Abstract
Candida albicans contains a functional mating response pathway that is similar to the well-studied system of Saccharomyces cerevisiae. We have characterized a regulator of G protein signaling (RGS) homolog in C. albicans with sequence similarity to the SST2 gene of Saccharomyces cerevisiae. Disruption of this gene, which had been designated SST2, causes an opaque MTLa/MTLa derivative of strain SC5314 to show hypersensitivity to the C. albicans α-factor. This hypersensitivity generates an enhanced cell cycle arrest detected in halo assays but reduces the overall mating efficiency of the cells. Transcriptional profiling of the pheromone-regulated gene expression in the sst2 mutant shows a pattern of gene induction similar to that observed in wild-type cells, but the responsiveness is heightened. This involvement of an RGS in the sensitivity to pheromone is consistent with the prediction that the mating response pathway in C. albicans requires the activation of a heterotrimeric G protein.
G protein-mediated signaling pathways are ubiquitous in eukaryotic cells. These pathways control a wide range of cellular functions in response to a variety of external signals (27). In general, these pathways involve a 7-transmembrane cell surface receptor protein that binds or detects the extracellular signal or ligand and an intracellular heterotrimeric G protein that is activated in response to the receptor activation (21). Ligand binding to the receptor stimulates conformational changes within the G protein that results in replacement, on the α subunit, of GDP with GTP. The conformational arrangement of the α and βγ subunits of the G protein is also modified, permitting the subunits to interact with other molecules to activate downstream signaling events (13). Both α and βγ subunits can transmit these signals (4).
A key point of regulation in G protein-mediated signaling is the rate of GTP hydrolysis associated with the α subunit. Slow hydrolysis causes persistent signaling, while rapid hydrolysis shuts the signal off quickly. Recently, a class of molecules has been characterized that act to control the rate of GTP hydrolysis on the Gα subunits of the heterotrimeric G proteins. These RGS (regulators of G protein signaling) proteins serve the equivalent function of the GTPase activating proteins that regulate the small GTPases (3); they accelerate the return to the “off” state by stimulating GTPase activity (15). Crystal structures of the RGS class of proteins have been obtained that suggest a molecular mechanism for this process (35), and structurally similar proteins have been identified in many cell types, suggesting that RGS proteins represent a conserved mechanism for controlling heterotrimeric G protein mediated pathways (14).
In the simple eukaryotic yeast Saccharomyces cerevisiae, there is a well-studied G protein-mediated pathway that controls mating. In this pathway, a heterotrimeric G protein consisting of the products of the GPA1, STE4, and STE18 genes transmits signals from pheromone receptors to a mitogen-activated protein kinase cascade that regulates gene expression and cell cycle arrest (11). An RGS protein encoded by the SST2 gene is critical for normal signaling in this pathway; loss-of-function mutants increase the sensitivity of cells to mating pheromone and reduce the specificity requirements of the pheromone (9), while overproduction of Sst2p reduces signaling capacity (10).
Recent evidence has been presented that the pathogenic fungus Candida albicans has a mating system that is similar to that of S. cerevisiae (18, 34). Efficient mating of C. albicans cells can be observed in cultures that have become homozygous for the mating type locus (16, 25) and have switched to the opaque cellular form from the more common white form (26). Although the requirement to switch to the opaque is a complexity not observed in S. cerevisiae, the overall signaling network for C. albicans mating appears analogous to that of the budding yeast. Homologs of most of the components of the yeast mating pathway can be detected in the C. albicans genome (19), and the functional requirement of several of these proteins in the C. albicans mating pathway has been confirmed (8, 24). Putative G protein subunits similar to the Gpa1p, Ste4p, and Ste18p elements of the yeast mating pathway are encoded in the C. albicans genome, suggesting that the fungal pheromone response pathway may have a G protein involvement similar to that of the yeast pathway. Intriguingly, the C. albicans genome lacks a strong homolog to the Ste5p scaffold that in yeast is required to link the G protein to the mitogen-activated protein kinase cascade. The response to pheromone also appears to differ between S. cerevisiae and C. albicans; pheromone treatment of C. albicans cells does not effectively arrest the cell cycle, and the timing of the induction of pheromone-responsive genes appears slower (2, 23, 30). However, among the C. albicans pheromone-induced genes is an RGS homolog (2), suggesting that a feedback regulation may reduce overall pheromone response. We have characterized cells containing a deletion of the gene encoding this RGS protein to see if regulation of G protein signaling is required for the normal mating behavior of C. albicans cells.
MATERIALS AND METHODS
Strains, culture conditions, and opaque selection.
Table 1 describes the strains and the plasmids used in this work. Disruption of the SST2 gene was done in strain 3294, an MTLa/MTLa derivative of strain CNC43 (29) obtained after growth on sorbose as the sole source of carbon (25). Strains were routinely grown in YPD medium (33) at 30°C, supplemented with uridine at 100 μg/ml and tryptophan at 60 μg/ml (YPD). For all experiments with cells in the opaque phase, cultures were grown at 24°C in modified synthetic complete medium (33) composed of 2% (wt/vol) dextrose, 0.67% (wt/vol) yeast nitrogen base without amino acids (Difco), and 0.15% amino acid mix without uracil and supplemented with arginine at 70 μg/ml and, after autoclave sterilization, uridine at 100 μg/ml, tryptophan at 60 μg/ml, and 0.1 μM zinc sulfate (SC). For solid media, 1.6% (wt/vol) Bacto agar was added. For petri plates at pH 4.5, succinic acid and dibasic potassium phosphate solutions were added to the sterilized medium at a final concentration of 50 mM each. For strain conversion from the white to the opaque phase, original strains in the white phase were grown for 28 h at 30°C in liquid SC and a 1-μl aliquot was diluted with sterile water and plated at low density (≈100 to 200 CFU/plate) on several SC, pH 4.5, petri plates containing 0.0005% (wt/vol) Phloxine B (catalog no. P 2759; Sigma, St. Louis, MO) (SC-Phloxine B petri). Petri plates at pH 4.5 with Phloxine B produce better color development and stability of the typical dark pink staining of opaque cells than plates at pH 6.5. These plates were incubated for about 1 week at 24°C to identify opaque cells that are stained dark pink. Dark pink and well-isolated sectors were selected for a second round of single-cell purification to identify well-defined populations of opaque cells. Cells from an opaque colony were analyzed by microscopy to ensure the typical elongated morphology associated with the opaque phenotype, and if no apparent smaller rounded cells were visible in the population, used to inoculate a liquid culture to make a −80°C glycerol stock. For each experiment, strains were routinely restreaked from the original −80°C glycerol stock on SC-Phloxine B plates, and cell morphology of the fresh opaque colony was reconfirmed by microscopy. For transformation, strains in the white phase were grown to stationary phase in YPD medium at 30°C, and the one-step lithium acetate/polyethylene glycol transformation protocol (7) was used with the following modifications. Cells were incubated overnight at 30°C with the transforming DNA before plating, and no heat shock treatment was done (the strains used in this work showed poor transformability when in opaque phase). Transformed cells were plated on SC plates with the proper amino acid dropout for specific selection of transformants for the introduced marker. For mating experiments, SC plates with a 5-amino-acid dropout (uridine, histidine, arginine, tryptophan, and lysine) (SC-5aa) were used for selection of mating products. For deletion of the URA3 marker by intrachromosomal recombination, selection was done by plating cells on SC plates containing uridine at 50 μg/ml and 0.1% (wt/vol) 5-fluoroorotic acid (catalog no. 1556; Diagnostic Chemicals).
