Abstract
Variations in vascular reactivity and morphology of proximal and distal saphenous vein might affect its performance as a bypass conduit. Because peri- or postoperative graft spasm or intimal hyperplasia reduces patency, we compared the reactivity and morphology of human proximal and distal saphenous vein conduits.
Isometric tension studies were performed in response to potassium chloride (80 mM), phenylephrine (10−8–10−5 M), norepinephrine (10−8–10−5 M), and angiotensin II (10−11–10−7 M). Relaxant responses were tested with acetylcholine (10−9–10−5 M), sodium nitroprusside (10−10–10−6 M), and diltiazem (10−10–10−4 M). Also, vein segments from proximal and distal leg saphenous vein grafts were collected for histopathologic investigation. In proximal and distal saphenous vein segments, we also examined the structure of intima, media, and adventitia, and we evaluated the smooth muscle cell/extracellular matrix ratio in the media.
There was no significant difference (P >0.05) between proximal and distal venous segments in response to vasoconstrictors or vasodilators. Similarly, investigation by light microscopy was unable to show any significant difference between proximal and distal conduits in vascular structure. The smooth muscle cell/extracellular matrix ratio was also similar in these graft materials.
Our failure to find functional or morphologic differences between proximal and distal saphenous vein segments suggests that there is no advantage in using one of these preparations over the other as a conduit in coronary artery bypass operations.
Key words: Comparative study; coronary artery bypass; extracellular matrix/ultrastructure; graft occlusion, vascular/prevention & control; muscle, smooth, vascular; saphenous vein/anatomy & histology/transplantation; spasm; vasoconstriction; vasodilation; vascular patency
Three types of blood vessels are currently chosen as conduit vessels in coronary artery bypass grafting (CABG) operations: the saphenous vein (SV), the internal mammary artery (IMA), and the radial artery (RA). Saphenous veins continue to constitute than 70% of coronary bypass grafts1 because of ready availability and suppleness. After implantation into the arterial circulation, coronary bypass graft materials can go into spasm, and constriction has led to premature occlusion during the perioperative or postoperative period.2 Saphenous vein grafts are more resistant to vasospasm than are IMA or RA grafts because of the relative absence of smooth muscle in the tunica media.3 However, when a vein is placed in the arterial circulation, the vein graft can over-distend due to its sudden exposure to arterial blood pressure.4 Thus, SV grafts, like arterial conduits, are susceptible to perioperative spasm. In addition, SV grafts are considered to be particularly susceptible to the development of atherosclerotic lesions because of intimal hyperplasia.5 Under high arterial pressures, the structural components of the vascular wall become mechanically strained, which promotes hyperplasia and long-term occlusion.6 Intimal hyperplasia is responsible for about a third of the vein graft failures that occur during the first 12 to 18 months after operation.7,8
The histological changes that are seen after implantation of a vein into the arterial system have been well documented, but little attention has been focused on the histological appearances of the donor SV before grafting.9 However, many of the changes that may be important in graft failure are present prior to grafting. Because the harvested SV is usually longer than required for CABG, it is possible to choose only the best part of the vessel. A difference in vascular responsiveness or histological appearance between proximal and distal venous graft sections might affect the performance of the bypass conduit. The histological structures of proximal and distal venous grafts may differ due to a higher distention range of the saphenous vein in the distal extent of the leg, compared with the proximal. In addition, receptor densities or signal transduction pathways, which govern the reactivity of the vascular smooth muscle to various vasoactive agents, also may differ in proximal or distal SV segments.
Saphenous vein grafts used in CABG may be taken from both the proximal and the distal leg sections. Because the morphology and responsiveness of SV to various vasoactive mediators are important factors contributing to the patency of coronary bypass grafts,2,6 comparison of proximal and distal SV grafts in these regards is of potential benefit. Therefore, we investigated possible functional and morphologic differences between proximal and distal SV grafts that might affect graft patency.
