Abstract
Heme biosynthesis involves a number of enzymatic steps which in eukaryotes take place in different cell compartments. Enzyme compartmentalization differs between photosynthetic and nonphotosynthetic eukaryotes. Here we investigated the structures and subcellular localizations of three enzymes involved in the heme pathway in Polytomella sp., a colorless alga evolutionarily related to the green alga Chlamydomonas reinhardtii. Functional complementation of Escherichia coli mutant strains was used to isolate cDNAs encoding three heme biosynthetic enzymes, glutamate-1-semialdehyde aminotransferase, protoporphyrinogen IX oxidase, and ferrochelatase. All three proteins show highest similarity to their counterparts in photosynthetic organisms, including C. reinhardtii. All three proteins have N-terminal extensions suggestive of intracellular targeting, and immunoblot studies indicate their enrichment in a dense cell fraction that is enriched in amyloplasts. These results suggest that even though the plastids of Polytomella sp. are not photosynthetically active, they are the major site of heme biosynthesis. The presence of a gene for glutamate-1-semialdehyde aminotransferase suggests that Polytomella sp. uses the five-carbon pathway for synthesis of the heme precursor 5-aminolevulinic acid.
Hemes are a very versatile group of iron-containing tetrapyrroles that are involved in many facets of cellular metabolism, including respiratory and photosynthetic electron transport-dependent phosphorylation, redox-dependent biosynthetic reactions, and oxidative stress responses (8). With few exceptions, heme is essential for and synthesized by all cells. Cytochromes are the predominant redox-active heme proteins in most organisms. Heme is the cofactor for cytochromes in various cell compartments, including mitochondria, chloroplasts, and the cytoplasm.
All hemes are biosynthesized from the precursor 5-aminolevulinic acid (ALA) (6). In most prokaryotes and in all photosynthetic algae and plants, ALA is made from glutamate in a three-step process involving a glutamyl-tRNA synthetase (EC 6.1.1.17), glutamyl-tRNA reductase (EC 1.2.1.70), and glutamate-1-semialdehyde aminotransferase (GSAT) (EC 5.4.3.8). In contrast, in animals, yeasts, fungi, and members of the α-proteobacteria, ALA is formed in a single step by condensation of glycine and succinyl coenzyme A catalyzed by the pyridoxal phosphate-dependent enzyme ALA synthase (EC 2.3.1.37). To date, the kinetoplastid Euglena gracilis is the only organism known to synthesize ALA via both routes (55). In animal cells, yeasts, and fungi, ALA synthase is localized in the mitochondria, whereas in photosynthetic eukaryotes, the enzymes involved in ALA synthesis from glutamate appear to be located exclusively in the chloroplast (5).
The biosynthetic steps from ALA to heme are common to nearly all organisms. In animal cells, mitochondrially synthesized ALA is exported to the cytosol, where it is converted to coproporphyrinogen III by a set of four enzymes. Coproporphyrinogen III is then transported to the mitochondria, where the last three steps of heme synthesis take place, namely, conversion of coproporphyrinogen III to protoporphyrinogen IX by coproporphyrinogen III oxidase (EC 1.1.1.3), oxidation of protoporphyrinogen IX to protoporphyrin IX by protoporphyrinogen IX oxidase (PPO) (EC 1.3.3.4), and the insertion of a Fe2+ atom into the macrocyclic ring by ferrochelatase (FeC) (EC 4.99.1.1). In yeasts and fungi, coproporphyrinogen III oxidase is cytoplasmic rather than mitochondrial (26), but the other enzymes are located as in animal cells. In contrast, in photosynthetic eukaryotes, the enzymes involved in the conversion of ALA to protoporphyrinogen IX are detected exclusively in the chloroplast (5). The localization of PPO and FeC is unresolved as yet. In angiosperm plants, PPO is usually encoded by two genes, one of whose products is believed to be targeted specifically to chloroplasts and the other to the mitochondria (28, 54). Plants also generally contain two genes for FeC, but there is a current debate concerning whether the products of these genes are located only in the chloroplasts or whether each isoform is targeted to a different organelle (9, 29, 30). In the unicellular green alga Chlamydomonas reinhardtii, PPO and FeC are each encoded by a single gene (51). Immunoblot analysis of chloroplast and mitochondrial fractions of C. reinhardtii cells indicated that both PPO and FeC are located exclusively in the chloroplast (51). Neither protein was detected in mitochondria isolated from cells grown under photosynthetic conditions. The results imply that heme is exported from the chloroplast for use in other parts of the cell. Heme export from isolated pea chloroplasts has been described previously (48). However, the mechanism of heme export from chloroplasts to the mitochondria and cytoplasm is not understood.
Polytomella sp. is a nonphotosynthetic, motile, unicellular protist that is believed to have evolved from a photosynthetic algal ancestor (16, 41). Polytomella sp. is viewed as a close relative of C. reinhardtii (1, 40, 52). Polytomella sp. cells are wall-less and have plastid-related organelles, known as amyloplasts, that contain starch. Virtually nothing is known about the metabolism of these organelles. Because Polytomella sp. does not contain a functional photosynthetic apparatus but does have active mitochondrial respiratory metabolism, it was of interest to determine which of the two known pathways it uses to synthesize ALA and whether the cells have evolved the ability to synthesize heme in the mitochondria instead of depending on the amyloplasts to synthesize heme for use in the mitochondria. Here we show the occurrence of a GSAT in Polytomella sp., suggesting that the cells use the glutamate route to ALA. Furthermore, analysis of Polytomella sp. GSAT, PPO, and FeC shows that these enzymes are most similar to their counterparts in C. reinhardtii and plants. Immunoblotting results indicate that all three enzymes are enriched in the amyloplasts.
MATERIALS AND METHODS
Algal strains, growth conditions, and subcellular fractionation.
C. reinhardtii cell wall-deficient strain CC400 was obtained from the Chlamydomonas culture collection (Duke University, Durham, NC) and maintained on Tris-acetate-phosphate medium (20) solidified with 1.5% (wt/vol) agar. The cells were routinely grown on Tris-acetate-phosphate medium at 25°C under aeration and continuous irradiance. Polytomella sp. Pringsheim 198.80 cells (obtained from the Culture Collection of Algae at the University of Göttingen, Germany) were grown aerobically at room temperature, on acetate as the sole carbon source, at pH 6.0 (52).
Polytomella sp. cell fractions were obtained as follows. Cells were disrupted with glass beads essentially as described previously (3). Polytomella sp. homogenate was centrifuged at 500 × g for 2 min, yielding a pellet enriched in amyloplasts (P1); the supernatant was centrifuged at 3,000 × g for 10 min, yielding pellet P2; and finally the supernatant was centrifuged at 15,000 × g for 10 min, yielding a pellet enriched in mitochondria (P3). Polytomella sp. cells were fractionated into soluble and membrane components as follows. Cells were harvested and resuspended in HEPES-KOH, 50 mM, pH 7.2, containing 1 mM 6-aminocaproic acid and 0.1 mM phenylmethylsulfonyl fluoride. Cells were sonicated four times for 10 s and centrifuged at 150,000 × g for 1 h. The supernatant, containing the soluble proteins, was removed, and the membrane-containing pellet was resuspended in HEPES-KOH, 50 mM, pH 7.2. The suspension was centrifuged again at 150,000 × g for 1 h, and the pellet was resuspended in HEPES-KOH, 50 mM, pH 7.2, containing 1 mM 6-aminocaproic acid and 0.1 mM phenylmethylsulfonyl fluoride.
Construction of a Polytomella sp. cDNA library and complementation of heme-deficient strains of Escherichia coli.
Total RNA of Polytomella sp. cells was isolated using an RNeasy kit (QIAGEN, Valencia, CA). Poly(A)+ RNA was isolated and used for construction of a cDNA library with a λZAPII cDNA synthesis kit (Stratagene, La Jolla, CA).