TABLE 1.
Candida albicans strains and plasmids used in this work
| Strain (parent) or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| SC5314 | MATa/α clinical isolate, wild type | 12 |
| 3294a (CNC43b) | MATa/a his1/his1 ura3/ura3 arg5,6/arg5,6 | P. T. Magee |
| 3315a (A505c) | MATα/α trp1/trp1 lys2/lys2 | P. T. Magee |
| 3740a (CNC43b) | MATα/α his1/his1 ura3/ura3 arg5,6/arg5,6 | P. T. Magee |
| 3745a (A505c) | MATa/a trp1/trp1 lys2/lys2 | 30 |
| CA3 (3294) | MATa/a SST2/Δsst2::HIS1 ura3/ura3 arg5,6/arg5,6 | This work |
| CA12/Δsst2 (CA3) | MATa/a Δsst2::HIS1 Δsst2::URA3 arg5,6/arg5,6 | This work |
| CA29/Δsst2 (CA12) | MATa/a Δsst2::HIS1 Δsst2::HIS1 ura3/ura3 arg5,6/arg5,6 | This work |
| CA35; CA37 (CA29) | MATa/a Δsst2::HIS1 Δsst2::p1374 arg5,6/arg5,6 | This work |
| CA40 (CA29) | MATa/a Δsst2::HIS1 Δsst2::p1376 arg5,6/arg5,6 | This work |
| Plasmids | ||
| pBS-cURA3 | C. albicans URA3 gene and promoter, in pBluescript | A. J. P. Brown |
| pBS-cHIS1 | C. albicans HIS1 gene and promoter, in pBluescript (unpublished) | C. Bachewich |
| p1374 | C. albicans wild-type SST2 gene and promoter, in pBS-cURA3 | This work |
| p1376 | C. albicans mutant sst2 and promoter, in pBS-cURA3 | This work |
Deletion of SST2.
The entire orf19.4222 coding sequence of 2,028 nucleotides was deleted from strain 3294. The first allele was replaced by the HIS1 marker, creating strain CA3, and the second allele was replaced by the URA3 marker, creating strain CA12. This was done in two steps by homologous recombination using a PCR-based cassette method (Fig. 1A). Table 2 describes the oligonucleotides used for PCR. The two cassettes could be amplified with the same oligonucleotide set because the HIS1 and URA3 markers are in vectors with the same backbone and polylinker sequences. Oligonucleotides 472 and 473, used for amplification of the PCR cassettes, both are 100-mer chimeric oligonucleotides, composed of 20 nucleotides from the vector polylinker sequence flanking the marker, plus an 80-nucleotide extension derived from the contig 19-10202 genomic sequence immediately flanking the 5′ and 3′ of orf19.4222, thus allowing the homologous recombination of the cassettes at the SST2 locus (Fig. 1A). For disruption of the first SST2 allele, the SST2-HIS1-SST2 PCR cassette was generated by amplification of the HIS1 marker from plasmid pBS-cHIS1. The PCR product was purified on a QIAquick PCR purification column (catalog no. 28106; QIAGEN, Valencia, CA), eluted in 1/10 TE buffer, concentrated to 10 μl using a SpeedVAC (Savant Instruments, Holbrook, NY), and transformed into strain 3294. Positive transformants were initially identified by colony PCR with oligonucleotides 1 and 2 positioned at the SST2 locus but external to the recombination sites of the integrating PCR cassette. A 2.4-kb band is expected for the wild-type SST2 allele, and a smaller band is predicted if SST2 was replaced with either the HIS1 or the URA3 marker (Fig. 1B). The second SST2 allele was disrupted and analyzed in a similar way (Fig. 1A and B). Strain CA3 was transformed with an SST2-URA3-SST2 cassette prepared from vector pBS-cURA3 to generate the CA12 strain. Genomic DNA was extracted from the disrupted strains for further characterization by PCR with oligonucleotides 1 and 2, at the SST2 locus but external to recombination sites, to determine the size of alleles; with oligonucleotides 3 and 4, inside the SST2 gene, to detect the presence or absence of the gene; with oligonucleotides H1 and H2, inside HIS1, and oligonucleotides U1 and U2, inside URA3, for the presence or absence of the markers or used in various combination with oligonucleotides 1 and 2 to confirm proper integration sites of the markers (Fig. 1B). In the situation of comigrating PCR fragments (Fig. 1B, lane 17), the presence of the two markers, HIS1 and URA3, was demonstrated by digestion with the following specific restriction enzymes for resolution: PstI, present in HIS1, absent in SST2 and URA3; NdeI, present in URA3, absent in SST2 and HIS1; NheI, present in SST2, absent in HIS1 and URA3 (data not shown). Finally, strains were also characterized by PCR at the MTL locus for confirmation of the mating type identity (data not shown).
FIG. 1.
Disruption of SST2. (A) PCR-based cassette method for disruption of SST2 in 2 steps. The thick black bar represents genomic DNA at the SST2 locus, and the white rectangles represent the SST2 gene coding sequence. The PCR cassettes used for the disruption are composed of a selectable marker (gray ovoid rectangle) flanked by two 80-nucleotide segments from the SST2 locus (small white rectangles) for the homologous recombination of the cassettes. The first allele of SST2 was replaced by the HIS1 marker, and the second allele was replaced by the URA3 marker. Small arrows represent orientation and approximate position of oligonucleotides (Table 2) used for PCR analysis and confirmation of the disruption. (B) Confirmation of disruption by PCR. The parent strain 3294 (lanes 1 to 8), the first allele disrupted strain CA3 (lanes 9 to 16), and the second allele disrupted strain CA12 (lanes 17 to 24) were analyzed by PCR and amplified with the oligonucleotides identified in white over the upper part of the agarose gel (PCR). A 1-kb DNA ladder (Invitrogen, Carlsbad, CA) was used for size reference (lanes M). PCR with oligonucleotides 1 and 2 produce a 2.44-kb DNA fragment for the SST2 wild-type allele, as seen for strains 3294 and CA3 (lanes 1 and 9) but absent in Δsst2 strain CA12 (lane 17), a 1.76-kb fragment when SST2 is replaced by the HIS1 marker, as seen for strains CA3 and CA12 (lanes 9 and 17), or a 1.86-kb fragment in strain CA12 when the second SST2 allele is replaced by the URA3 marker (lane 17). The 1.76-kb and the 1.86-kb PCR fragments from the two markers were not well resolved on this gel (lane 17), so the presence of the two markers in this PCR product was confirmed by digestion with specific restriction enzymes, as described in Materials and Methods. The deletion of the SST2 gene was also confirmed by PCR with SST2 internal oligonucleotides 3 and 4. No PCR band is visible for Δsst2 strain CA12 (lane18), confirming that no other copy of the SST2 gene is detectable, while the 1.1-kb internal fragment is visible for strains 3294 and CA3 (lanes 2 and 10). The proper integration sites of the two markers are demonstrated in the other lanes.