Patients and Methods
In Vitro Organ Bath Studies
Samples of redundant SV were obtained from 30 patients who underwent coronary artery bypass graft surgery. Grafts were taken from 25 men and 5 women, from 52 to 73 years of age (mean age, 63 ± 11 years). Patients who had venous insufficiency as detected by venous Doppler ultrasonography were excluded, as were patients who had systemic hypertension, diabetes mellitus, or dyslipidemia. Those patients who were not receiving an angiotensin-converting enzyme inhibitor, an angiotensin-receptor blocker, or a statin were selected for the studies. All patients were placed on the same therapy (long-acting nitrates and aspirin). The study protocol conformed to the declaration of Helsinki and was approved by our institutional ethics committee; written informed consent was obtained from each patient. After harvesting the SV, we carefully excised ∼2− cm segments from both proximal and distal ends of the conduit, which samples we transported to the laboratory in cold (4 °C) physiologic salt solution (PSS). All the proximal and distal SV rings that were used in this study were obtained from the same patients. The SV segments were cleaned of connective tissue and cut into 3-mm-wide rings. Two rings (1 proximal and 1 distal) were obtained from each vessel and were labeled as a set, so that proximal and distal rings of the same patient would be compared.
Each ring was carefully suspended in an organ bath by 2 stainless steel clips passed through the vascular lumen. Each 20-mL organ bath was filled with PSS (mM: NaCl, 118; KCl, 5; NaHCO3, 25; KH2PO4, 1.0; MgSO4, 1.2; CaCl2, 2.5; and glucose, 11.2), which was maintained at 37 °C and infused with 95% O2 and 5% CO2 in order to obtain a pH of 7.4. Isometric tension was continuously measured with an isometric force transducer (FDT10-A, Commat Ltd.; Ankara, Turkey), which was connected to a computer-based data acquisition system (TDA 97, Commat Ltd.). Two g of tension was progressively applied to each SV ring and then maintained for 60 min.
After the application of tension, the rings were assessed with respect to their ability to vasodilate or vasoconstrict in response to a range of vasoactive mediators. In the 1st set of experiments, responses to 80-mM KCl were obtained in both proximal and distal SV rings. Then, tissues were challenged with norepinephrine (NE, 10−8–10−5 M), phenylephrine (Phe, 10−8–10−5 M), or angiotensin II (AT-II, 10−11–10−7 M) by adding concentrations of each agonist to the baths in a stepwise manner, during which the isometric tension developed by the tissue was recorded. The tissue response was allowed to reach a stable plateau (2–4 min) before each successive addition of agonist. In separate experiments, increasing concentrations of sodium nitroprusside (SNP, 10−10–10−6 M), diltiazem (10−11–10−4 M), and acetylcholine (ACh, 10−9–10−5 M) were administered to both proximal and distal SV segments that had been contracted by means of 10−6 M phenylephrine, and the ability of the SV rings to relax was assessed.
Histology
Ten paired proximal and distal SV samples were routinely fixed in 4% formalin solution and subsequently embedded in paraffin. Paraffin-embedded vascular tissue sections (4 μm) were mounted on microscope slides, deparaffinized for 10 minutes in xylene at room temperature, and rehydrated through descending concentrations of ethanol. Sections were then stained with hematoxylin-eosin or elastica van Gieson to define the intimal and medial layers. Sections were evaluated by light microscopy and analyzed with an image analyzer (SAMBA 2000; North Sioux City, Iowa) to measure the vascular diameter and the thickness of the intima and the media.
Immunohistochemistry
Subsequent to deparaffinization and rehydration, sections were treated with 0.3% H2O2 in methanol for 10 minutes to block endogenous peroxidase activity. Sections then were preincubated with blocking serum (DakoCytomation; Glostrup, Denmark) for 10 minutes at room temperature and incubated for 60 minutes with anti-a-SMA (smooth muscle cell actin antibody, 1:100; M0851; DakoCytomation). After a wash in PBS (phosphate buffered saline, pH 7.4), sections were incubated for 15 minutes with biotin-labeled antibody at room temperature and subsequently washed in PBS. After incubation with biotin-labeled streptavidin-horseradish peroxidases (K0675, DakoCytomation) for 15 minutes at room temperature, the horseradish peroxidases was visualized with DAB (diaminobenzidine; K3465, DakoCytomation) for 3 to 5 minutes.
Immunoquantification
Smooth muscle cell/extracellular matrix (SMC/ECM) ratios of the medial layer were quantified by using an image analyzer (SAMBA 2000), as described in more detail by Vermeulen and coworkers.10
Materials
L-Phenylephrine hydrochloride, acetylcholine chloride, sodium nitroprusside, angiotensin II, norepinephrine, diltiazem, and the salts for the PSS were purchased from Sigma Chemical (St. Louis, Mo). All drugs were prepared fresh daily during experiments, and were dissolved in distilled water before use.