For complementation, a sample of the library was excised with helper phage VCMS13 in XL1-Blue MRF′ E. coli cells (Stratagene) and recovered as pBlueScript plasmids. E. coli GSAT-deficient (hemL) strain GE 1377 (23) was obtained from the E. coli Genetic Stock Center (Yale University, New Haven, CT). E. coli strains deficient in PPO (hemG) (43) and FeC (hemH) (32) were obtained from H. A. Dailey (University of Georgia, Athens, GA). Batches of electrocompetent E. coli hemL cells were transformed with 1 μg of plasmid and selected for ALA-independent growth on LB-ampicillin plates. Batches of electrocompetent E. coli hemH and hemG cells were transformed with 1 μg of plasmid and selected for hemin-independent growth on LB-ampicillin plates. For each transformation, approximately 2 × 108 to 3 × 108 cells were used.
DNA analysis.
Total DNA from Polytomella sp. was isolated according to Newman et al. (34). The DNA was digested with restriction enzymes, separated on a 1% (wt/vol) agarose gel, and transferred onto Hybond-N+ membranes (Amersham, Piscataway, NJ) by using standard protocols (42). An Alkphos direct labeling and detection system kit (Amersham Pharmacia Biotech) was used to label the Polytomella sp. GSAT DNA probe, and subsequent hybridization was carried out following the recommended protocol. Polytomella sp. PPO and Polytomella sp. FeC DNA probes were labeled with [α-32P]dCTP by use of a random primer labeling kit (Gibco BRL, Carlsbad, CA). Membranes were hybridized overnight at 65°C and washed twice for 20 min at 65°C in 0.2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and 0.5% sodium dodecyl sulfate (SDS).
Construction of expression vectors, overexpression, and antibody production.
The region of Polytomella sp. GSAT cDNA (from M36 to A475) was amplified using PCR primers 5′-GACGTCGACTGATGAAGGCTGCATCTAAGC-3′and 5′-GTCCTGCAGGCGTTTGCGGCGGCGCTAATC-3′(the cloning restriction sites are underlined). The region of Polytomella sp. PPO cDNA (from I54 to K577) was amplified using PCR primers 5′-GACCCATGGGAATCTCTGGGTTGTCTACTGCC-3′and 5′-GTCAGATCTTTTTTTTCTCGCCAATTC-3′.The region of Polytomella sp. FeC cDNA (from E61 to S437) was amplified using PCR primers 5′-GACGAGCTCGAGAAGCTGGGTGTGTTC-3′and 5′-GTCCTGCAGGGACCAAAGGTAGGTCGA-3′. The amplified products were ligated into pGEM-T-easy (Promega, Madison, WI).
The product for Polytomella sp. GSAT was digested with SalI and PstI and ligated into expression vector pQE-30 (QIAGEN) predigested with SalI and PstI. The product for Polytomella sp. PPO was digested with BglII and NcoI and ligated into expression vector pQE-60 (QIAGEN) predigested with BglII and NcoI. The product for Polytomella sp. FeC was digested with SacI and PstI and ligated into expression vector pQE-30 (QIAGEN) predigested with SacI and PstI. The resulting plasmids were used to transform E. coli XL1-Blue MRF′.
The pQE-30 and pQE-60 vectors introduced His6 tags at the N- and C-terminal ends of the expressed proteins, respectively. Overexpression of the His-tagged proteins was induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside). Overexpressed Polytomella sp. GSAT in inclusion bodies was purified on a Ni-nitrilotriacetic acid affinity column, according to the QIAGEN product manual, after denaturation in 8 M urea. The expressed Polytomella sp. PPO and Polytomella sp. FeC proteins were purified on Ni-nitrilotriacetic acid columns under denaturing conditions after cell proteins were denatured in 6 M guanidinium hydrochloride. Antibodies were raised against the purified Polytomella sp. GSAT, Polytomella sp. PPO, and Polytomella sp. FeC proteins in rabbits (Animal Pharm Services, Cloverdale, CA).
Protein analysis.
Resuspended cells were solubilized in 2% (wt/vol) SDS in the presence of 1 mM β-mercaptoethanol and heated at 90°C for 2 min. Proteins were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) with a 12% (wt/vol) gel and subsequently transferred onto nitrocellulose membranes (Schleicher and Schuell, Keene, NH) for immunodetection. Blots were incubated for 1 h with primary antibodies at 1:5,000 for anti-Polytomella sp. GSAT and anti-Polytomella sp. PPO and at 1:2,000 for anti-Polytomella sp. FeC, anti-Cox2a (obtained from D. González-Halphen, Autonomous National University of Mexico, Mexico City), and anti-α-amylase (Sigma-Aldrich, St. Louis, MO) and then for 1 h with horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (Pierce, Rockford, IL). Signals were visualized using an enhanced chemiluminescence system (SuperSignal West Pico kit; Pierce). A benchmark prestained protein ladder (Invitrogen, Carlsbad, CA) was used to estimate molecular masses.
Sequence analysis.
Molecular masses and pI values were calculated using Compute pI/MW (18) (available at ExPASy Tools, Swiss Institute of Bioinformatics [http://www.expasy.ch]). Prediction of targeting to mitochondria and chloroplasts was done using TargetP v 1.1 (17, 35) (available at the Center for Biological Sequence Analysis, Technical University of Denmark [http://www.cbs.dtu.dk/services/TargetP/]) and Predotar v. 1.03 (at ExPASy Tools). Prediction of transmembrane segments was performed using HMMTOP, TMHMM, and SOSUI (all at ExPASy Tools). Sequence identity calculations were done using SIM (at ExPASy Tools), with the gap open penalty set at 12 and the gap extension penalty set at 4 (22). Multiple sequence alignments were done using CLUSTALWv. 1.82 (49), and bootstrapped neighbor-joining trees were constructed using CLUSTALX v. 1.83 (50) with standard settings (1,000 trials) and visualized with TreeView v. 1.6.6 (37) (available at http://taxonomy.zoology.gla.ac.uk/rod/treeview.html).
Nucleotide sequence accession numbers.
The sequence of Polytomella sp. GSAT cDNA was deposited in GenBank under accession number AY152854, the sequence of Polytomella sp. PPO cDNA under accession number AF332964, and the sequence of Polytomella sp. FeC cDNA under accession number AF332963.
RESULTS
Characterization of GSAT, PPO, and FeC of Polytomella sp.
cDNAs for GSAT, PPO, and FeC of the Polytomella sp. heme biosynthesis pathway were isolated by functional complementation of E. coli mutant strains lacking functional GSAT, PPO, and FeC. All retrieved cDNAs contained poly(A) tails, indicating that the cDNAs were derived from nuclear transcripts.
Complementation of an E. coli hemL strain, which lacks GSAT, led to the retrieval of a high number of colonies, all containing a single type of Polytomella sp. cDNA. The isolated 1,756-bp cDNA exhibits a 1,440-bp open reading frame (ORF) for a protein of 480 amino acids with a predicted molecular mass of 50,794 Da. Polytomella sp. GSAT has 80% similarity with C. reinhardtii GSAT (31) (GenBank accession number Q39566) and 67% similarity with E. coli GSAT (GenBank accession number P23893). Compared to bacterial GSAT, cDNA-predicted GSATs of photosynthetic eukaryotes exhibit an N-terminal extension which serves as a chloroplast-targeting peptide. Polytomella sp. GSAT also exhibits a 40-residue extension at its N terminus (Fig. 1A), which appears to be an intracellular targeting sequence. Interestingly, this extension exhibits typical features of mitochondrial targeting sequences: a high content of hydroxylated residues and positively charged residues as well as a potential amphiphilic α-helix (G16 through A32). The targeting prediction programs Predotar and TargetP gave higher scores for targeting to chloroplasts than to mitochondria (Table 1). The most-N-terminal region of the predicted mature Polytomella sp. GSAT that has high similarity to both eukaryotic and prokaryotic GSATs begins at residue T46 of the predicted precursor protein. The invariant active-site Lys occurs at position 181 of the precursor protein (see Fig. S1 in the supplemental material). The predicted 442-residue mature Polytomella sp. GSAT has a molecular mass of 46,617 Da and a pI of 7.01. Mature Polytomella sp. GSAT was predicted to be soluble by the programs HMMTOP, TMHMM, and SOSUI. Polytomella sp. GSAT has a short insertion, not present in other GSATs, containing mostly charged residues (EELGKEVEKK), beginning at residue E407 of the preprotein (see Fig. S1 in the supplemental material). Modeling based on the X-ray crystallographic structure of GSAT from Synechococcus sp. PCC 6301 (21) (Protein Data Bank accession number 2GSA) indicates that the insert lies within a solvent-exposed loop near the distal apices of the homodimeric protein (results not shown).