TABLE 2.
Oligonucleotides used in this work
| Name | Description | No. of nucleotides | Sequencea |
|---|---|---|---|
| 472 | 5′-SST2 PCR cassette | 100 | AAGTACTTTTCAAATTCCCCTTTGGAGGTGGTTTTTGTTATGGGAAAT |
| ACTATTGTACATCTTTTTCATAATTAATTAATtatagggcgaattggagctc | |||
| 473 | 3′-SST2 PCR cassette | 100 | AAACCACCAAGCATACATTTGTAGATTAGTTATTGCATTTATATATTC |
| GAATATATAAAAGTAAAATAAATACAGTACTAgacggtatcgataagcttga | |||
| 1 | SST2 foward, external | 25 | TTAAATTAAATGTGACATGGATCTG |
| 2 | SST2 reverse, external | 23 | GAAAAAATTGCGTTGTCTAGACG |
| 3 | SST2 foward, internal | 24 | GTTGATTGAACAGTTTTACAAAGC |
| 4 | SST2 reverse, internal | 23 | ATATCACTTAAATCCTCCCAAGC |
| H1 | HIS1 foward | 24 | TTTAGTCAATCATTTACCAGACCG |
| H2 | HIS1 reverse | 22 | TCTATGGCCTTTAACCCAGCTG |
| U1 | URA3 foward | 22 | TTGAAGGATTAAAACAGGGAGC |
| U2 | URA3 reverse | 24 | ATACCTTTTACCTTCAATATCTGG |
| 500 | 5′-SST2 external + XmaI | 39 | ATTTTTCGcccGGGCAAATACTTAATTGGTTTAAATTCG |
| 501 | 3′-SST2 external + KpnI | 33 | TTGTCCggtACCCCAGACTTCTGAGCATTTGGC |
| 508 | 3′ reintegration, mapping | 22 | TACTATGTTGTCAGGTGCTGCC |
| 509 | 5′ reintegration, mapping | 24 | TTTAGTTTTATCCAATGGGCTGTC |
Oligonucleotide sequence. Uppercase letters indicate wild-type SST2 sequence, lowercase letters indicate vector sequence. Underlined lowercase letters indicate modification of the SST2 wild-type sequence to create the restriction site (underlined) to facilitate SST2 cloning.
DNA sequencing.
The contig and open reading frame (ORF) numbers discussed in this paper refer to the nomenclature from the Stanford Genome Technology Center database for the C. albicans genome (http://www-sequence.stanford.edu/group/candida/), which was used as the primary source of the DNA sequence. DNA sequencing was done by PCR with the BigDye Terminator version 3.1 cycle sequencing kit (catalog no. 4337457; Applied Biosystems, Foster City, CA) on an ABI Prism 377 DNA sequencer. The SST2 gene from strains SC5314, 3745, and 3294 was amplified from genomic DNA with oligonucleotides 1 and 2, and the PCR fragments were sequenced directly. The sequences were compared with the reference sequence from the Stanford database for the SC5314 genome and with supplementary data tables for allelic polymorphism in the C. albicans genome (http://genome-www.stanford.edu/candida-pnas2004-supplement/) (19). SST2 sequences from strains 3294 and 3745 are identical and represent only one of the two polymorphic alleles present in strain SC5314.
Complementation of Δsst2 strain.
The SST2 gene was reintegrated, for complementation experiments, at the SST2 locus in Δsst2 strain CA29. Strain CA29 is a ura3− version of strain CA12 obtained after selection on 5-fluoroorotic acid. To clone the SST2 gene for reintegration, genomic DNA was extracted from strain 3294, and a 3.6-kb fragment was amplified by PCR with oligonucleotides 500 and 501 (Table 2). This fragment, corresponding to contig 19-10202 nucleotides 6267 to 9924, is flanked by XmaI and KpnI restrictions sites to facilitate cloning in the vector pBS-cURA3 and has a unique BglII site. Several clones were sequenced to confirm the integrity of the SST2 gene. SST2 from plasmid 374 (pl374) is identical to the reference sequence from strains 3294 and 3745. However SST2 from plasmid 376 (pl376) has a C to A point mutation, changing codon TAC of residue tyrosine-160 into a TAA stop codon, and generating a truncated version of the SST2 gene. For reintegration of the wild-type copy of the SST2 gene, pl374 was linearized at the unique BglII site and transformed in strain CA29 to create strains CA35 and CA37 used for Δsst2 complementation experiments. For reintegration of the sst2 mutant, pl376 was also linearized at the BglII site and transformed in strain CA29, creating strain CA40, used as a positive control in the Δsst2 complementation experiments. These new strains were also mapped by PCR as described above for deletion of the SST2 gene and also with oligonucleotides 508 and 509 at the SST2 locus but external to the reintegrated 3.6-kb fragment (data not shown) (Table 2).
Peptide synthesis of α-factor.
Peptide synthesis was done on a model 396 multiple-peptide synthesizer (Advanced Chemtech) with the standard 9-fluorenylmethoxy solid-phase method, and the peptides were purified by preparative high-performance liquid chromatography. Two α-factor peptides were synthesized: a 13-amino-acid version (13α, GFRLTNFGYFEPG) with high biological activity and a 14-amino-acid variant of a single residue extension (14α, GFRLTNFGYFEPGK) with a reduced biological activity. The lyophilized peptides were dissolved at 1 mg/ml in 50% water-methanol.
Halo assay.