Statistical Analysis
All values are expressed as mean ± SEM. The curves were analyzed with the aid of Graph Pad Prism version 3.0 for Windows (GraphPad Software; San Diego, Calif). Changes in tension in response to vasoactive agents are normalized to the maximal response induced by 80-mM KCl and are expressed as percentages of that maximum. Responses to ACh, SNP, and diltiazem are expressed as percentages of the reversal of the tension developed in response to Phe. The concentrations of agents required to elicit 50% of their maximum responses (EC50) are calculated for each ring to compare the sensitivities and are expressed as negative log M. Statistical analysis of the results was performed by ANOVA for repeated measures followed by Tukey's post hoc test or Student's t-test where appropriate. A P value less than 0.05 was considered significant.
Results
Reactivity Studies
The maximum contractile response to 80-mM KCl for segments in the proximal SV was 5.66 ± 0.82 g, and for segments in the distal SV was 6.09 ± 0.63 g. No significant difference was observed in this response between proximal and distal SV grafts (Fig. 1). Concentration–response curves to the variety of constrictor agents also showed no significant differences between proximal and distal SV grafts in the range of contraction and sensitivity. The cumulative addition of norepinephrine, phenylephrine, and angiotensin II to the organ bath resulted in the generation of sigmoidal dose-response curves for each agonist (Fig. 2). The maximal contractile responses (expressed in percentage of KCl contraction) induced by norepinephrine, phenylephrine, or angiotensin II were not significantly different when SV segments obtained from proximal and distal regions of the leg were compared (Table I). Similarly, the potency values of all contractile agents in their interaction with SV were not significantly different between proximal and distal rings (Table I).

Fig. 1 Contractile effect of 80-mM KCl on proximal and distal saphenous vein segments (n = 7 for all groups). Values are mean ± SEM.
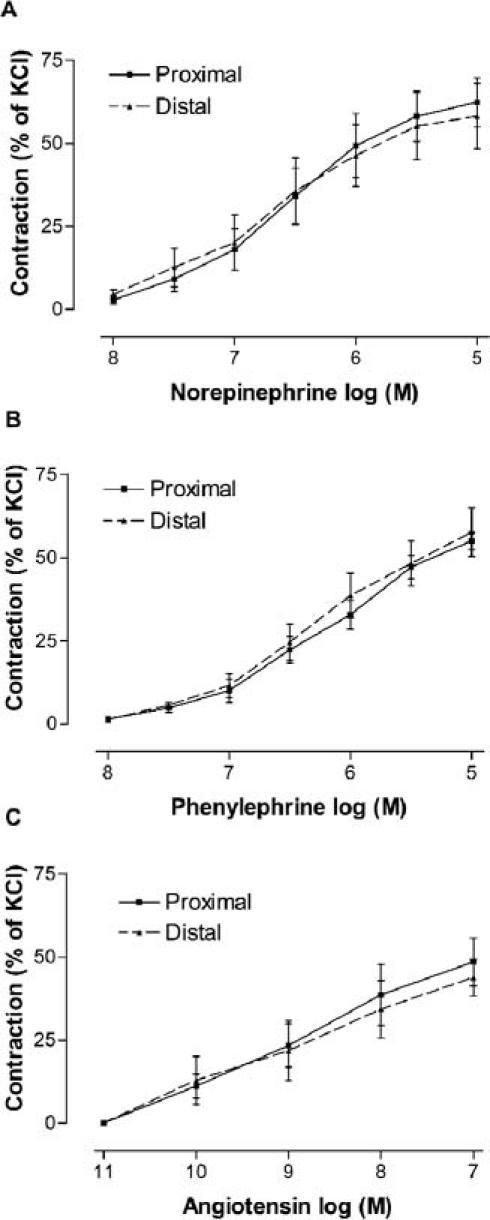
Fig. 2 Contractile responses to norepinephrine (A), phenylephrine (B), and angiotensin II (C) in the proximal and distal saphenous vein segments (n = 7–8 for all groups). Values are mean ± SEM.