FIG. 1.
Sequence alignments of the N termini of predicted GSAT, PPO, and FeC of Polytomella sp. with representative prokaryotic and eukaryotic counterparts. The full-length, unprocessed eukaryotic precursors are shown. The alignments were generated using the CLUSTALW algorithm and refined manually. Residues that are similar or identical are shaded by gray or black, respectively; photosynthetic organisms are indicated in bold; and ↓ indicates the N termini of the mature proteins, predicted on the basis of the start of similarities with eukaryotic and prokaryotic sequences. (A) ARATH, Arabidopsis thaliana GSAT-II (GenBank accession number Q42522); CHLRE, C. reinhardtii (Q39566); CHLTE, Chlorobium tepidum TLS (NP_662973); ECOLI, E. coli (P23893); POLSP, Polytomella sp. (AY152854); THEEL, Thermosynechococcus elongatus BP-1 (Q8DLK8); TOBAC, N. tabacum (P31593). (B) ARATH-I, A. thaliana PPO-I (P55826); BACSU, Bacillus subtilis (P32397); CHLRE, C. reinhardtii (Q9ZTA7); HUMAN, Homo sapiens (P50336); POLSP, Polytomella sp. (AF332964); SOLTU, Solanum tuberosum (O64384); THEEL, T. elongatus BP-1 (Q8DLV2); TOBAC-I, N. tabacum PPO-I (O24163); TOBAC-II, N. tabacum PPO-II (O24164); YEAST, S. cerevisiae (P40012). (C) ARATH-I, A. thaliana FeC-I (P42043); ARATH-II, A. thaliana FeC-II (O04921); BACSU, B. subtilis (P32396); CHLRE, C. reinhardtii (Q9ATG8); ECOLI, E. coli (P23871); HUMAN, H. sapiens (P22830); POLSP, Polytomella sp. (AAK16729); SOLTU, S. tuberosum (O64391); THEEL, T. elongatus BP-1 (Q8DLV2); YEAST, S. cerevisiae (P16622).
TABLE 1.
Polytomella sp. heme biosynthetic enzymes
| Polytomella sp. enzyme | % Identitya
|
Targeting prediction score
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C. reinhardtii | Arabidopsis thaliana | Thermosynechococcus elongatus | E. coli | Human | S. cerevisiae | Predotar
|
TargetP
|
|||
| Chloroplast | Mitochondria | Chloroplast | Mitochondria | |||||||
| GSAT | 69 | 63 | 66 | 55 | 0.06 | 0.01 | 0.44 | 0.06 | ||
| PPO | 42 | 43 | 40 | 07 | 28 | 16 | 0.16 | 0.64 | 0.52 | 0.38 |
| FeC | 61 | 57 | 56 | 28 | 40 | 37 | 0.36 | 0.22 | 0.03 | 0.44 |
Percent identity between Polytomella sp. predicted proteins and their homologs in C. reinhardtii, A. thaliana (GSAT-I or GSAT-II, PPO-I, and FeC-I), T. elongatus BP-1, E. coli, human (FeC isoform a), and S. cerevisiae (human and S. cerevisiae do not possess GSAT).
Complementation of an E. coli hemG strain, which lacks PPO activity, led to the retrieval of four colonies. The 2,060-bp cDNA for Polytomella sp. PPO exhibits an ORF of 1,731 bp encoding a precursor protein of 577 amino acids and a calculated molecular mass of 62,086 Da. Polytomella sp. PPO shows highest sequence identity to PPOs from photosynthetic eukaryotes (∼40%). The identity of Polytomella sp. PPO to mitochondrial PPOs is significantly lower, ranging between 16% and 28%, and the identity to E. coli PPO is very low (Table 1). As is found for PPOs from photosynthetic eukaryotes, Polytomella sp. PPO exhibits an N-terminal extension compared to prokaryotic PPOs (Fig. 1B). Of note, the N-terminal extensions in Polytomella sp. and C. reinhardtii differ in both length and amino acid content. The targeting prediction programs TargetP and Predotar gave inconclusive results (Table 1). The TargetP-predicted presequence (111 residues) includes residues that are within the region that has significant similarity to both eukaryotic and prokaryotic PPOs, and therefore it is likely that this predicted presequence is too long. A plausible mature Polytomella sp. PPO would begin at residue F41 of the preprotein, which is the beginning of the most-N-terminal region that has similarity to both eukaryotic and prokaryotic PPOs. Starting from this residue, the predicted mature Polytomella sp. PPO has 537 residues, a molecular mass of 57,635 Da, and a pI of 8.66. Polytomella sp. PPO contains an invariant GXGXXG motif near the N terminus (residues 51 through 56 of the preprotein) (Fig. 1B; see also Fig. S2 in the supplemental material) that has been proposed to be a dinucleotide-binding motif for binding the flavin adenine dinucleotide (FAD) cofactor (12, 13). This proposed functional assignment has been partially confirmed by the recently available crystal structure of PPO-II from Nicotiana tabacum, which indicates that one of these residues, which corresponds to S55 of the Polytomella sp. PPO preprotein, is hydrogen bonded to the cofactor (25) (Protein Data Bank accession number 1SEZ). Polytomella sp. PPO exhibits an atypical insertion of approximately 28 residues (RMSHQGDDDSSRTAGAVPRTAEGDVAAG) that begins at or near residue R326 of the preprotein, which is not present in other PPOs and is characterized by a high content of charged residues (see Fig. S2 in the supplemental material). This sequence had no matches in the GenBank database. Modeling based on the X-ray crystallographic structure of PPO-II from N. tabacum indicates that the insert lies within a solvent-exposed loop in the FAD-binding domain, distal to the homodimer interface and away from the membrane-binding domain and putative domain for interaction with FeC (results not shown).
Complementation of an E. coli hemH strain, which lacks FeC activity, led to the retrieval of four colonies. The 2,227-bp cDNA for Polytomella sp. FeC exhibits a 1,320-bp ORF encoding a precursor protein of 440 amino acids and a calculated molecular mass of 48,571 Da. Comparison with bacterial FeC sequences indicates that Polytomella sp. FeC has a long N-terminal sequence, likely an intracellular targeting peptide (Fig. 1C). The N-terminal extension of Polytomella sp. FeC shares no obvious features with that of C. reinhardtii FeC. As for Polytomella sp. PPO, the targeting prediction programs TargetP and Predotar gave inconclusive results (Table 1). The most-N-terminal region of the predicted mature Polytomella sp. FeC that has high similarity to both eukaryotic and prokaryotic FeCs begins at residue K5 of the predicted mature protein. The predicted 394-residue mature Polytomella sp. FeC has a molecular mass of 43,584 Da and a pI of 5.76. An invariant active-site His, which is required for activity (56), is located at position 257 of the preprotein (see Fig. S3 in the supplemental material). Polytomella sp. FeC also exhibits a conserved, mostly hydrophobic 46-residue loop, YNLFNDPDIIRMPPVANMFQPIVAKIISSTRASKSAKGYESIGGGS, beginning at residue Y84 of the preprotein, and a 12-residue loop, AYQSRVGPTEWL, beginning at residue A298 of the preprotein. These loops form upper and lower “lips,” respectively, at the opening of the active site pocket and are reported to confer membrane association of FeCs (14, 46, 56). The C-terminal portions of FeCs show characteristics specific to certain groups of organisms (11, 14). The FeCs of animals, yeasts, and plants contain a C-terminal extension that is absent from most bacterial FeCs (11). Animal FeCs and those of some yeasts and bacteria are characterized by the presence of a [2Fe-2S] cluster (10, 14). Three of the four Cys ligands required to bind the [2Fe-2S] cluster are found at the C terminus of these FeCs (10). No Cys residues for a [2Fe-2S] cluster are found in FeCs of Saccharomyces cerevisiae or photosynthetic eukaryotes. The C terminus is also involved in the dimerization of FeCs, although some FeCs that contain a C-terminal extension are nonetheless monomeric (14, 56). In many FeCs from photosynthetic organisms, the C terminus exhibits an LHC motif, a consensus sequence of light-harvesting chlorophyll-binding proteins, which could play a role in directing the protein to the thylakoid membranes or anchoring the protein in the membranes (24). The C terminus of Polytomella sp. FeC has an intermediate length, longer than those of FeCs of photosynthetic organisms and shorter than those of FeCs of yeasts and mammals (see Fig. S3 in the supplemental material). Analysis of the C terminus of Polytomella sp. FeC did not reveal the presence of either the LHC motif or the [2Fe-2S] cluster-binding Cys residues (see Fig. S3 in the supplemental material).