In the halo assay, a lawn of cells is treated with a localized source of pheromone to determine if cell growth is inhibited (1). A small amount of cell mass was taken from a fresh opaque colony and transferred to a 1.5-ml Eppendorf tube containing 1 ml of sterile water. This was diluted to about 5 × 105 cells/ml, and 200 μl was spread on a SC plate and allowed to dry for about 30 min before spotting 5 μl of α-factor peptide 13α or 14α or solvent, as a negative control, on the lawn of cells. The plate was then incubated at 24°C for 2 days before scanning for photodocumentation. Cells in the white phase were also tested to confirm their unresponsiveness to α-factor. Halos may be difficult to detect for the wild-type strain 3294 and for SST2 reintegrated strains CA35 and CA37, while Δsst2 strains are more tolerant of experimental variation such as cell density or incubation time during the assays.
Mating assay.
Qualitative mating was done as a patch mating experiment using auxotrophy complementation with strain 3315 as the MTLα tester strain. All strains were maintained in the opaque phase, except in some negative-control experiments to demonstrate the absence of complementation for strains in the white phase (data not shown). Assay and tester strains were restreaked as straight lines on separate YPD plates. After 24 h of incubation, the two sets of streaks were crossed onto a single fresh YPD plate. After 24 h of incubation, cells were replicated to an SC-5aa plate for selection of mating products, and colonies of tetraploid cells were visible after 2 days. At this stage, it was possible to assess qualitatively the relative mating efficiency between strains.
Quantitative mating.
Mating efficiency was estimated by titration on plates of the number of cells that form mating products in a known number of cells. Opaque starter cultures were inoculated from a fresh colony and grown overnight at 24°C in liquid SC, and the cell number was determined at an optical density at 600 nm (OD600). Cells (4 × 106) of the tester strain 3315 were mixed together with 2 × 105 cells of the assay strain in 0.5 ml of water, for an approximate cell number ratio of 20 to 1. The cells were transferred to a 1.5-ml Eppendorf tube containing 0.5 ml of solidified YPD-2% agar (without uridine to minimize growth during the experiment). The cells were gently spun down onto the YPD-agar plug surface by centrifugation at a 90° angle (in a swinging bucket adapted for Eppendorf tubes) for 2 min at 1,000 rpm. The supernatant was removed, and the tubes with the cells deposited on the solid YPD surface were incubated for 5 h at 24°C. Cells were recovered by resuspension with 1.0 ml of sterile water (two 0.5-ml aliquots combined) and were plated on two types of plates: SC-tryptophan plates to titer the total number of assay cells and SC-5aa plates on which only mating products are able to form colonies. Tester strain 3315 is unable to grow on either of these plates.
Morphology assay.
Strains grown in liquid medium were treated with various concentrations of synthetic α-factor from C. albicans (30). Cells from overnight liquid cultures grown at 24°C were diluted with fresh medium to an OD600 of 1.0 for the time zero (t0) reference time point, and α-factor was added. Typically, 5-ml cultures were grown in 50-ml conical tubes, and 5 μl of 1,000× peptide stock solutions was added, either once at t0, or, in a second set of experiments, every 2 h at t0, 2 h, and 4 h. Experiments were repeated for cells grown in YPD or in SC and for 13α peptide concentrations at t0 of 0, 0.001, 0.01, 0.1, and 1.0 μg/ml. An aliquot was taken from the cultures at different time points (t0, 2 h, 4 h, 6 h, and 24 h) to monitor the cell morphology with a 1,000× oil immersion objective on a Leica DMIRE2 microscope.
Microarray analysis.
Transcription profiling was performed using custom arrays (28) and was determined for 13 conditions with emphasis for pheromone-induced genes in Δsst2 (Fig. 2). Standard methods, as described previously (28), were used for RNA isolation, probe construction, and hybridization to DNA microarrays, except for the following modifications. Glass beads (catalog no. G-8772; Sigma, St. Louis, MO) were added to samples for the hot phenol extraction steps, amino-allyl-UTP (catalog no. A-0410; Sigma, St. Louis, MO) was added to the deoxynucleoside triphosphate mix for cDNA synthesis, and the cDNAs probes were subsequently labeled with monoreactive cyanine dyes (Cy3 catalog no. PA23001, Cy5 catalog no. PA25001; Amersham), and purified on QIAquick PCR columns (QIAGEN, Valencia, CA) before hybridization. All cultures were grown in liquid SC medium at 24°C. For α-factor induction of the three MTLa strains (3294, CA12, and 3745) (Fig. 2, conditions 4 to 7), cells were grown to an OD600 of 0.5 before the addition of the 13α peptide solution at 1 μg/ml final concentration for a further 2 h of incubation before harvesting cells at an OD600 of ≈0.8. Gene induction by factors that would be secreted and present in the culture supernatant was also tested under one condition (Fig. 2, condition 3). Cultures of both mating types were grown to an OD600 of 0.5 and transferred into sterile bottles for 5 min of centrifugation at 3,000 rpm, the supernatants were decanted, and the cell pellets were resuspended with the supernatant from the opposite mating type culture and transferred back into the original culture flasks for a further 2-h incubation before cell collection at an OD600 of ≈0.8. GeneSpring software (Silicon Genetics, Redwood City, CA) was used for analysis, with a P value set at <0.05 for statistical significance. The complete data set for the 40 DNA-microarray chips, covering the 13 conditions, is accessible at http://candida.bri.nrc.ca/chipdata/sst2/sst2-chipdata.xls. For a matter of clarity, only a data subset for 11 conditions is presented in Fig. 2. Each condition was covered by a minimum of 3 DNA microarrays (except conditions 7 and 11, both with only 2 DNA microarrays) and with dye swaps.
FIG. 2.
DNA microarray data for genes induced by α-factor in Δsst2 strain. Each of the 11 conditions consists of an experiment (EXP) compared to a reference (ref) whose description is summarized above the column of values. The description includes the strain name (full genotypes are given in Table 1; CA12 is designated Δsst2), the strain mating type (MTL) as either MTLa (a) or MTLα (alpha), and the strain phase as either opaque (Op) or white (wh). In conditions 3 to 7, cultures were induced by pheromone, either the synthetic α-factor peptide 13α (+13) (conditions 4 to 7) or, in condition 3, by factors in the supernatant from the opposite mating type culture: supernatant from MTLa culture (+“a”) or supernatant from MTLα culture (+“α”). Genes induced more than twofold by α-factor in the Δsst2 strain are shown for the 11 conditions as signal intensity EXP/ref ratios in black boxes if the ratio is >2.0 and in gray-shaded boxes if the ratio is <0.5. Genes are identified by their orf19 number and annotation names. Controls were added for an opaque-phase-specific gene ops4, and opaque MTLα-specific gene MFalpha1. The data were clustered for conditions having similar transcription profiles as depicted by the dendrogram above the table.
RESULTS
RGS genes in C. albicans.
