 100% of Bacillus subtilis spores after 3 and 6 h.8 H2O2 pulsed plasma (0.208 mg/L) has been reported to kill 100% of 3.4×105 B. subtilis subsp. globgii spores on paper disks after 15 min.9 H2O2 probably exerts its effect by producing destructive hydroxyl-free radicals that may attack lipid membranes, DNA and other cell components. These radicals may affect the spore core, oxidize thiol groups in proteins and enzymes of viruses and bacteria, and act on ribosomes in fungi.10 At a high concentration, H2O2 liquid will corrode metals such as copper, zinc and brass.5, 6, 7 Vapour-phase H2O2 technology sterilization is, however, a promising alternative to more toxic and potentially environmentally hazardous methods of sterilization of heat-labile materials.
The use of H2O2 vapour may be an effective way to decontaminate critical enclosed areas, such as isolation units, clean rooms, technical equipment and devices, and general areas contaminated with pathogens. Recently, French et al. 11 showed that an H2O2 vapour decontamination system, using a gas generator that evaporates 30% liquid H2O2 via a distribution system with high kinetic energy and even distribution throughout the room, was highly effective in eradicating environmental methicillin-resistant Staphylococcus aureus (MRSA). Before decontamination, 72% of room swabs yielded MRSA; afterwards, only one of 85 samples (1.2%) yielded MRSA. The decontamination cycles lasted for 5 h in total and gave a concentration of 500 ppm for 40 min.11
In the present study, we used an H2O2 dry gas system programmed to a pre-set concentration of 12–60 ppm per treatment cycle, dependent on the volume of the rooms and garages. One to three cycles were used for repetitive experiments, followed by increasing contact times of 30, 60 and 120 min, respectively; i.e. a total of 4–5 h. Medical equipment and devices, and an ambulance containing medical equipment were placed in test rooms and garages, respectively. Repetitive experiments were performed using B. atrophaeus (former subtilis) spores (Raven Biological, Nebraska, USA) to control the effect of decontamination. Spore strips were placed in various places in the rooms, ambulances, and inside and outside the pieces of medical equipment. Decontamination was effective in 87% of 146 tests in special test rooms, and in 100% of 48 tests in rooms in a surgical department, using three decontamination cycles. One or two cycles had no effect on any of the spore tests (18/18). The sporicidal effect on internal parts of the medical equipment was lower; approximately 62% of all 220 tests. However, when the medical device was run and ventilated during the disinfection period, all of the spore tests (57/57) from inside the devices were decontaminated. In ambulances, the penetration of H2O2 into equipment, devices and the driver's cabin was 100% effective (60/60 tests) when using three cycles of decontamination.
In conclusion, an H2O2 dry gas system, programmed to a pre-set concentration per treatment cycle, seemed to have a good sporicidal effect when used in rooms, ambulances and internal parts of ventilated equipment when three cycles were run. Further studies need to be performed concerning concentration, diffusion and contact times of H2O2, and the number of treatment cycles. This is especially important for inner parts of medical equipment that cannot be run and therefore ventilated during the decontamination process.
References
- 1.Norwegian Medicines Agency . Norwegian Medicines Agency; Oslo: 2002. Chemical disinfection liquids for technical use in medicine. [Google Scholar]
- 2.Andersen B.M., Hochlin K., Solheim N. Technical disinfection of medical instruments and devices. In: Andersen B.M., editor. Handbook in hygiene and infection control. Ullevaal University Hospital; Oslo: 2003. pp. 347–355. [Google Scholar]
- 3.Andersen B.M., Bergh K., Steinbakk M. A Norwegian nosocomial outbreak of methicillin-resistant Staphylococcus aureus resistant to fusidic acid and susceptible to other antistaphylococcal agents. J Hosp Infect. 1999;41:123–132. doi: 10.1016/s0195-6701(99)90049-x. [DOI] [PubMed] [Google Scholar]
- 4.Andersen B.M., Lindemann R., Bergh K. Spread of methicillin-resistant Staphylococcus aureus in a neonatal intensive unit associated with understaffing, overcrowding and mixing of patients. J Hosp Infect. 2002;1:1–7. doi: 10.1053/jhin.2001.1128. [DOI] [PubMed] [Google Scholar]
- 5.Whitney E.A.S., Beatty M.E., Taylor T.H. Inactivation of Bacillus anthracis spores. Emerg Infect Dis. 2003;9:623–627. doi: 10.3201/eid0906.020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rutala W.A. Selection and use of disinfectants in healthcare. In: Mayhall C.G., editor. Hospital epidemiology and infection control. 2nd ed. William and Wilkins; London: 1999. pp. 1161–1187. [Google Scholar]
- 7.Weber D.J., Rutala W.A. The emerging nosocomial pathogens Cryptosporidium, Escherichia coli O 157:H7, Helicobacter pylori, and hepatitis C: epidemiology, environmental survival, efficacy of disinfection, and control measures. Infect Control Hosp Epidemiol. 2001;22:306–315. doi: 10.1086/501907. [DOI] [PubMed] [Google Scholar]
- 8.Baldry M. The bactericidal, fungicidal, and sporicidal properties of hydrogen peroxide and peracetic acid. J Appl Bacteriol. 1983;54:417–423. doi: 10.1111/j.1365-2672.1983.tb02637.x. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs P., Lin S. Hydrogen peroxide plasma sterilization system. Surgikos Inc.; Arlington, TX: 1987. p. 13. [Google Scholar]
- 10.Russel A.D., Furr J.R., Maillard J.-Y. Microbial susceptibility and resistance to biocides. AMS News. 1997;63:481–487. [Google Scholar]
- 11.French G.L., Otter J.A., Shannon K.P. Tackling contamination of the hospital environment by methicillin-resistant Staphylococcus aureus (MRSA): a comparison between conventional terminal cleaning and hydrogen peroxide vapour decontamination. J Hosp Infect. 2004;57:31–37. doi: 10.1016/j.jhin.2004.03.006. [DOI] [PubMed] [Google Scholar]