Southern hybridizations.
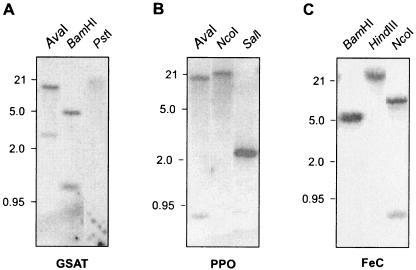
To determine the numbers of gene copies for the enzymes of interest, total Polytomella sp. genomic DNA was digested with different restriction enzymes. Southern analysis utilizing DNA probes corresponding to the predicted coding regions of Polytomella sp. GSAT, Polytomella sp. PPO, and Polytomella sp. FeC was carried out under high-stringency hybridization conditions. The hybridization patterns support the presence of one gene each for GSAT, PPO, and FeC in Polytomella sp. (Fig. 2).
FIG. 2.
Southern blot analysis of GSAT, PPO, and FeC in Polytomella sp. Total DNA (20 μg) was digested with restriction enzymes and subjected to DNA blot analysis. Hybridization was performed using Polytomella sp. predicted ORFs for GSAT, PPO, and FeC. Positions of size markers (kbp) are indicated at the left of each blot. GSAT cDNA contains one restriction site for AvaI, one for BamHI, and none for PstI; PPO cDNA contains three restriction sites for AvaI, none for NcoI, and two for SalI; and FeC cDNA contains one restriction site for BamHI, none for HindIII, and two for NcoI.
Heterologous expression of Polytomella sp. proteins in E. coli and antibody production.
Putative mature Polytomella sp. GSAT, Polytomella sp. PPO, and Polytomella sp. FeC were efficiently expressed in the E. coli wild-type strain for these proteins (not shown). The recombinant proteins were purified under denaturing conditions and used for antibody production.
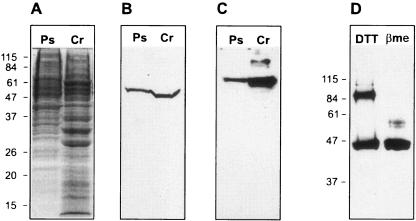
Anti-Polytomella sp. GSAT antiserum recognized in Polytomella sp. cell extract a single protein with an apparent molecular mass of ∼54 kDa (Fig. 3B), which is somewhat higher than the molecular mass of predicted mature Polytomella sp. GSAT (∼47 kDa). The anti-Polytomella sp. GSAT antibodies also recognized C. reinhardtii GSAT as a slightly smaller protein (molecular mass of ∼48 kDa).
FIG. 3.
Immunoblots identifying the enzymes involved in the heme biosynthetic pathway in Polytomella sp. Protein samples were separated by 12% (wt/vol) SDS-PAGE and transferred to nitrocellulose membranes. Immunodetection was performed with specific polyclonal antibodies produced in this work. (A) Coomassie blue-stained SDS-PAGE loaded with cell extracts (40 μg each) from Polytomella sp. (Ps) and C. reinhardtii (Cr). (B) Immunodetection of GSAT in cell extracts (40 μg each). (C) Immunodetection of PPO in cell extracts (40 μg). (D) Immunodetection of FeC in Polytomella sp. cell extracts (100 μg) in the presence of 1 mM β-mercaptoethanol (βme) or 50 mM dithiothreitol (DTT). Positions of molecular mass markers (kDa) are indicated at the left of the gel and blots.
Anti-Polytomella sp. PPO recognized in Polytomella sp. cell extract a single protein with an apparent molecular mass of ∼65 kDa, larger than the molecular mass calculated for the predicted mature Polytomella sp. PPO (∼58 kDa) (Fig. 3C). The antibody also recognized C. reinhardtii PPO as a slightly shorter protein (∼63 kDa), consistent with the fact that Polytomella sp. PPO contains a 24-residue insertion compared to its counterpart in C. reinhardtii.
Anti-Polytomella sp. FeC recognized in cell extract from Polytomella sp. a protein with an apparent molecular mass of ∼45 kDa. With β-mercaptoethanol in the gel loading buffer, a single band was detected, whereas in the presence of 50 mM dithiothreitol, an additional band at ∼90 kDa was observed (Fig. 3D). It is likely that the 90-kDa form corresponds to a Polytomella sp. FeC homodimer. However, any role of Cys residues in holding the dimer together is unlikely to be direct. Modeling based on the X-ray crystallographic structure of a human FeC (56) (Protein Data Bank accession number 1HRK) indicates that the four Cys residues present in the predicted mature form of Polytomella sp. FeC are spaced too far apart to form intra- or intersubunit disulfide bonds in the native enzyme (results not shown).
Subcellular localization of Polytomella sp. GSAT, PPO, and FeC.
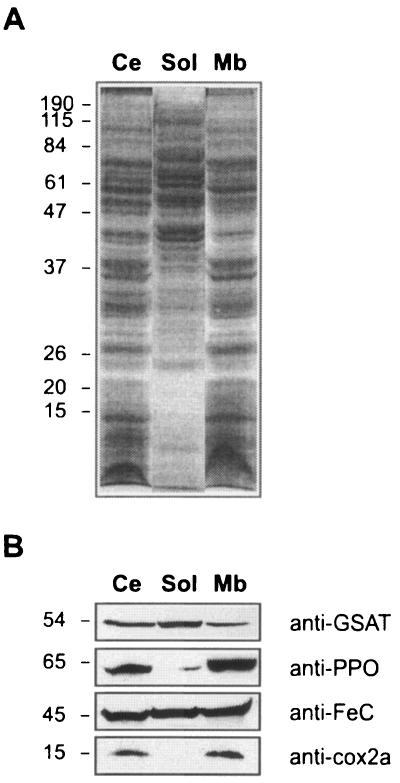
Association of Polytomella sp. proteins with total cell membranes was examined. Cells grown on acetate at pH 6.0 were fractionated into their soluble and membrane components after sonication and ultracentrifugation. Cell fractions were loaded on an SDS-PAGE gel and transferred to a nitrocellulose membrane for immunoblotting. To assess the effectiveness of the fractionation, cell fractions were probed with a specific antibody raised against Cox2a, a mitochondrial cytochrome c oxidase subunit which has two transmembrane helices (38). The 15-kDa Cox2a protein was detected only in the membrane fraction (Fig. 4B). GSAT was found mostly in the soluble fraction. In contrast, PPO was found mostly in the membrane fractions and was barely detectable in the soluble fraction. Surprisingly, FeC was distributed between the soluble and membrane fractions, suggesting that the protein is loosely attached to the membranes.
FIG. 4.
Distribution of GSAT, PPO, and FeC between membrane and soluble fractions of Polytomella sp. Whole Polytomella sp. cells grown on acetate as the sole carbon source were sonicated and fractionated into their membrane and soluble components. Proteins in cell fractions were electrophoresed on a 12% (wt/vol) SDS-PAGE gel and transferred to nitrocellulose membranes. (A) SDS-PAGE gel stained with Coomassie blue. (B) Immunoblot analysis with the indicated antibody probes. Ce, whole cells; Sol, soluble fraction; Mb, membrane fraction. Positions of molecular mass markers (kDa) are indicated at the left of the gel and blots.