TABLE I. Maximum Responses (Emax) and Potency (EC50) Values for Responses to Several Vasoactive Agents in the Proximal and Distal SV Grafts

In vascular segments that had been contracted with 10−6 M phenylephrine, a similar concentration-dependent relaxation response to the endothelium-dependent agonist ACh was seen in all groups (Fig. 3A). Also, the endothelium-independent agonists sodium nitroprusside and diltiazem relaxed vein segments from the proximal and distal SV with similar potency and efficacy (Figs. 3B and 3C). The sensitivities of the SV grafts to each of the vasodilators were not significantly different in proximal SV and distal SV rings, nor were the maximum relaxation responses to these agents (Table I).

Fig. 3 Effects of acetylcholine (A), sodium nitroprusside (B), and diltiazem (C) on the tone of isolated human proximal and distal saphenous vein that had been contracted with phenylephrine (n = 14 for all groups). Agonists were added cumulatively after reaching a stable contraction. Values are mean ± SEM.
Histological Studies
The vein sections were stained with hematoxylin-eosin and elastica van Gieson to examine the intima and media, and no morphologic difference was found between the proximal and distal SV sections. Also, there was no significant difference in vascular diameter between the proximal and distal SV conduits (data not shown). The thicknesses of intima and media were similar in proximal and distal segments (Fig. 4A). Although the SMC/ECM ratio of the medial layer was found to be slightly higher in distal than in proximal SV sections, this difference was not significant (Fig. 4B).

Fig. 4 Thickness of intima and media (A), and smooth muscle cell/extracellular matrix (SMC/ECM) ratio (B) of proximal and distal saphenous vein segments (n = 7 for all groups). Values are mean ± SEM.
Discussion
It has been clearly demonstrated that variations in the morphology and vascular reactivity of grafts can influence their performance as bypass conduits.11 Because graft closure due to spasm or intimal hyperplasia limits the term of patency, factors that can affect vascular reactivity or morphology are thought to be important in determining short- and long-term graft performance.12 Therefore, possible variations along the length of vessel may be an important factor when choosing a segment of SV before grafting.
Various vasoactive substances are implicated in the generation of vasospasm, including sympathomimet-ic substances such as norepinephrine and the renin-angiotensin-system–related substance angiotensin II.13,14 In the present study, we mimicked the spasm by increasing vascular tension with norepinephrine, phenylephrine, and angiotensin II, which are possibly involved in vasospasm. In a previous study, Stooker and colleagues4 reported a difference in distention characteristics between upper- and lower-leg saphenous vein graft segments, which might have implications for future patency. However, no comparative study of vascular reactivity to vasoactive agents has been performed to date. In the present study, a comparison of maximal contractile responses and sensitivities to these spasmogens demonstrated that the reactivity of proximal and distal SV segments is not significantly different. High KCl-induced contractile responses are also similar. On the basis of these results, we have concluded that there are no significant differences in the profiles of various receptors, such as adrenoreceptors and angiotensin II receptors, along the SV vessel. However, further receptor-binding studies are required to define the distribution and variability of these receptors in SV. In addition, it is important to know the responsiveness of graft vessels to vasodilator agents for choosing the vasodilator most effective in the prevention or treatment of graft spasm.15 In the present study, there was no statistically significant difference in vascular reactivity to SNP or to diltiazem, which are common in postoperative use. As a result of these observations, we suggest that the responsiveness of proximal and distal SV graft segments to vasoactive agents is not significantly different.
Abnormalities within the venous wall may affect venous graft patency. Such variations in the vascular smooth muscle and the endothelial cells lining the vascular wall have been implicated in influencing the short- and long-term performance of different bypass grafts.11 Venous graft intimal hyperplasia is believed to be an early event in atherosclerotic lesion formation, which is a significant cause of graft failure.16 Intimal hyperplasia is believed to be initiated by endothelial injury or dysfunction, followed by smooth muscle cell proliferation and connective tissue deposition.5 The lesion results from smooth muscle cell proliferation and migration from the media into the intima.17 Increased pressure appears to enhance intimal hyperplasia.
Although we expected to find a difference in intimal thickness due to higher intraluminal pressure within the distal SV segment compared with the proximal segment, we failed to discover any. The effect of shear on the structure of the vascular wall may alter the endothelium's production of vasoactive factors such as endothelium-derived relaxing factor (EDRF). Indeed, significant decreases in EDRF response have been found in the presence of severe intimal lesions (poor-quality vein grafts).18 Because EDRF is known to inhibit smooth muscle cell proliferation and platelet adhesion, a loss of EDRF could also contribute to intimal proliferation after a graft has been inserted.19 However, we saw no significant difference between proximal and distal SV segments in their response to endothelium-dependent agonist acetylcholine. In view of the findings of this study, we think it likely that proximal and distal SV conduits experience similar post-implantation development of intimal hyperplasia upon application of high arterial pressure.
