Abstract
We present the case of a 72-year-old woman who had an acute massive pulmonary embolism after abdominal surgery. The patient had undergone a right hemicolectomy and pancreaticoduodenectomy for locally invasive colonic adenocarcinoma. Six hours postoperatively, she required emergent intubation when she suddenly became cyanotic, severely hypotensive, and tachypneic, with an oxygen saturation of 50%. An acute massive pulmonary embolism was suspected, and an emergency transesophageal echocardiogram confirmed the diagnosis. On the basis of the patient's clinical condition and the echocardiographic findings, we performed an emergent pulmonary embolectomy, with the patient on cardiopulmonary bypass. We evacuated multiple large clots from both pulmonary arteries. The patient recovered and was discharged from the hospital 61 days postoperatively.
Herein, we review the current literature on open surgical pulmonary embolectomy. This case supports the use of open pulmonary embolectomy for the treatment of hemodynamically unstable patients on the basis of clinical diagnosis. We discuss the role of emergent transesophageal echocardiography in the diagnosis and management of massive pulmonary embolism.
Key words: Embolectomy, emergencies, pulmonary embolism/diagnosis/therapy
In the United States, deep venous thrombosis and pulmonary embolism are associated with approximately 250,000 hospitalizations each year, and as many as 50,000 individuals die each year as a result of pulmonary embolism.1 Pulmonary embolism has always been a major source of morbidity and mortality and is a topic that has received renewed attention in recent publications.2,3 Massive pulmonary embolism is defined as obstruction of the pulmonary arterial tree that exceeds 50% of the cross-sectional area, causing acute and severe cardiopulmonary failure from right ventricular overload. Depending on the series reviewed, up to 50% of patients with pulmonary embolism experience a massive pulmonary embolism. Studies show that approximately 70% of patients who die of a pulmonary embolus die within the 1st hour after onset of symptoms, thus advocating rapid evaluation and intervention.4 Definitive diagnosis is made on the basis of imaging studies (ventilation-perfusion scanning, contrast pulmonary angiography, computed tomographic [CT] angiography, and echocardiography). Anticoagulation and thrombolysis are the basic methods of treatment of pulmonary embolism. Inotropic support for hemodynamic optimization completes the axis of medical therapy. Surgical embolectomy has also been described in extreme cases. Massive pulmonary embolism with cardiopulmonary collapse at times precludes time-consuming imaging studies and requires urgent pulmonary embolectomy on the basis of clinical criteria and a high index of suspicion for pulmonary embolism.2 Urgent pulmonary embolectomy in the surgical treatment of pulmonary embolism has received mixed reviews in terms of efficacy and associated morbidity and mortality. Opinions range from no need for pulmonary embolectomy in massive pulmonary embolism to pulmonary embolectomy for massive pulmonary embolism in patients without hemodynamic disturbances.5
We report herein a case of a massive pulmonary embolism that required an urgent pulmonary embolectomy on the basis of clinical impression and emergent transesophageal echocardiography (TEE). This report highlights the early use of open pulmonary embolectomy in the surgical treatment of acute massive pulmonary embolism.
Case Report
A 72-year-old woman underwent right hemicolectomy and pancreaticoduodenectomy for locally advanced colonic adenocarcinoma. She became acutely cyanotic and went into acute respiratory distress 6 hours postoperatively. She was also hypotensive (systolic blood pressure as low as 40 mmHg) and cyanotic (SaO2 of 50% and PaO2 of 46.8 mmHg) and had to be intubated and started on high-dose inotropic support. At this time, given the acuity of the hemodynamic instability and the risk factors for deep venous thrombosis, an acute massive pulmonary embolism was suspected. The patient became hemodynamically stable for a short period of time after intubation and required high-dose inotropic support; however, she became more hemodynamically labile despite additional inotropic agents, with a blood pressure of 63/39 mmHg and a central venous pressure of 28 mmHg on neosynephrine, epinephrine, dobutamine, and vasopressin support. The patient did not require cardiac massage throughout the resuscitative efforts. A TEE revealed a severely dilated right ventricle with decreased systolic function. The proximal pulmonary artery was also visualized and appeared dilated with a large floating thrombus within its lumen (Fig. 1).