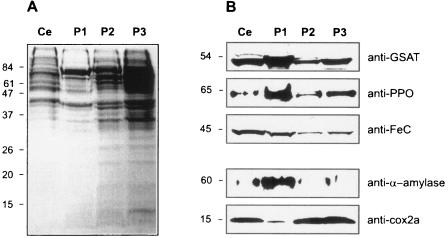
Protocols to obtain pure organelles from Polytomella sp. are not currently available. So far, studies carried out with mitochondria were done with a fraction obtained by differential centrifugations of glass-bead-disrupted cells (3). Blue native-PAGE analysis has shown that this fraction contains primarily enzymes of mitochondrial metabolism and oxidative phosphorylation (4, 52). To determine the intracellular localization of the proteins of interest, mitochondria and amyloplasts were separated on the basis of the difference in their densities. Amyloplasts are dense organelles, due to their high content in starch, and can be sedimented by a 15-s centrifugation at 500 × g. The pellets resulting from differential centrifugation of glass-bead-disrupted cells were analyzed by SDS-PAGE. Coomassie blue-stained gels showed different protein profiles of the pellets from low- and high-speed centrifugations (Fig. 5A). The first pellet (P1), resulting from the short, low-speed centrifugation, was whitish and enriched in α-amylase, but the mitochondrial protein Cox2a was barely detectable in this pellet (Fig. 5B). These results indicate that this fraction contains amyloplasts and is largely devoid of mitochondria but that it might contain some unbroken cells. In contrast, the higher-speed pellets (P2 and P3) showed abundant amounts of Cox2a but very low amounts of α-amylase, indicating that these fractions are enriched in mitochondria and largely devoid of amyloplasts. Immunoblots show that GSAT, PPO, and FeC were all enriched in the fraction that contained predominantly amyloplasts compared to the fraction that contained predominantly mitochondria. These results indicate that all three of these proteins involved in heme biosynthesis are concentrated in the amyloplasts. However, the results do not rule out the possibility that the mitochondria might contain minor amounts of these proteins.
FIG. 5.
Subcellular localization of GSAT, PPO, and FeC in Polytomella sp. Proteins in cell fractions were electrophoresed on a 12% (wt/vol) SDS-PAGE gel and transferred to nitrocellulose membranes. (A) SDS-PAGE gel stained with Coomassie blue. (B) Immunoblot analysis with the indicated antibody probes. Samples were as follows: extract of whole cells (Ce) (50 μg), a fraction enriched in amyloplasts (P1, 50 μg), and fractions enriched in mitochondria (P2, 50 μg, and P3, 75 μg). Positions of molecular mass markers (kDa) are indicated at the left of the gel and blots.
DISCUSSION
This work reports for the first time the use of functional complementation of E. coli mutant strains to isolate specific genes of the colorless alga Polytomella sp. All three cDNAs that complemented the heme biosynthesis-defective mutants encode proteins which have N-terminal extensions compared to their homologs in prokaryotes. Although the proteins are distinct from their E. coli counterparts, particularly in containing targeting sequences and various insertions, they were still sufficiently related to functionally complement the E. coli mutant cells.
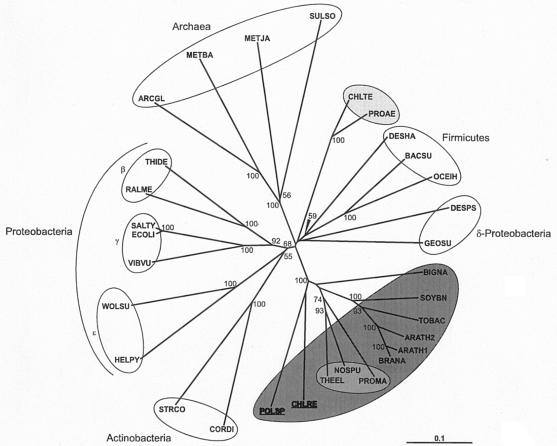
By use of complementation of E. coli hemL mutants, a single class of cDNA was isolated. Polytomella sp. cDNA encodes a typical GSAT which shows high similarity with C. reinhardtii GSAT. Complementation did not retrieve any clones for an ALA synthase, in contrast to the results of complementing ALA synthesis-defective E. coli mutants with α-proteobacterial and human cDNAs (27, 45). The isolation of Polytomella sp. cDNA for GSAT, but not for ALA synthase, indicates that there exists in this colorless alga, as in all photosynthetic eukaryotes, the five-carbon pathway for ALA synthesis. Phylogenetic analysis of the available GSAT sequences shows three major groups: the archaea, the bacteria except the cyanobacteria, and the cyanobacteria plus photosynthetic eukaryotes. Polytomella sp. GSAT groups with GSATs of cyanobacteria and photosynthetic eukaryotes (Fig. 6). It is thus hypothesized that Polytomella sp., which has apparently evolved from a photosynthetic C. reinhardtii-like ancestor, has retained the glutamate pathway to synthesize its hemes.
FIG. 6.
Bootstrapped neighbor-joined rootless trees showing the relatedness of GSATs from various sources. For the eukaryotic enzymes, full-length preproteins were used in the analyses. Bootstrap values above 50 are shown at the nodes. The bar labeled “0.1” is the branch length representing a mean difference of 0.1 per residue along each branch. Photosynthetic organisms are shaded as follows: dark gray, eukaryotes; medium gray, cyanobacteria; light gray, chlorobia. ARATH1, Arabidopsis thaliana GSAT-I (GenBank accession number P42799); ARATH2, A. thaliana GSAT-II (Q42522); ARCGL, Archaeoglobus fulgidus DSM 4304 (AAB90001); BACSU, Bacillus subtilis (NP_390690); BIGNA, Bigelowiella natans (Q7XYK0); BRANA, Brassica napus (Q85WB7); CHLRE, C. reinhardtii (Q39566); CHLTE, Chlorobium tepidum TLS (NP_662973); CORDI, Corynebacterium diphtheriae (Q6NJJ2); DESHA, Desulfitobacterium hafniense DCB-2 (ZP_00098839); DESPS, Desulfotalea psychrophila LSv54 (YP_064548); ECOLI, E. coli (P23893); GEOSU, Geobacter sulfurreducens PCA (NP_951397); HELPY, Helicobacter pylori (P56115); METBA, Methanosarcina barkeri (ZP_00297199); METJA, Methanocaldococcus jannaschii (C64375); NOSPU, Nostoc punctiforme PCC 73102 (ZP_00111760); OCEIH, Oceanobacillus iheyensis HTE831 (NP_692986); POLSP, Polytomella sp. Pringsheim 198.80 (AAN74531); PROAE, Prosthecochloris aestuarii DSM 271 (ZP_00591188); PROMA, Prochlorococcus marinus (NP_874875); RALME, Ralstonia metallidurans CH34 (ZP_00275477); SALTY, Salmonella enterica (NP_804085); SOYBN, Glycine max (P45621); STRCO, Streptomyces coelicolor (Q9F2S0); SULSO, Sulfolobus solfataricus (Q980U5); THEEL, Thermosynechococcus elongatus BP-1 (Q8DLK8); THIDE, Thiobacillus denitrificans ATCC 25259 (ZP_00334465); TOBAC, N. tabacum (P31593); VIBVU, Vibrio vulnificus YJ016 (Q7MHY9); WOLSU, Wolinella succinogenes (Q7M847).
Recently, two other colorless algae, Helicosporidium sp. and Prototheca wickerhamii, were reported to contain genes encoding GSAT (7, 15). In contrast, the malaria parasite, Plasmodium falciparum, even though possessing a plastid remnant, apparently does not contain a gene for GSAT. Instead, P. falciparum synthesizes ALA in the mitochondria via the ALA synthase route (44, 53).