Candida albicans has two genes that appear to encode members of the RGS superfamily of proteins. One of these genes, Orf19.695, has no striking similarity with any S. cerevisiae gene and only weak similarity with a human RGS domain. In overall domain structure, it resembles ScRGS2 in having a primarily N-terminally located RGS domain, but otherwise the proteins have only minor similarities in sequence. The other sequence, Orf19.4222, shows good similarity to the SST2 gene of S. cerevisiae (Fig. 3). Orf19.4222 contains, in addition to the RGS motif from amino acids 469 to 670, a DEP motif (motif found in Dishevelled, Egl10, and Pleckstrin) (17) from amino acids 369 to 434 (Fig. 3). This motif arrangement is similar to that of the SST2 gene of yeast, which has two N-terminal DEP domains and a C-terminal RGS element. Therefore, we have designated Orf19.695 as RGS2, while Orf19.4222 had been named SST2. Because of the convincing similarity of Sst2p to other G protein regulators, we investigated its function in C. albicans by constructing a disruption of this gene.
FIG. 3.
RGS genes in C. albicans. Analysis of the C. albicans genome revealed 2 ORFs with an RGS domain. Schematic representation of the 2 ORFs, orf19.695 and orf19.4222, aligned with the most similar gene from S. cerevisiae. Rectangles represent RGS domains, and ovoid rectangles represent DEP domains, identified using SMART (32). The first DEP domain of ScSST2 is part of a more generalized fungal DR domain (31). The length of each ORF, based on the predicted amino acid sequence, is shown at the right of each arrow.
SST2 disruption.
We disrupted the SST2 gene using a standard two-step strategy (Fig. 1). The SST2 gene of the parental strain 3294, a derivative of CNC43, was first disrupted with the HIS1 marker, and the second allele of the resulting heterozygote was disrupted with URA3, as described in Materials and Methods. This created the homozygous disruption strain CA12 that was then used to isolate its ura3 derivative CA29 (Table 1). We also created two replacement derivatives of CA29. First, we reintroduced a wild-type copy of the SST2 gene by integration at the SST2 locus; this process generated strain CA37. Second, we reintroduced a defective sst2 gene with a stop codon at amino acid 160 of the SST2 locus to create strain CA40 (see Materials and Methods for details).
After each strain was constructed, we identified opaque form derivatives to permit the analysis of the mating type-specific behavior of the strains, and derivatives of strains 3294, CA12, and CA37 were tested for enhanced sensitivity to pheromone in a halo assay. The opaque version of strain 3294 showed poor response to the 13α pheromone as measured by this assay; treatment of lawns of 3294 grown on SC medium gave evidence of a weak halo of growth inhibition in response to pheromone. No response was identified at all to the less active 14α form (30) of the mating pheromone. However, treatment of CA12, the sst2 derivative of 3294, gave strong halos of growth inhibition whose size was dependent on the amount of pheromone added, and even the 14α peptide was able to generate halos in this strain. Reintegration of the functional wild-type gene eliminated this heightened level of response (Fig. 4).
FIG. 4.
Halo assay. Improved growth arrest of Δsst2 strains in the presence of mating pheromone α-factor. For the assay, SC plates were seeded with 1 × 105 cells from a colony, and 5 μl of α-factor peptide 13α at 1 μg/μl was spotted over the black dot (13) or 5 μl of solvent for the negative control (control). (A) Wild-type parent strain 3294; (B) Δsst2 strain CA12; (C) Δsst2 strain CA12 in the white phase; (D) Δsst2 strain CA29; (E) strain CA37, Δsst2 plus wild-type SST2; (F) strain CA40, Δsst2 plus mutant sst2. Cells were in the opaque phase, except for those in panel C. The wild-type parent strain 3294 produces weak halos only (A). The halos are significantly better after deletion of the SST2 gene (B and D), and reintegration of a wild-type copy of the SST2 gene reverts this phenotype (E). Reintegration of a mutant sst2 gene (see Materials and Methods) does not suppress the phenotype of Δsst2 strains (F).
We determined if the enhanced cell cycle arrest response determined in the halo assay was coupled to any other changes in the mating capacity of the cells. We compared the overall mating ability of the sst2 disruption strain with its wild-type progenitor and the SST2 reintegrant in a qualitative mating assay. The strains were crossed with the opaque mating tester strain 3315 on plates, and the relative mating levels were determined by the appearance of prototrophic cells that were able grow on minimal plates. The sst2 mutant showed reduced formation of prototrophic colonies, while the reintegrants containing a functional SST2 gene showed wild-type levels of mating (Fig. 5). The reduced mating of the Δsst2 strain CA12 was also confirmed in quantitative mating experiments as described in Materials and Methods. In 8 experiments with strain 3294, mating efficiency ranged from 0.21% to 1.20%, for a median value of 0.51%. In 9 experiments with strain CA12, the mating efficiency ranged from 0.01% to 0.22%, for a median value of 0.06%. The relative mating ratio, from experiment to experiment, varied from 5- to 38-fold reduction in mating efficiency for the strain CA12, with a median value of 14-fold reduction.
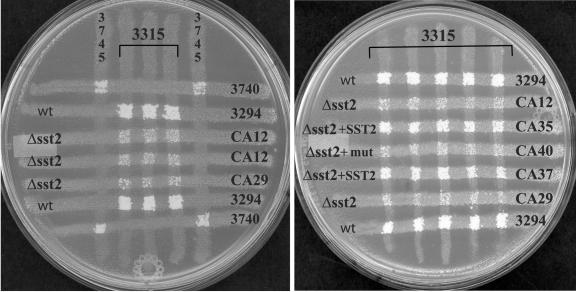
FIG. 5.
Reduced mating of Δsst2 strains. Strains of opposite mating types were crossed for 24 h on YPD plates for mating by auxotrophic marker complementation (see Materials and Methods), and cells were transferred by replica to plates lacking 5 amino acids for selection of the mating products. The selective plates are shown after 2 days incubation. The petri plates are labeled with each strain used in the assays and are described in Table 1. Reduced mating, relative to wild-type parent strain 3294, is observed for strains CA12, CA29, and CA40, and near wild-type mating efficiency is observed for the SST2-reintegrated strains CA35 and CA37. Strains 3740 and 3745 were included as controls; they have, respectively, the same auxotrophic markers as strains 3294 and 3315 but are of opposite mating types.
We also examined the morphology of the wild-type and disruptant strains in the presence of mating pheromone. Cells were treated with various doses of mating pheromone at 0, 2, and 4 h, and the cellular morphology determined microscopically at 6 h after the initial pheromone treatment. Both the wild type and the sst2 disruptant showed extensive morphological changes at the higher pheromone concentrations, with 51 of 141 wild-type cells measured and 71 of 140 of the sst2 cells measured showing projections. However, as shown in Fig. 6, at the 0.01 μg/ml pheromone concentration, the sst2 mutant exhibited greater responsiveness. When several representative fields were scored, 36 of 57 mutant cells but only 4 of 140 wild-type cells were observed with projections.