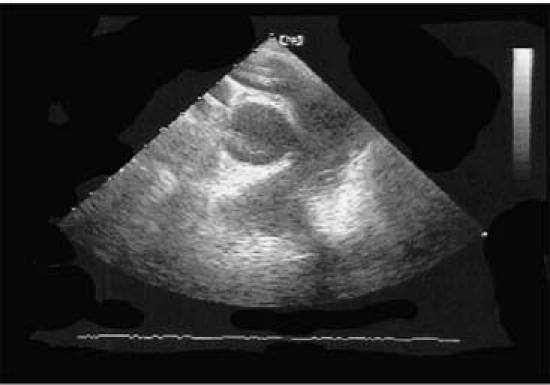
Fig. 1 Transesophageal echocardiogram shows right ventricular dilatation and large thrombus within the pulmonary artery.
The patient was taken to the operating room, and an emergent sternotomy with aortic and bicaval cannulation was performed. Full normothermic cardiopulmonary bypass on a beating heart was instituted. A longitudinal pulmonary arteriotomy was made, and a #7 Fogarty catheter was passed multiple times down the left and right pulmonary arteries. A large amount of clot was removed from the lumen of the left and right pulmonary arteries (Fig. 2). Intraoperative TEE confirmed no residual pulmonary emboli, and the arteriotomy site was closed with 4-0 Prolene sutures. Following embolectomy, severe right ventricular failure was noted. Right ventricular assist device (RVAD) support was discussed with the family intraoperatively; however, it was not recommended due to the advanced stage of the colon cancer. The patient was very slowly weaned off cardiopulmonary bypass over approximately 2 hours. The sternum could not be closed during this operation due to a distended right ventricle, and the patient required closure of the sternotomy along with inferior vena caval filter placement, 2 days later. Postoperatively, the patient did well and slowly recovered. She was discharged from the hospital on the 61st postoperative day, taking an oral anticoagulant. She was seen 8 months later and had no recurrent pulmonary embolism nor congestive heart failure.
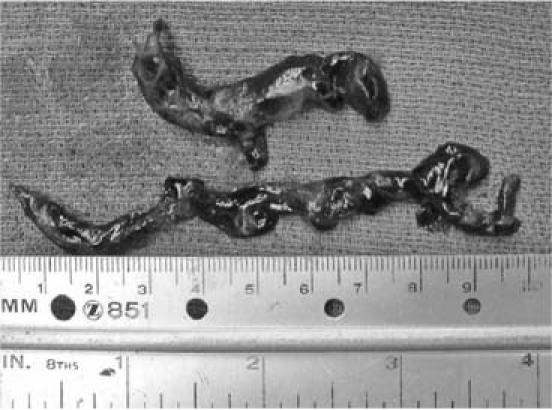
Fig. 2 Surgical specimens extracted from the left and right pulmonary arteries.