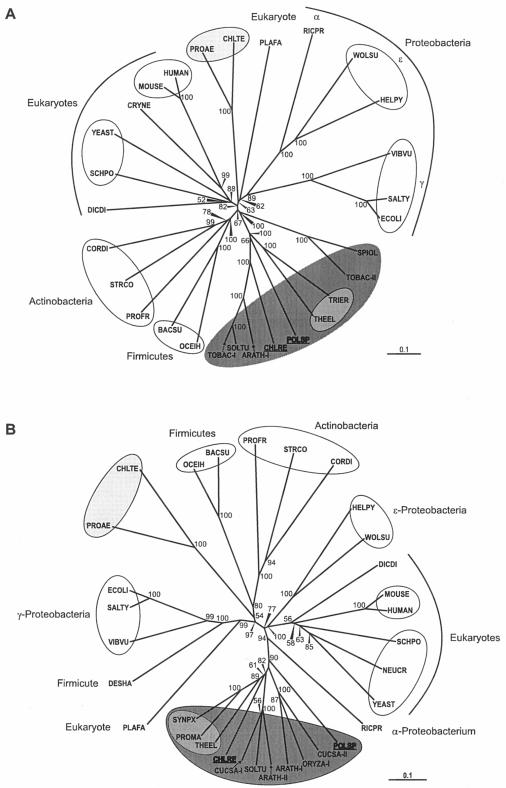
Of the three Polytomella sp. proteins analyzed in this work, PPO is the most divergent from that of C. reinhardtii (Table 1). Nevertheless, PPO phylogenetic analysis showed that Polytomella sp. PPO clusters with the group formed by photosynthetic eukaryotes and cyanobacteria (Fig. 7A). Furthermore, the analysis showed two distinct clusters of PPOs among photosynthetic organisms, which seem to correspond to the two forms of PPO identified to date in plants: PPO-I, a form present in chloroplasts of photosynthetic tissues, and PPO-II, a form that was variously reported to be located in the mitochondria or dually targeted to mitochondria and chloroplasts (28, 54). For C. reinhardtii, a single form of PPO, which was localized to the chloroplast and shown to group with the PPO-I forms expressed in photosynthetic tissues, was identified (51). Polytomella sp. PPO also groups with the photosynthetic tissue form (Fig. 7A). Polytomella sp. PPO was found to be membrane bound, as is the PPO of C. reinhardtii (51) as well as those of plants, animals, and most bacteria (12, 13).
FIG. 7.
Bootstrapped neighbor-joined rootless trees showing the relatedness of PPO (A) and FeC (B) enzymes from various sources. For the eukaryotic enzymes, full-length preproteins were used in the analyses. Bootstrap values above 50 are shown at the nodes. The bar labeled “0.1” is the branch length representing a mean difference of 0.1 per residue along each branch. Photosynthetic organisms are shaded as described in the legend for Fig. 6. (A) ARATH-I, Arabidopsis thaliana (GenBank accession number P55826); BACSU, Bacillus subtilis (P32397); CHLRE, C. reinhardtii (Q9ZTA7); CHLTE, Chlorobium tepidum (Q8KB91); CORDI, Corynebacterium diphtheriae (Q6NJJ3); CRYNE, Cryptococcus neoformans (Q5KDI9); DICDI, Dictyostelium discoideum (Q54DT8); ECOLI, E. coli (P27863); HELPY, Helicobacter pylori (O25143); HUMAN, Homo sapiens (P50336); MOUSE, Mus musculus (P51175); OCEIH, Oceanobacillus iheyensis HTE831 (NP_692090); PLAFA, P. falciparum isolate 3D7 (Q8IJC3); POLSP, Polytomella sp. (Q9ATG6); PROAE, Prosthecochloris aestuarii DSM 271 (ZP_00591000); PROFR, Propionibacterium freudenreichii (O32434); RICPR, Rickettsia prowazekii strain Madrid E (NP_221195); SALTY, Salmonella enterica subsp. enterica serovar Typhi (CAD07906); SCHPO, Schizosaccharomyces pombe (P40012); SOLTU, Solanum tuberosum (O64384); SPIOL, Spinacia oleracea (Q94IG7); STRCO, Streptomyces coelicolor (Q8CJP6); THEEL, Thermosynechococcus elongatus (Q8DLV2); TOBAC-I, N. tabacum PPOI (O24163); TOBAC-II, N. tabacum PPOII (O24164); TRIER, Trichodesmium erythraeum IMS101 (ZP_00325289); VIBVU, Vibrio vulnificus YJ016 (NP_932826); WOLSU, Wolinella succinogenes (Q7MAI5); YEAST, S. cerevisiae (P40012). (B) ARATH-I, A. thaliana HEMH1 (P42043); ARATH-II, A. thaliana HEMH2 (O04921); BACSU, B. subtilis (P32396); CHLRE, C. reinhardtii (Q9ATG8); CHLTE, C. tepidum (Q8KEC6); CORDI, C. diphtheriae (Q6NH66); CUCSA-I, Cucumis sativus (Q9FEK8); CUCSA-II, C. sativus (P42044); DESHA, Desulfitobacterium hafniense DCB-2 (ZP_00102659); DICDI, D. discoideum (Q54IA8); ECOLI, (P23871); HELPY, H. pylori (P56107); HUMAN, H. sapiens (P22830); MOUSE, M. musculus (P22315); NEUCR, Neurospora crassa (Q7SA94); OCEIH, O. iheyensis (Q8ERX9); ORYZA-I, Oryza sativa (Q69TB1); PLAFA, P. falciparum (Q8IFR0); POLSP, Polytomella sp. (AAK16729); PROAE, P. aestuarii DSM 271 (ZP_00591682); PROFR, P. freudenreichii subsp. shermanii (P72183); PROMA, Prochlorococcus marinus (Q7VD58); RICPR, R. prowazekii (Q9ZC84); SALTY, S. enterica serovar Typhimurium (P37408); SCHPO, S. pombe (O59786); SOLTU, S. tuberosum (O64391); STRCO, S. coelicolor (O50533); SYNPX, Synechococcus sp. WH8102 (Q7U5G0); THEEL, T. elongatus (Q8DGU6); VIBVU, V. vulnificus (Q8DFM2); WOLSU, W. succinogenes (Q7M7P9); YEAST, S. cerevisiae (P16622).
Phylogenetic analysis indicates that Polytomella sp. FeC branches with FeCs of photosynthetic eukaryotes, away from the strong group of nonphotosynthetic eukaryotes (Fig. 7B). For plants, two forms of FeC have been described. FeC-I is found in chloroplasts but is mainly expressed in nonphotosynthetic tissues. FeC-II is found predominantly in chloroplasts and is expressed in photosynthetic tissues in a light-responsive manner (30, 47). C. reinhardtii contains a single FeC form that was localized to the chloroplast, which branches with the plant FeCs expressed in photosynthetic tissues (51). The resolution of Polytomella sp. FeC is not clear in our analysis because it is rooted close to the branching point between FeC-I and FeC-II. Nevertheless, Polytomella sp. FeC differs from these FeCs in amino acid sequence and likely in solubility. Particularly, the C-terminal domain of Polytomella sp. FeC is shorter than the corresponding domain in C. reinhardtii FeC and lacks the LHC motif. The role of the LHC motif in FeCs, found to date only in the forms of FeC expressed in photosynthetic tissues (51), is not understood. The possibility that the LHC motif plays a role in light regulation or targeting to the thylakoid membranes is strengthened by its absence from the FeC of Polytomella sp., which does not contain thylakoid membranes (33). The C-terminal domains of plant and animal FeCs are responsible for dimerization (11). Polytomella sp. FeC, which has a C-terminal domain intermediate in length between those of animals and plants, tends to dimerize even under the denaturing conditions of SDS-PAGE. Generally, FeCs of plant and animal cells are firmly attached to the membranes of chloroplasts and mitochondria, respectively, via conserved, mostly hydrophobic loops that form lips at the entry to the active site pocket (11, 46, 56). Despite possessing these conserved domains, Polytomella sp. FeC is unusual for a eukaryotic FeC in being relatively loosely associated with membranes.
