Steric effects have become widely cited as important factors in recognition and replication of DNA. Because the DNA backbone is relatively rigid, selectivity in hybridization against mismatches having different sizes and geometry is significant. However, the pairing fidelity observed in the enzymatic replication of DNA is markedly higher.1 It is hypothesized that polymerases achieve this by tightly surrounding the new base pairs being synthesized, thus providing an even stricter steric environment.2
In order to test these proposed effects, it would be useful to evaluate polymerase activities with DNA base analogs having varied sizes. In one recent test of halogen-substituted nonpolar DNA base analogs with gradually increasing sizes,3 the replication enzymes of E. coli were highly sensitive to even sub-Angstrom alterations of sterics. Surprisingly, it was observed that DNA polymerase I prefers analogs that are larger than natural DNA bases. However, this apparently enlarged steric preference has not yet been tested with hydrogen-bonding nucleotide analogs. Thus we turned to the thiocarbonyl group as a way to increase size by a small increment. This substitution, 0.45 Å longer than the carbonyl group,4 has previously been used on several nucleoside analogs, but has not yet been generally studied for its effects on DNA base pairing and replication. Here we report the results of base pairing studies and of polymerase kinetics studies of 2-thio- and 4-thio-thymidine.5 We find that the larger size can lead to increased efficiency and selectivity in pairing and replication.
Both the 2-thio- and 4-thiothymidine deoxyribosides (2S and 4S, Figure 1) are known,5 and have been employed especially for the unique chemistry of the thiocarbonyl group, including nucleophilic reactivity6 and photocrosslinking.7 Surprisingly, although a few preliminary tests of their hybridization have been carried out,8 their pairing selectivity and quantitative replication properties are largely unknown. In light of their possible utility in evaluating steric effects on nucleic acid systems, we undertook a general and quantitative analysis of base pairing and replication properties of these two analogs. We carried out a base pairing study of both thiothymidine isomers in a 12-base-pair duplex. Thermal denaturation experiments confirmed8a that 4S was slightly destabilizing to the duplex compared to natural thymidine, while the 2S isomer was more stabilizing than the natural congener in this sequence context, increasing Tm by 1.4 °C with one substitution (Fig. 2, and Table S1 in the Supporting file). The pairing selectivity of the thio analogs was also evaluated; results showed that the 2S isomer gave higher pairing specificity than thymine itself, with a 12.0 °C decrease in Tm for the most stable mismatch (2S-G) compared to a 7.5 °C drop for the natural T-G mismatch.
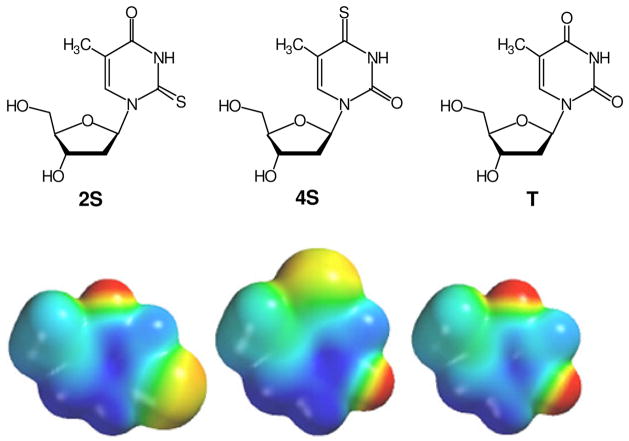
Figure 1.
Structures of 2-thiothymidine (2S) and 4-thiothymidine (4S), with unmodified thymidine shown at right for comparison. Space-filling models of the bases alone are shown below each structure.
Figure 2.

Histogram showing pairing stability (as Tm) and selectivity of 2S and 4S placed opposite each of the natural bases in a 12 base pair DNA duplex. The sequence is dAAGAAXGAAAAG · dCTTTTCYTTCTT, where X is 2S, 4S, or T, and Y is each of the four natural bases as shown. Conditions: 2 M DNA, 100 mM NaCl, 10 mM MgCl2, 10 mM phosphate (pH 7.0). Uncertainty in Tm values is less than ± 0.5 °C.
Interestingly, the 4S isomer showed very different behavior, forming a 4S-G pair with Tm even higher than a natural T-A base pair. Thus, in summary, 2S forms pairs with high stability and selectivity, while 4S is slightly destabilizing in this context and shows dual pairing ability with A and G. Models suggest that 4S should have somewhat unfavorable steric interactions with the amino group of adenine in a 4S-A base pair (see Fig. S1), and gas phase AM1 calculations suggest that 4S can exist in a relatively stable enethiol tautomer that could adopt a triply-hydrogen-bonded Watson-Crick pair geometry with G (Fig. S2). It is possible that the enhanced pairing properties of 2S-A and 4S-G arise from stronger stacking of the larger and more polarizable species,9 or from altered solvation and/or cation interactions; more studies will be needed to test these effects.
Few pairing data for 2S and 4S exist in the literature for comparison to the current results. Thermal denaturation studies have been reported for both compounds paired opposite adenine,8a generally showing some stabilization (relative to T) by 2S, and weak destabilization by 4S, consistent with the current results. Studies were not previously carried out with the three different mismatches of each compound; however, there does exist one previous experiment for the 4S-G mismatch,8b which showed strong destabilization, in marked contrast to the current results. The origin of this difference might be due to a difference in sequence context, or to 4S degradation under some DNA synthesis conditions.10
To test the effects of the added size on DNA replication, we carried out steady-state kinetics experiments on replication of the thio analogs by the Klenow fragment of E. coli DNA polymerase I (Kf exo-). The larger-than-natural analogs were tested both as template bases and as incoming dNTP analogs (see Table 1 and Table S2). When replicated opposite adenine, both thio analogs showed high efficiency compared to natural thymidine. This is especially the case where the analogs were used as triphosphate derivatives, where 2S-triphosphate was 2.2-fold more efficient than dTTP, and 4S-triphosphate was 3.7-fold more efficient. In the template strand, the analogs had the same efficiency as thymidine when paired with dATP. Thus the added size of the 2-thio and 4-thio groups had no deleterious effects on efficiency of this polymerase, and indeed, the increased bulk was associated with generally increased efficiency for incoming nucleotides, consistent with the recent observation that the Kf enzyme generally shows a kinetic preference for base analogs larger than the natural ones.3
Table 1.