Discussion
First described in the 1800s, pulmonary embolism can be classified as acute or chronic, as submassive (25%–50% obstruction) or massive (>50% obstruction), and as central or peripheral.6 Acute pulmonary embolism leads to an abrupt rise in pulmonary vascular resistance. Right ventricular contractile function is compromised, and right ventricular failure ensues. This vicious cycle of cardiogenic shock is augmented by concomitant hypoxia, which inevitably leads to cardiovascular collapse.7 The interval from the onset of symptoms to death is relatively short. In patients with massive pulmonary embolism, 50% die within 30 minutes, 70% die within 1 hour, and more than 85% die within 6 hours of the onset of symptoms.8 Therefore, the window for obtaining a definitive diagnosis is small. In an optimal setting, the diagnosis of pulmonary embolism can be made on the basis of the history and physical examination along with selective tests, such as electrocardiography (ECG) to rule out myocardial infarction, chest radiography to rule out pneumothorax, and an arterial blood gas analysis to bolster the diagnosis.2 Electrocardiographic signs of pulmonary embolism are seen in approximately 75% of cases; however, ECG changes, along with radiographic findings consistent with pulmonary embolism, are often not present.9 Pulmonary angiography and spiral CT pulmonary angiography, the diagnostic gold standards for pulmonary embolism, are precluded by hemodynamic instability in many patients. A delay in treatment in order to complete a lengthy and invasive diagnostic procedure is not justified. Transesophageal echocardiography is a method that is beneficial and is gaining acceptance in demonstrating, noninvasively, right ventricular dilatation and the presence of emboli within the pulmonary arteries.6 When the diagnosis of massive pulmonary embolism is made, medical or surgical treatment must be initiated immediately. If the patient is in extremis, the decision to perform embolectomy may be made primarily on clinical impression. Ullmann and colleagues6 reviewed a series of 40 patients who underwent urgent pulmonary embolectomy. Those authors demonstrated that in 20 cases the hemodynamic instability of the patients precluded the establishment of pulmonary embolism by a diagnostic study. In these 20 cases, the diagnosis of pulmonary embolism was made on clinical findings alone (75% on ECG changes). Operative findings confirmed the clinical impression in 95% of those patients. Furthermore, that study demonstrated that echocardiography is also a useful tool in confirming the diagnosis of massive pulmonary embolism. In 18 of 40 cases, echocardiography was successful in demonstrating massive or fulminant pulmonary embolism.6
Treatment options for massive pulmonary embolism vary, depending on the clinical picture of the patient. Although anticoagulation and thrombolysis are the standard for treatment of acute massive pulmonary embolism, these treatments are limited to patients who are hemodynamically stable and do not have contraindications. Furthermore, data suggest that patients treated with thrombolysis have a higher death rate, increased risk of major hemorrhage, and increased rates of recurrence of pulmonary embolism, compared with patients treated by means of pulmonary embolectomy.10 The International Cooperative Pulmonary Embolism Registry found a surprisingly high intracranial hemorrhage rate of 3% among patients with pulmonary embolism who were treated with thrombolytic therapy.11 Regardless, the risk of fatal hemorrhagic complication of thrombolysis restricts the use of these agents in the immediate postoperative course, as was in the case of our patient. Although interventional catheter-based catheter fragmentation and suction embolectomy are also available for pulmonary embolectomy in some institutions, open surgical embolectomy is indicated in patients who have contraindications to thrombolytic therapy, persistence of thrombi in the right heart or pulmonary arteries after pulmonary embolism, or severe hemodynamic compromise with cardiovascular collapse. Early surgical treatment must also be considered in patients whose course deteriorates in spite of aggressive medical therapy.12 Depending on the series, the overall mortality rate after pulmonary embolectomy varies from 16% to 46%, with a mean mortality rate of 26%.6 Ullmann's group reported an operative mortality of 35% in their series.6 The high mortality rate, for the most part, is due to the fact that most patients who undergo surgical embolectomy are hemodynamically compromised and arrive at the operating room in cardiac arrest with cardiopulmonary resuscitation (CPR) in progress, or else they have had CPR performed beforehand. Data suggest that preoperative hemodynamic status is the most important prognostic indicator of postoperative outcome after surgical pulmonary embolectomy, and cardiac arrest and CPR are independent factors predictive of postoperative death.6,12 These findings suggest that earlier surgical intervention may result in improved survival. The present report describes a case in which severe hemodynamic compromise mandated a surgical embolectomy.