FIG. 6.
Pheromone-induced morphological changes. The α-factor peptide 13α was added at different concentrations to liquid culture for the wild-type parent strain (3294) and for the Δsst2 strain (CA12). The concentration of α-factor in the cultures at t0 is indicated at the left; the reference, consisting of the addition of solvent without peptide, is marked 0 μg/ml. Typical unconstricted projections (shmoos) are highlighted with white arrows. Many cells develop projections in the presence of α-factor at 0.1 μg/ml, and the two strains are undistinguishable. However, morphological change is difficult to detect for strain 3294 at a lower concentration of α-factor (0.01 μg/ml), while Δsst2 cells are still responsive at 0.001 μg/ml. Pictures are shown at a magnification of ×1,000, 6 h after a single-dose addition of peptide to cells grown in SC medium.
Intriguingly, the cell cycle arrest triggered by mating pheromone is not complete even for the sst2 mutant strain. The halos of growth inhibition were consistently cloudy, due to the frequent appearance of growing colonies within the zone of inhibition. These colonies occasionally represented white revertants, but the majority of colonies were formed by opaque cells that were able to proliferate in the presence of pheromone. This behavior of the sst2 mutant strain CA12 was similar to that of previously analyzed strain 3745, which was also capable of generating cloudy halos when assayed for pheromone-mediated arrest (30). One possible explanation for this behavior is that strain 3745 contains an inactivating mutation in the SST2 gene. We cloned and sequenced the SST2 gene from strain 3745; both alleles were identical and contained 6 nucleotide substitutions relative to the reference sequence provided by the Stanford sequencing project (19). Three of these changes are silent and do not modify the coding sequence, but the other three differences introduce amino acid substitutions that could be the cause of inactivation of function. However, further analysis shows that these substitutions simply represent allelic variation within the SST2 gene, as both the halo-forming strain 3745 and the nonresponsive strain 3294 are homoallelic and contain this same sequence. The reference strain SC5314 actually contains both the allele represented by strains 3745 and 3294 and the allele selected as the reference sequence (D. Dignard, unpublished). Because the SST2 gene is found on chromosome 5 (unpublished data), the selection of homozygosis of the MTL locus also causes homozygosis of the SST2 gene; selection for MTLa/a selects the 3745/3294 allele, while presumably selection for MTLα/α selects for the allele represented in the Stanford sequence.
Recently, it has been established that C. albicans cells responding to mating pheromone exhibit significant changes in gene expression (2). We examined the transcriptional response of the CA12 strain to pheromone as well as the response of the progenitor wild-type strain 3294 and the halo-responsive wild-type strain 3745 (Fig. 2). All three strains were treated with 1 μg/ml of α-pheromone, and the transcriptional profile was determined after 2 h of pheromone addition. Twenty genes showed a greater-than-twofold induction under at least one of the conditions investigated; these genes were all represented in the previously identified data set of pheromone-induced genes (2). Several genes are more responsive to α-factor in Δsst2 strain CA12 than in other MTLa strains (e.g., PRM1, CEK2, POL, GPX1, KAR5); this is also confirmed when α-factor-induced Δsst2 cells are directly compared to α-factor-induced wild-type cells.
The most highly induced gene was orf19.3801 (orf6.2919). This gene has no significant similarity to any sequence in other annotated genomes, and we have designated it FAV1 for factor activated 1. Two other activated genes, orf19.1120 and orf19.1914, have not been previously named, so we have designated them FAV2 and FAV3, respectively. In general, the pheromone-induced genes showed an enhanced responsiveness in the sst2 disruptant. This was either measured by comparing the absolute levels of expression in the disruptant and the wild-type strain when the treated and untreated strains were compared (Fig. 2, lane 5 versus lane 6) or when the treated mutant was compared to the treated wild type directly (Fig. 2, lane 7). Intriguingly, some of the pheromone-sensitive genes were normally repressed in opaque cells relative to white cells (RBT4/PRY4 and GPX1), while others were expressed to a greater extent in opaque cells even in the absence of pheromone stimulation (CEK1).
DISCUSSION
We have characterized the product of C. albicans orf19.4222 as an RGS protein involved in the control of the pheromone response of this fungal pathogen. The structure of the C. albicans predicted protein corresponds to that of the S. cerevisiae SST2 gene product; both proteins have the RGS motif at the C terminus and both contain a centrally located DEP (Disheveled, EGL-10, Pleckstrin) homology domain. This DEP motif is involved in the function of many signaling proteins, and is often found in RGS family members (37). The N terminus of the C. albicans orf19.4222 also has similarity to the N terminus of other fungal RGS proteins. This N-terminal region has been termed a fungal-DR domain (31), but in several fungal RGS proteins, such as Sst2p of S. cerevisiae (9), Rgs1 from Schizosaccharomyces pombe (36) and FlbA of Aspergillus nidulans (22), this region also fits the consensus of the DEP motif. The overall structure of orf19.4222 is similar to a typical fungal RGS protein, with a centrally located classical DEP domain and a C-terminal RGS motif. The orf19.4222 gene has been designated SST2 to reflect its overall structural similarity to the SST2 gene of S. cerevisiae.
The C. albicans SST2 gene product also has functional similarity to RGS proteins. Loss of RGS function has been linked to heightened signaling responses; for example, in both S. cerevisiae cells lacking Sst2p (5) and S. pombe cells lacking Rgs1p (36), G protein-mediated signaling pathways involved in mating and pheromone response are hyperactivated. We have found that opaque-form cells homozygous for the MTLa locus become hypersensitive to α mating factor peptides (30) when they lack the orf19.4222 that encodes SST2. In halo assays, strain CA12 was responsive to even the relatively inactive 14α form of the C. albicans pheromone and showed significant cell cycle arrest in response to the 13α molecule compared to the SST2+ parental strain. This behavior would be expected for strains that have lost the ability to down-regulate a G protein-mediated mating signal due to loss of an inactivating regulator. We have only examined this behavior in MTLa cells; the absence of a defined a-factor protein or even an a-factor gene precludes the direct analysis of the role of Sst2p in pheromone response in MTLα cells.
Although strain CA12 exhibited large zones of inhibition of growth in response to the 13α mating pheromone, these zones were cloudy. Cloudy halos could result from at least two different modes of cellular behavior. Either all the cells within the zone of pheromone response are responding uniformly but are not totally blocked in growth or the cells are not uniform in response, with some of the cells arresting strongly and others showing little or no cell cycle arrest. Examination of the cells within the zone of growth inhibition suggested that the response was not uniform: there were many cells that had arrested with aberrant cell morphology without division, interspersed with colonies of cells that showed little evidence for a morphological response. A small percentage of these unresponsive cells had switched to the white phenotype, but a majority of cells were still opaque and, on subculturing, were still capable of generating halos when retested. This behavior contrasts with that of S. cerevisiae cells lacking SST2 function; S. cerevisiae cells generate clear halos, within which essentially all the cells are arrested at start in an unbudded but morphologically aberrant form (6).