A 2nd structural change that occurs in SV grafts is the development of medial thickening. Medial thickening may occur in response to arterial pressure itself or to the deformation or wall stress that pressure produces.20 Increased synthesis and deposition of connective tissue by smooth muscle cells in vitro have been demonstrated to occur in response to cyclic stretching.21 Davies and colleagues22 reported that there was significantly more moderate or severe focal hyperplasia and circular muscle hypertrophy in distal long SV than in its proximal counterpart. It is generally accepted that the pathophysiological impact of a changed SMC/ECM ratio in the medial layer of muscular arteries might result in an altered, possibly decreased, vascular elastic compliance.23,24 Although it is possible to discover a difference in SMC/ECM ratio to explain the consistent finding of a higher distention range for lower-leg SV segments than for upper-leg SV segments, our study found medial thickness and SMC/ECM ratio to be similar in lower and upper segments. Our results confirm the findings of Stooker and associates,4 who suggest that histopathologic investigation reveals no significant difference in SMC/ECM ratio and who appear to support our results, which show similar vasoreactivity of proximal and distal SV grafts to high KCl concentrations. In addition to these explanations, Buxton and coworkers25 have said that best results after bypass surgery were obtained using veins with a large internal diameter and a thin wall. The luminal diameter and wall thickness of the vein grafts appear to influence graft patency, and this may influence the choice of material for a bypass graft. However, we did not find any difference between proximal and distal SV segments in internal diameter and wall thickness.
In conclusion, this study may have practical application in the selection of SV grafts for CABG operation. We demonstrate that proximal and distal SV segments show similar reactivity to receptor- and nonreceptor-mediated agonists, show no significant difference in morphologic structure, and display similar SMC/ECM ratios in the media. Therefore, we expect no difference between the proximal and distal SV segments in respect to graft patency: they can be considered equally applicable for use in the patient who requires CABG operation.
Footnotes
Address for reprints: Ilhan Golbasi, MD, Akdeniz University, Faculty of Medicine, Department of Cardiovascular Surgery, 07070 Antalya, Turkey
E-mail: Golbasi@akdeniz.edu.tr
This study was supported by Akdeniz University Research Foundation.
References
- 1.Izzat MB, West RR, Bryan AJ, Angelini GD. Coronary artery bypass surgery: current practice in the United Kingdom. Br Heart J 1994;71:382–5. [DOI] [PMC free article] [PubMed]
- 2.Chanda J, Canver CC. Reversal of preexisting vasospasm in coronary artery conduits. Ann Thorac Surg 2001;72:476–80. [DOI] [PubMed]
- 3.Reardon MJ, Conklin LD, Reardon PR, Baldwin JC. Coronary artery bypass conduits: review of current status. J Cardiovasc Surg (Torino) 1997;38:201–9. [PubMed]
- 4.Stooker W, Gok M, Sipkema P, Niessen HW, Baidoshvili A, Westerhof N, et al. Pressure-diameter relationship in the human greater saphenous vein. Ann Thorac Surg 2003;76: 1533–8. [DOI] [PubMed]
- 5.Dilley RJ, McGeachie JK, Prendergast FJ. A review of the histologic changes in vein-to-artery grafts, with particular reference to intimal hyperplasia. Arch Surg 1988;123:691–6. [DOI] [PubMed]
- 6.Canham PB, Finlay HM, Boughner DR. Contrasting structure of the saphenous vein and internal mammary artery used as coronary bypass vessels. Cardiovasc Res 1997;34: 557–67. [DOI] [PubMed]