We believe that surgical embolectomy is warranted in patients with severe hemodynamic instability on the basis of clinical impression, after other causes of hemodynamic collapse have been ruled out. During the preoperative or intraoperative period, TEE is extremely reliable for diagnosis by evaluating right ventricular function and localizing thrombi within the pulmonary arterial tree. Furthermore, RVAD implantation, which was considered for our patient, is a viable option to support patients who have ongoing right ventricular dysfunction after pulmonary embolectomy. Right ventricular assist device implantation has produced successful results in patients who could not be weaned from CPB, despite maximal inotropic support.13 With the emergence of TEE as a reliable tool for the rapid diagnosis of massive pulmonary embolism, surgical embolectomy is proving to be the gold standard for the treatment of patients in extremis.
Footnotes
Address for reprints: Gregory R. Brevetti, MD, FACS, Division of Cardiothoracic Surgery, SUNY–Downstate Medical Center, 450 Clarkson Ave, Box 40, Brooklyn, NY 11203-2098
E-mail: gregorybrevetti@hotmail.com
References
- 1.Goldhaber SZ. Pulmonary embolism. N Engl J Med 1998; 339:93–104. [DOI] [PubMed]
- 2.Brevetti GR, O'Brien B, Coomer CL, Hall TS, Brevetti LS, Jablons DM. Emergent surgery for massive pulmonary embolism on the basis of clinical diagnosis. Tex Heart Inst J 2003;30:149–51. [PMC free article] [PubMed]
- 3.Funakoshi Y, Kato M, Kuratani T, Shigemura N, Kaneko M. Successful treatment of massive pulmonary embolism in the 38th week of pregnancy. Ann Thorac Surg 2004;77: 694–5. [DOI] [PubMed]
- 4.Hsieh PC, Wang SS, Ko WJ, Han YY, Chu SH. Successful resuscitation of acute massive pulmonary embolism with extracorporeal membrane oxygenation and open embolectomy. Ann Thorac Surg 2001;72:266–7. [DOI] [PubMed]
- 5.Aklog L, Williams CS, Byrne JG, Goldhaber SZ. Acute pulmonary embolectomy: a contemporary approach. Circulation 2002;105:1416–9. [DOI] [PubMed]
- 6.Ullmann M, Hemmer W, Hannekum A. The urgent pulmonary embolectomy: mechanical resuscitation in the operating theatre determines the outcome. Thorac Cardiovasc Surg 1999;47:5–8. [DOI] [PubMed]
- 7.Ohteki H, Norita H, Sakai M, Narita Y. Emergency pulmonary embolectomy with percutaneous cardiopulmonary bypass. Ann Thorac Surg 1997;63:1584–6. [DOI] [PubMed]
- 8.Stulz P, Schlapfer R, Feer R, Habicht J, Gradel E. Decision making in the surgical treatment of massive pulmonary embolism. Eur J Cardiothorac Surg 1994;8:188–93. [DOI] [PubMed]
- 9.Tayama E, Ouchida M, Teshima H, Takaseya T, Hiratsuka R, Akasu K, et al. Treatment of acute massive/submassive pulmonary embolism. Circ J 2002;66:479–83. [DOI] [PubMed]
- 10.Gulba D, Schmid C, Borst HG, Lichtlen P, Dietz R, Luft FC. Medical compared with surgical treatment for massive pulmonary embolism. Lancet 1994;343:576–7. [DOI] [PubMed]
- 11.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999; 353:1386–9. [DOI] [PubMed]
- 12.Meyer G, Tamisier D, Sors H, Stern M, Vouhe P, Makowski S, et al. Pulmonary embolectomy: a 20-year experience at one center. Ann Thorac Surg 1991;51:232–6. [DOI] [PubMed]
- 13.Kaltenbock F, Gombotz H, Tscheliessnigg KH, Matzer C, Winkler G, Auer T. Right ventricular assist device (RVAD) in septic, fulminating pulmonary artery embolism [in German]. Anaesthesist 1993;42:807–10. [PubMed]