We had previously investigated a nonmutated C. albicans strain (3745) that was also capable of generating cloudy halos when challenged by pheromone (30). The sensitivity of strain 3745 to pheromone-induced arrest cannot be attributed to a nonfunctional SST2 gene. Sequence analysis established that the amino acid sequence of the SST2 gene of strain 3745 differed from the sequence deposited in the Stanford database at 3 sites. However, sequence analysis also established that the SC5314 background contains two distinct alleles of SST2: one allele identical to that deposited as the standard sequence in the Stanford database and the other allele with the 3 amino acid differences noted in strains 3745 and 3294. Because SST2 is found on the same chromosome as the MTL locus, selection for MTL homozygosis will tend to fix one or the other allele of SST2 as well. Thus, the differences between the Stanford SST2 sequence and the 3745 sequence represent the two alleles that exist in the Candida genome. The observation that both CA12, with a defective SST2 gene, and 3745, with a functional SST2 gene, show cell cycle arrest that is detected by halo assays suggests that there is not a single modulator of the arrest process.
The hyperactivation of response to pheromone exhibited in the halo assays was found as well when the sst2 mutant strain was assayed for morphological changes exhibited by cells responding to mating pheromone. When cells were treated with 0.01 μg/ml of 13α pheromone in liquid culture, most of the sst2 cells exhibited the morphological changes characteristic of pheromone-treated cells, while few of the SST2+ parental strain were responsive. At higher concentrations of pheromone, most of the cells in both cultures appeared responsive, and there was little difference in the morphology of the responding sst2 and SST2 cells. This behavior contrasts with that of the sst2 mutant of S. cerevisiae; yeast sst2 mutants and wild-type cells arrested by mating pheromone are morphologically distinct (5).
A previous investigation of the transcriptional profile generated by α-factor in C. albicans used concentrations of mating pheromone of 10 μg/ml and examined the response after up to 4 h of pheromone treatment; this study detected approximately 60 pheromone-induced genes (2). In the current work, when we examined sst2 mutant cells treated for 2 h with a pheromone concentration of only 1 μg/ml, we saw a very similar profile of genes induced, suggesting that the expression profile in the mutant is qualitatively similar to that of the wild type. The most highly induced gene in the current study, encoded by orf19.3801, was noted in proof in the previous paper examining pheromone-responsive gene expression (2). This transcript, which we have named factor activated 1 (FAV1), has no strong similarity to any other gene. Intriguingly, a very weak match was found to the S. cerevisiae pheromone-induced gene FUS2. FUS2 is implicated in karyogamy in the mating process of S. cerevisiae, and karyogamy-related KAR4 and KAR5 homologs are among the other pheromone induced genes in C. albicans.
Although mutation in the SST2 gene enhanced pheromone sensitivity, it reduced the mating competence of the cells. The SST2 gene is itself a pheromone-inducible gene, so it is likely that down-regulation of the response pathway is triggered as a direct consequence of its activation. In both S. cerevisiae (6) and S. pombe (36), similar loss of function of the pheromone response pathway RGS proteins compromised mating. This shows that the mating process itself is sensitive to overstimulation; desensitization is not required simply to extricate responding cells that have not found a mating partner, it is a critical component of the mating process itself.
This observation that an RGS homolog is important in the C. albicans pheromone response pathway is consistent with a model in which heterotrimeric G protein signaling is involved in C. albicans mating, although biochemical conformation of this remains to be determined. A convincing homolog of a Gβ subunit is encoded by C. albicans orf19.799, and deletion of this gene blocks mating in C. albicans (D. Dignard, D. Andre, and M. Whiteway, unpublished). This provides further evidence for the overall similarity among fungal mating pathway regulatory circuits; the use of a G protein-mediated signaling process that requires a functional RGS for efficient deactivation appears to be a common mechanism to control the mating process of these organisms.
Acknowledgments
We thank B. B. Magee and P. T. Magee for providing several strains used in this work. We thank members of the Genetics Group for advice and support and, in particular, Ursula Oberholzer and Doreen Harcus for comments on the manuscript.
This work was supported in part by the Genomics and Health Initiative of the National Research Council of Canada, and CIHR grant MOP-42516 to M.W.
Footnotes
This is NRC publication no. 47485.
REFERENCES
- 1.Achstetter, T. 1989. Regulation of alpha-factor production in Saccharomyces cerevisiae: a-factor pheromone-induced expression of the MFα1 and STE13 genes. Mol. Cell. Biol. 9:4507-4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett, R. J., M. A. Uhl, M. G. Miller, and A. D. Johnson. 2003. Identification and characterization of a Candida albicans mating pheromone. Mol. Cell. Biol. 23:8189-8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernards, A., and J. Settleman. 2004. GAP control: regulating the regulators of small GTPases. Trends Cell Biol. 14:377-385. [DOI] [PubMed] [Google Scholar]
- 4.Cabrera-Vera, T. M., J. Vanhauwe, T. O. Thomas, M. Medkova, A. Preininger, M. R. Mazzoni, and H. E. Hamm. 2003. Insights into G protein structure, function, and regulation. Endocr. Rev. 24:765-781. [DOI] [PubMed] [Google Scholar]
- 5.Chan, R. K., and C. A. Otte. 1982. Isolation and genetic analysis of Saccharomyces cerevisiae mutants supersensitive to G1 arrest by a factor and α factor pheromones. Mol. Cell. Biol. 2:11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan, R. K., and C. A. Otte. 1982. Physiological characterization of Saccharomyces cerevisiae mutants supersensitive to G1 arrest by a factor and α factor pheromones. Mol. Cell. Biol. 2:21-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, D. C., B. C. Yang, and T. T. Kuo. 1992. One-step transformation of yeast in stationary phase. Curr. Genet. 21:83-84. [DOI] [PubMed] [Google Scholar]