The three Polytomella sp. heme biosynthetic enzymes all exhibit predicted long N-terminal extensions relative to their prokaryotic counterparts, and these extensions likely serve as targeting peptides. These targeting sequences are different from the sequences for mitochondrial and chloroplast targeting in C. reinhardtii and plants, and predictions based on current targeting prediction programs are inconclusive. Although a few mitochondrial presequences are known for Polytomella sp. (4, 39), no targeting peptide that serves as an amyloplast targeting sequence has been identified as yet. The set of experiments carried out in the present work showed a clear abundance of GSAT, PPO, and FeC in a fraction that is enriched in starch and α-amylase, but all three proteins were detected, albeit at lower abundance, in a fraction that is enriched in the mitochondrial protein Cox2a. This might result from a slight contamination of mitochondria by amyloplast membranes. Although the products of GSAT-encoding genes of the nonphotosynthetic algae Helicosporidium sp. and P. wickerhamii were predicted on the basis of phylogenetic analysis to be targeted to the plastids, direct determination of their intracellular locations has not been reported (7, 15). PPO and FeC have not been analyzed for these algae.
An obvious function of the amyloplasts in Polytomella sp. is starch synthesis and accumulation, as for the chloroplasts in photosynthetic eukaryotes. Other possible metabolic functions of Polytomella sp. amyloplasts are not well understood. As with all respiring eukaryotes, hemes are required in the Polytomella sp. mitochondrial electron transport chain. Mitochondrial cytochromes in Polytomella sp. have been extensively characterized (1, 2, 19, 38, 39, 52). In contrast, there is no evidence to date for cytochromes or other hemoproteins in the amyloplasts. From the present results, it is inferred that amyloplasts are the major site of heme synthesis in Polytomella sp., thus resembling the chloroplasts of C. reinhardtii and plants. At present, it cannot be concluded whether GSAT, PPO, and FeC are located exclusively in the amyloplasts of Polytomella sp., as they are in the chloroplast of C. reinhardtii (36, 51). Further knowledge about the intracellular localization of Polytomella sp. heme biosynthetic enzymes will benefit from the development of protocols for isolation of pure mitochondria and amyloplasts as well as for in vivo localization of the proteins.
Supplementary Material
Acknowledgments
This work was supported by National Science Foundation grant MCB-9808578 and Department of Energy grant DE-FG02-88ER13918 to S.I.B.
We thank H. A. Dailey for providing E. coli hemG and hemH strains, D. González-Halphen for anti-Cox2a antibody, and L. A. Nogaj for critical comments on the manuscript.
Footnotes
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Antaramian, A., R. Coria, J. Ramírez, and D. González-Halphen. 1996. The deduced primary structure of subunit I from cytochrome c oxidase suggests that the genus Polytomella shares a common mitochondrial origin with Chlamydomonas. Biochim. Biophys. Acta 1273:198-202. [DOI] [PubMed] [Google Scholar]
- 2.Antaramian, A., S. Funes, M. Vásquez-Acevedo, A. Atteia, R. Coria, and D. González-Halphen. 1998. Two unusual amino acid substitutions in cytochrome b of the colorless alga Polytomella spp.: correlation with the atypical spectral properties of the bH heme. Arch. Biochem. Biophys. 354:206-214. [DOI] [PubMed] [Google Scholar]
- 3.Atteia, A., R. van Lis, J. Ramirez, and D. González-Halphen. 2000. Polytomella spp. growth on ethanol. Extracellular pH affects the accumulation of mitochondrial cytochrome c550. Eur. J. Biochem. 267:2850-2858. [DOI] [PubMed] [Google Scholar]
- 4.Atteia, A., R. van Lis, G. Mendoza-Hernández, K. Henze, W. Martin, H. Riveros-Rosas, and D. González-Halphen. 2003. Bifunctional aldehyde/alcohol dehydrogenase (ADHE) in chlorophyte algal mitochondria. Plant Mol. Biol. 53:175-188. [DOI] [PubMed] [Google Scholar]
- 5.Beale, S. I. 1999. Enzymes of chlorophyll biosynthesis. Photosynth. Res. 60:43-73. [Google Scholar]
- 6.Beale, S. I. Biosynthesis of 5-aminolevulinic acid. In B. Grimm, R. Porra, W. Rüdiger, and H. Scheer (ed.), Chlorophylls and bacteriochlorophylls: biochemistry, biophysics, functions and applications, in press. Springer, Dordrecht, The Netherlands.
- 7.Borza, T., C. E. Popescu, and R. W. Lee. 2005. Multiple metabolic roles for the nonphotosynthetic plastid of the green alga Prototheca wickerhamii. Eukaryot. Cell 4:253-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapman, S. K., S. Daff, and A. W. Munro. 1997. Heme: the most versatile redox centre in biology? Struct. Bonding 88:39-70. [Google Scholar]
- 9.Chow, K. S., D. P. Singh, A. R. Walker, and A. G. Smith. 1998. Two different genes encode ferrochelatase in Arabidopsis: mapping, expression and subcellular targeting of the precursor proteins. Plant J. 15:531-541. [DOI] [PubMed] [Google Scholar]
- 10.Dailey, H. A., M. G. Finnegan, and M. K. Johnson. 1994. Human ferrochelatase is an iron-sulfur protein. Biochemistry 33:403-407. [DOI] [PubMed] [Google Scholar]
- 11.Dailey, H. A., T. A. Dailey, C.-K. Wu, A. E. Medlock, K.-F. Wang, J. P. Rose, and B.-C. Wang. 2000. Ferrochelatase at the millennium: structures, mechanisms and [2Fe-2S] clusters. Cell. Mol. Life Sci. 57:1909-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dailey, H. A., and T. A. Dailey. 1996. Protoporphyrinogen oxidase of Myxococcus xanthus. Expression, purification, and characterization of the cloned enzyme. J. Biol. Chem. 271:8714-8718. [DOI] [PubMed] [Google Scholar]
- 13.Dailey, T. A., and H. A. Dailey. 1996. Human protoporphyrinogen oxidase: expression, purification, and characterization of the cloned enzyme. Protein Sci. 5:98-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dailey, T. A., and H. A. Dailey. 2002. Identification of [2Fe-2S] clusters in microbial ferrochelatases. J. Bacteriol. 184:2460-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Koning, A. P., and P. J. Keeling. 2004. Nucleus-encoded genes for plastid-targeted proteins in Helicosporidium: functional diversity of a cryptic plastid in a parasitic alga. Eukaryot. Cell 3:1198-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De la Cruz, V. F., and S. M. Gittleson. 1981. The genus Polytomella: a review of classification, morphology, life cycle, metabolism, and motility. Arch. Protistenkd. 124:1-28. [Google Scholar]
- 17.Emanuelsson, O., H. Nielsen, S. Brunak, and G. von Heijne. 2000. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J. Mol. Biol. 300:1005-1016. [DOI] [PubMed] [Google Scholar]
- 18.Gasteiger, E., C. Hoogland, A. Gattiker, S. Duvaud, M. R. Wilkins, R. D. Appel, and A. Bairoch. 2005. Protein identification and analysis tools on the ExPASy server, p. 571-607. In J. M. Walker (ed.), The proteomics handbook. Humana Press, Totowa, N.J.