- 7.Campeau L, Enjalbert M, Lesperance J, Bourassa MG, Kwiterovich P Jr, Wacholder S, Sniderman A. The relation of risk factors to the development of atherosclerosis in saphenous-vein bypass grafts and the progression of disease in the native circulation. A study 10 years after aortocoronary bypass surgery. N Engl J Med 1984;311:1329–32. [DOI] [PubMed]
- 8.Bourassa MG, Fisher LD, Campeau L, Gillespie MJ, McConney M, Lesperance J. Long-term fate of bypass grafts: the Coronary Artery Surgery Study (CASS) and Montreal Heart Institute experiences. Circulation 1985;72:(6 Pt 2): V71–8. [PubMed]
- 9.Milroy CM, Scott DJ, Beard JD, Horrocks M, Bradfield JW. Histological appearances of the long saphenous vein. J Pathol 1989;159:311–6. [DOI] [PubMed]
- 10.Vermeulen EG, Niessen HW, Bogels M, Stehouwer CD, Rauwerda JA, van Hinsbergh VW. Decreased smooth muscle cell/extracellular matrix ratio of media of femoral artery in patients with atherosclerosis and hyperhomocysteinemia. Arterioscler Thromb Vasc Biol 2001;21:573–7. [DOI] [PubMed]
- 11.Chester AH, Marchbank AJ, Borland JA, Yacoub MH, Taggart DP. Comparison of the morphologic and vascular reactivity of the proximal and distal radial artery. Ann Thorac Surg 1998;66:1972–7. [DOI] [PubMed]
- 12.He GW, Rosenfeldt FL, Angus JA. Pharmacological relaxation of the saphenous vein during harvesting for coronary artery bypass grafting. Ann Thorac Surg 1993;55:1210–7. [DOI] [PubMed]
- 13.Robertson RM, Bernard Y, Robertson D. Arterial and coronary sinus catecholamines in the course of spontaneous coronary artery spasm. Am Heart J 1983;105:901–6. [DOI] [PubMed]
- 14.Romero JC, Reckelhoff JF. State-of-the-Art lecture. Role of angiotensin and oxidative stress in essential hypertension. Hypertension 1999;34(4 Pt 2):943–9. [DOI] [PubMed]
- 15.He GW, Rosenfeldt FL, Buxton BF, Angus JA. Reactivity of human isolated internal mammary artery to constrictor and dilator agents. Implications for treatment of internal mammary artery spasm. Circulation 1989;80(3 Pt 1):I141–50. [PubMed]
- 16.el-Sanadiki MN, Cross KS, Murray JJ, Schuman RW, Mikat E, McCann RL, Hagen PO. Reduction of intimal hyperplasia and enhanced reactivity of experimental vein bypass grafts with verapamil treatment. Ann Surg 1990; 212:87–96. [DOI] [PMC free article] [PubMed]
- 17.Kohler TR. Intimal hyperplasia. In: White RA, Hollier LH, editors. Vascular surgery: basic science and clinical correlations. Philadelphia: Lippincott; 1994. p. 217–25.
- 18.Ku DD, Caulfield JB, Kirklin JK. Endothelium-dependent responses in long-term human coronary artery bypass grafts. Circulation 1991;83:402–11. [DOI] [PubMed]
- 19.Radomski MW, Palmer RM, Moncada S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br J Pharmacol 1987; 92:639–46. [DOI] [PMC free article] [PubMed]
- 20.Dobrin PB, Littooy FN, Golan J, Blakeman B, Fareed J. Mechanical and histologic changes in canine vein grafts. J Surg Res 1988;44:259–65. [DOI] [PubMed]
- 21.Leung DY, Glagov S, Mathews MB. Cyclic stretching stimulates synthesis of matrix components by arterial smooth muscle cells in vitro. Science 1976;191:475–7. [DOI] [PubMed]
- 22.Davies AH, Magee TR, Baird RN, Sheffield E, Horrocks M. Vein compliance: a preoperative indicator of vein morphology and of veins at risk of vascular graft stenosis. Br J Surg 1992;79:1019–21. [DOI] [PubMed]
- 23.Tyagi SC. Homocyst(e)ine and heart disease: pathophysiology of extracellular matrix. Clin Exp Hypertens 1999;21: 181–98. [DOI] [PubMed]
- 24.Tyagi SC, Smiley LM, Mujumdar VS, Clonts B, Parker JL. Reduction-oxidation (Redox) and vascular tissue level of homocyst(e)ine in human coronary atherosclerotic lesions and role in extracellular matrix remodeling and vascular tone. Mol Cell Biochem 1998;181:107–16. [DOI] [PubMed]
- 25.Buxton B, Lambert RP, Pitt TT. The significance of vein wall thickness and diameter in relation to the patency of femoropopliteal saphenous vein bypass grafts. Surgery 1980; 87:425–31. [PubMed]