- 8.Chen, J., S. Lane, and H. Liu. 2002. A conserved mitogen-activated protein kinase pathway is required for mating in Candida albicans. Mol. Microbiol. 46:1335-1344. [DOI] [PubMed] [Google Scholar]
- 9.Dietzel, C., and J. Kurjan. 1987. Pheromonal regulation and sequence of the Saccharomyces cerevisiae SST2 gene: a model for desensitization to pheromone. Mol. Cell. Biol. 7:4169-4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietzel, C., and J. Kurjan. 1987. The yeast SCG1 gene: a G alpha-like protein implicated in the a- and alpha-factor response pathway. Cell 50:1001-1010. [DOI] [PubMed] [Google Scholar]
- 11.Dohlman, H. G., J. Song, D. M. Apanovitch, P. R. DiBello, and K. M. Gillen. 1998. Regulation of G protein signalling in yeast. Semin. Cell Dev. Biol. 9:135-141. [DOI] [PubMed] [Google Scholar]
- 12.Gillum, A. M., E. Y. Tsay, and D. R. Kirsch. 1984. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 198:179-182. [DOI] [PubMed] [Google Scholar]
- 13.Hamm, H. E., and A. Gilchrist. 1996. Heterotrimeric G proteins. Curr. Opin. Cell Biol. 8:189-196. [DOI] [PubMed] [Google Scholar]
- 14.Hepler, J. R. 1999. Emerging roles for RGS proteins in cell signalling. Trends Pharmacol. Sci. 20:376-382. [DOI] [PubMed] [Google Scholar]
- 15.Hollinger, S., and J. R. Hepler. 2002. Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacol. Rev. 54:527-559. [DOI] [PubMed] [Google Scholar]
- 16.Hull, C. M., R. M. Raisner, and A. D. Johnson. 2000. Evidence for mating of the “asexual” yeast Candida albicans in a mammalian host. Science 289:307-310. [DOI] [PubMed] [Google Scholar]
- 17.Hurley, J. H., E. D. Anderson, B. Beach, B. Canagarajah, Y. S. J. Ho, E. Jones, G. Miller, S. Mistra, M. Pearson, L. Saidi, S. Suer, R. Trievel, and Y. Tsujishita. 2002. Structural genomics and signaling domains. Trends Biochem. Sci. 27:48-53. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, A. 2003. The biology of mating in Candida albicans. Nat. Rev. Microbiol. 1:106-116. [DOI] [PubMed] [Google Scholar]
- 19.Jones, T., N. A. Federspiel, H. Chibana, J. Dungan, S. Kalman, B. B. Magee, G. Newport, Y. R. Thorstenson, N. Agabian, P. T. Magee, R. W. Davis, and S. Scherer. 2004. The diploid genome sequence of Candida albicans. Proc. Natl. Acad. Sci. USA 101:7329-7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kakar, S. N., R. M. Partridge, and P. T. Magee. 1983. A genetic analysis of Candida albicans: isolation of a wide variety of auxotrophs and demonstration of linkage and complementation. Genetics 104:241-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karnik, S. S., C. Gogonea, S. Patil, Y. Saad, and T. Takezako. 2003. Activation of G-protein-coupled receptors: a common molecular mechanism. Trends Endocrinol. Metab. 14:431-437. [DOI] [PubMed] [Google Scholar]
- 22.Lee, B. N., and T. H. Adams. 1994. Overexpression of flbA, an early regulator of Aspergillus asexual sporulation, leads to activation of brlA and premature initiation of development. Mol. Microbiol. 14:323-334. [DOI] [PubMed] [Google Scholar]
- 23.Lockhart, S. R., R. Zhao, K. J. Daniels, and D. R. Soll. 2003. α-Pheromone-induced “shmooing” and gene regulation require white-opaque switching during Candida albicans mating. Eukaryot. Cell 2:847-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magee, B. B., M. Legrand, A. M. Alarco, M. Raymond, and P. T. Magee. 2002. Many of the genes required for mating in Saccharomyces cerevisiae are also required for mating in Candida albicans. Mol. Microbiol. 46:1345-1351. [DOI] [PubMed] [Google Scholar]
- 25.Magee, B. B., and P. T. Magee. 2000. Induction of mating in Candida albicans by construction of MTLa and MTLalpha strains. Science 289:310-313. [DOI] [PubMed] [Google Scholar]
- 26.Miller, M. G., and A. D. Johnson. 2002. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell 110:293-302. [DOI] [PubMed] [Google Scholar]
- 27.Morris, A. J., and C. C. Malbon. 1999. Physiological regulation of G protein-linked signaling. Physiol. Rev. 79:1373-1430. [DOI] [PubMed] [Google Scholar]
- 28.Nantel, A., D. Dignard, C. Bachewich, D. Harcus, A. Marcil, A.-P. Bouin, C. W. Sensen, H. Hogues, M. van het Hoog, P. Gordon, T. Rigby, F. Benoit, D. C. Tessier, D. Y. Thomas, and M. Whiteway. 2002. Transcription profiling of Candida albicans cells undergoing the yeast-to-hyphal transition. Mol. Biol. Cell 13:3452-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Negredo, A., L. Monteoliva, C. Gil, J. Pla, and C. Nombela. 1997. Cloning, analysis and one-step disruption of the ARG5,6 gene of Candida albicans. Microbiology 143:297-302. [DOI] [PubMed] [Google Scholar]
- 30.Panwar, S. L., M. Legrand, D. Dignard, M. Whiteway, and P. T. Magee. 2003. MFα1, the gene encoding the α mating pheromone of Candida albicans. Eukaryot. Cell 2:1350-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pereira, P. S., and N. C. Jones. 2001. The RGS domain-containing fission yeast protein, Rgs1p, regulates pheromone signalling and is required for mating. Genes Cells 6:789-802. [DOI] [PubMed] [Google Scholar]
- 32.Schultz, J., F. Milpetz, P. Bork, and C. P. Ponting. 1998. SMART, a simple modular architecture research tool: identification of signaling domains. Proc. Natl. Acad. Sci. USA 95:5857-5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sherman, F. 2002. Getting started with yeast. Methods Enzymol. 350:3-41. [DOI] [PubMed] [Google Scholar]
- 34.Soll, D. R. 2004. Mating-type locus homozygosis, phenotypic switching and mating: a unique sequence of dependencies in Candida albicans. Bioessays 26:10-20. [DOI] [PubMed] [Google Scholar]
- 35.Tesmer, J. J., D. M. Berman, A. G. Gilman, and S. R. Sprang. 1997. Structure of RGS4 bound to AlF4-activated G(i alpha1): stabilization of the transition state for GTP hydrolysis. Cell 89:251-261. [DOI] [PubMed] [Google Scholar]
- 36.Watson, P., K. Davis, M. Didmon, P. Broad, and J. Davey. 1999. An RGS protein regulates the pheromone response in the fission yeast Schizosaccharomyces pombe. Mol. Microbiol. 33:623-634. [DOI] [PubMed] [Google Scholar]
- 37.Zhong, H., and R. R. Neubig. 2001. Regulator of G protein signaling proteins: novel multifunctional drug targets. J. Pharmacol. Exp. Ther. 297:837-845. [PubMed] [Google Scholar]