- 19.Gutiérrez-Cirlos, E.-B., A. Antaramian, M. Vásquez-Acevedo, R. Coria, and D. González-Halphen. 1994. A highly active ubiquinol-cytochrome c reductase (bc1 complex) from the colorless alga Polytomella spp., a close relative of Chlamydomonas. Characterization of the heme binding site of cytochrome c1. J. Biol. Chem. 269:9147-9154. [PubMed] [Google Scholar]
- 20.Harris, E. H. 1989. The Chlamydomonas sourcebook. Academic Press, San Diego, Calif. [DOI] [PubMed]
- 21.Hennig, M., B. Grimm, R. Contestabile, R. A. John, and J. H. Jansonius. 1997. Crystal structure of glutamate-1-semialdehyde aminomutase: an α2-dimeric vitamin B6-dependent enzyme with asymmetry in structure and active site reactivity. Proc. Natl. Acad. Sci. USA 94:4866-4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang, X., and W. Miller. 1991. A time-efficient, linear-space local similarity algorithm. Adv. Appl. Math. 12:337-357. [Google Scholar]
- 23.Ilag, L. L., D. Jahn, G. Eggertsson, and D. Söll. 1991. The Escherichia coli hemL gene encodes glutamate 1-semialdehyde aminotransferase. J. Bacteriol. 173:3408-3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jansson, S. 1999. A guide to the Lhc genes and their relatives in Arabidopsis. Trends Plant Sci. 4:236-240. [DOI] [PubMed] [Google Scholar]
- 25.Koch, M., C. Breithaupt, R. Kiefersauer, J. Freigang, R. Huber, and A. Messerschmidt. 2004. Crystal structure of protoporphyrinogen IX oxidase: a key enzyme in haem and chlorophyll biosynthesis. EMBO J. 23:1720-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Labbe, P. 1997. Purification and properties of coproporphyrinogen III oxidase from yeast. Methods Enzymol. 281:367-378. [DOI] [PubMed] [Google Scholar]
- 27.Leong, S. A., G. S. Ditta, and D. R. Helinski. 1982. Heme biosynthesis in Rhizobium: identification of a cloned gene coding for δ-aminolevulinic acid synthetase from Rhizobium meliloti. J. Biol. Chem. 257:8724-8730. [PubMed] [Google Scholar]
- 28.Lermontova, I., E. Kruse, H.-P. Mock, and B. Grimm. 1997. Cloning and characterization of a plastidal and a mitochondrial isoform of tobacco protoporphyrinogen IX oxidase. Proc. Natl. Acad. Sci. USA 94:8895-8900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lister, R., O. Chew, C. Rudhe, M.-N. Lee, and J. Whelan. 2001. Arabidopsis thaliana ferrochelatase-I and -II are not imported into Arabidopsis mitochondria. FEBS Lett. 506:291-295. [DOI] [PubMed] [Google Scholar]
- 30.Masuda, T., T. Suzuki, H. Shimada, H. Ohta, and K. Takamiya. 2003. Subcellular localization of two types of ferrochelatase in cucumber. Planta 217:602-609. [DOI] [PubMed] [Google Scholar]
- 31.Matters, G. L., and S. I. Beale. 1994. Structure and light-regulated expression of the gsa gene encoding the chlorophyll biosynthetic enzyme, glutamate 1-semialdehyde aminotransferase, in Chlamydomonas reinhardtii. Plant Mol. Biol. 24:617-629. [DOI] [PubMed] [Google Scholar]
- 32.Miyamoto, K., K. Nakahigashi, K. Nishimura, and H. Inokuchi. 1991. Isolation and characterization of visible light-sensitive mutants of Escherichia coli K12. J. Mol. Biol. 219:393-398. [DOI] [PubMed] [Google Scholar]
- 33.Moore, J., M. H. Cantor, P. Sheeler, and W. Kahn. 1970. The ultrastructure of Polytomella agilis. J. Protozool. 17:671-676. [Google Scholar]
- 34.Newman, S. M., J. E. Boynton, N. W. Gillham, B. L. Randolph-Anderson, A. M. Johnson, and E. H. Harris. 1990. Transformation of chloroplast ribosomal RNA genes in Chlamydomonas: molecular and genetic characterization of integration events. Genetics 126:875-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 36.Nogaj, L. A., A. Srivastava, R. van Lis, and S. I. Beale. 2005. Cellular levels of glutamyl-tRNA reductase and glutamate-1-semialdehyde aminotransferase do not control chlorophyll synthesis in Chlamydomonas reinhardtii. Plant Physiol. 139:389-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Page, R. D. M. 1996. TREEVIEW: an application to display phylogenetic trees on personal computers. Computer Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 38.Pérez-Martínez, X., A. Antaramian, M. Vásquez-Acevedo, S. Funes, E. Tolkunova, J. d'Alayer, M. G. Carlos, E. Davidson, M. P. King, and D. González-Halphen. 2001. Subunit II of cytochrome c oxidase in chlamydomonad algae is a heterodimer encoded by two independent nuclear genes. J. Biol. Chem. 276:11302-11309. [DOI] [PubMed] [Google Scholar]
- 39.Pérez-Martínez, X., M. Vásquez-Acevedo, E. Tolkunova, S. Funes, M. G. Carlos, E. Davidson, M. P. King, and D. González-Halphen. 2000. Unusual location of a mitochondrial gene—subunit III of cytochrome c oxidase is encoded in the nucleus of chlamydomonad algae. J. Biol. Chem. 275:30144-30152. [DOI] [PubMed] [Google Scholar]
- 40.Pérez-Martínez, X., S. Funes, E. Tolkunova, E. Davidson, M. P. King, and D. González-Halphen. 2002. Structure of nuclear-localized cox3 genes in Chlamydomonas reinhardtii and its colorless close relative Polytomella sp. Curr. Genet. 40:399-404. [DOI] [PubMed] [Google Scholar]
- 41.Round, F. E. 1980. The evolution of pigmented and unpigmented unicells: a consideration of the protista. Biosystems 12:61-69. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 43.Sasarman, A., J. Letowski, G. Czaika, V. Ramirez, M. A. Nead, J. M. Jacobs, and R. Morais. 1993. Nucleotide sequence of the hemG gene involved in the protoporphyrinogen oxidase activity of Escherichia coli K12. Can. J. Microbio1. 39:1155-1161. [DOI] [PubMed] [Google Scholar]
- 44.Sato, S., B. Clough, L. Coates, and R. J. M. I. Wilson. 2004. Enzymes for heme biosynthesis are found in both the mitochondrion and plastid of the malaria parasite Plasmodium falciparum. Protist 155:117-125. [DOI] [PubMed] [Google Scholar]
- 45.Schoenhaut, D. S., and P. J. Curtis. 1986. Nucleotide sequence of mouse 5-aminolevulinic acid synthase cDNA and expression of its gene in hepatic and erythroid tissues. Gene 48:55-63. [DOI] [PubMed] [Google Scholar]
- 46.Shi, Z., and G. C. Ferreira. 2004. Probing the active site loop motif of murine ferrochelatase by random mutagenesis. J. Biol. Chem. 279:19977-19986. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki, T., T. Masuda, D. P. Singh, F.-C. Tan, T. Tsuchiya, H. Shimada, H. Ohta, A. G. Smith, and K. Takamiya. 2002. Two types of ferrochelatase in photosynthetic and nonphotosynthetic tissues of cucumber: their difference in phylogeny, gene expression, and localization. J. Biol. Chem. 277:4731-4737. [DOI] [PubMed] [Google Scholar]
- 48.Thomas, J., and J. D. Weinstein. 1990. Measurement of heme efflux and heme content in isolated developing chloroplasts. Plant Physiol. 94:1414-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Lis, R., A. Atteia, L. A. Nogaj, and S. I. Beale. Subcellular localization and light-regulated expression of protoporphyrinogen IX oxidase and ferrochelatase in Chlamydomonas reinhardtii. Plant Physiol., in press. [DOI] [PMC free article] [PubMed]
- 52.Van Lis, R., D. González-Halphen, and A. Atteia. 2005. Divergence of the mitochondrial electron transport chains from the green alga Chlamydomonas reinhardtii and its colorless relative Polytomella sp. Biochim. Biophys. Acta 1708:23-34. [DOI] [PubMed] [Google Scholar]
- 53.Varadharajan, S., S. Dhanasekaran, Z. Q. Bonday, P. N. Rangarajan, and G. Padmanaban. 2002. Involvement of δ-aminolevulinate synthase encoded by the parasite gene in de novo haem synthesis by Plasmodium falciparum. Biochem. J. 367:321-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watanabe, N., F. S. Che, M. Iwano, S. Takayama, S. Yoshida, and A. Isogai. 2001. Dual targeting of spinach protoporphyrinogen oxidase II to mitochondria and chloroplasts by alternative use of two in-frame initiation codons. J. Biol. Chem. 276:20474-20481. [DOI] [PubMed] [Google Scholar]
- 55.Weinstein, J. D., and S. I. Beale. 1983. Separate physiological roles and subcellular compartments for two tetrapyrrole biosynthetic pathways in Euglena gracilis. J. Biol. Chem. 258:6799-6807. [PubMed] [Google Scholar]
- 56.Wu, C.-K., H. A. Dailey, J. P. Rose, A. Burden, V. M. Sellers, and B.-C. Wang. 2001. The 2.0 Å structure of human ferrochelatase, the terminal enzyme of heme biosynthesis. Nat. Struct. Biol. 8:156-160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.