Steady-state kinetics data for processing of base pairs and mismatches involving thiothymidine nucleotides by Kf pol I (exo-). a See Table S1 for data with 2S and 4S as the template base.
| nucleoside triphosphate | template base (X) | Km (μM) | Vmax (% min−1)b | efficiency (Vmax/Km) | relative efficiency |
|---|---|---|---|---|---|
| 2S | A | 3.7 (0.8) | 12.9 (2.9) | 3.5 × 106 | 2.2 |
| 4S | A | 2.0 (0.1) | 11.8 (0.1) | 5.9 × 106 | 3.7 |
| T | A | 7.0 (1.8) | 11.0 (2.3) | 1.6 × 106 | 1 |
| 2S | T | 103 (18) | 0.802 (0.051) | 7.9 × 103 | 4.9 × 10−3 |
| 4S | T | 104 (27) | 0.203 (0.042) | 2.0 × 103 | 1.3 × 10−3 |
| T | T | 80 (13) | 0.015 (0.005) | 1.8 × 102 | 1.1 × 10−4 |
| 2S | C | 75 (22) | 0.022 (0.007) | 3.0 × 102 | 1.9 × 10−4 |
| 4S | C | 226 (20) | 0.138 (0.013) | 6.1 × 102 | 3.8 × 10−4 |
| T | C | 279 (6) | 0.018 (0.001) | 6.6 × 101 | 4.1 × 10−5 |
| 2S | G | 15 (7) | 0.006 (0.002) | 4.0 × 102 | 2.5 × 10−4 |
| 4S | G | 59 (13) | 0.246 (0.048) | 5.9 × 103 | 3.7 × 10−3 |
| T | G | 136 (60) | 0.139 (0.049) | 1.1 × 103 | 6.9 × 10−4 |
Conditions: 200 nM 23mer/28mer primer-template duplex and varied polymerase concentrations in a buffer containing 50 mM Tris·HCl (pH 7.5), 10 mM MgCl2, 50 ug/mL BSA and 1 mM dithiothreitol, incubated at 37°C in a reaction volume of 10 μL. Standard deviations are given in parentheses.
Normalized for the lowest enzyme concentration used.
Although the enzymatic incorporation of thiothymidines into DNA has been observed previously,11 we are aware of only one previous kinetics study of either isomer. It was reported12 that 4S dNTP derivative was incorporated opposite adenine with efficiency 1.3-fold higher than was dTTP by the Kf enzyme; that is generally consistent with the current result. No previous kinetics data exist for 2S as the dNTP derivative to our knowledge, nor for either isomer in a template strand of DNA.
We assessed the effects of the larger thio groups on replication selectivity by carrying out studies with all mismatched partners. Our data show that, consistent with its effects in hybridization, the analog 4S gave increased efficiency of 4S-G mismatched pair synthesis, whether in a template strand or as a dNTP analog. With the 4S dNTP analog, the overall fidelity of base pair synthesis was the same as the natural nucleotide as a result of the fact that both the 4S-A and 4S-G pairs were equally increased in efficiency (Fig. S3). With 4S in the template, however, the pair fidelity was decreased by the (possibly tautomeric) G-4S pair efficiency. The 2S isomer behaved differently than 4S; as a dNTP analog, 2S showed a high efficiency for being mispaired opposite T, leading to a lowered selectivity compared to natural thymidine. However, in a template strand, 2S displayed higher fidelity than natural T, since the 2S-G mispairing was suppressed significantly.
In summary, we find that the increased sizes of the thio groups at the 2- and 4-position have significant effects on the hybridization properties of DNAs containing them, and on DNA replication as well. Some of the effects, including the increased stability and hybridization selectivity of 2S, and the high efficiency of polymerase replication of both isomers, may prove useful in a number of applications, and may have relevance to the biological activity of other thionucleosides (thiopurines) in the treatment of leukemia.13 Thio-substitution of guanine also is known to affect hybridization and replication properties.14 It remains to be seen whether other enzymes would respond similarly to such steric effects.15
Supplementary Material
Details of DNA synthesis and characterization, thermal denaturation, and enzyme kinetics (10 pages). See any masthead page for Internet access information.
Acknowledgments
This work was supported by the U.S. National Institutes of Health (GM067201 and GM63587).
References
- 1.Petruska J, Goodman MF, Boosalis MS, Sowers LC, Cheong C, Tinoco I., Jr Proc Natl Acad Sci USA. 1988;85:6252–6256. doi: 10.1073/pnas.85.17.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Moran S, Ren RXF, Rumney S, Kool ET. J Am Chem Soc. 1997;119:2056–2057. doi: 10.1021/ja963718g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Goodman MF. Proc Natl Acad Sci USA. 1997;94:10493–10495. doi: 10.1073/pnas.94.20.10493. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Beard WA, Wilson SH. Chem Biol. 1998;5:R7–13. doi: 10.1016/s1074-5521(98)90081-3. [DOI] [PubMed] [Google Scholar]; (d) Kunkel TA, Bebenek K. Annu Rev Biochem. 2000;69:497–529. doi: 10.1146/annurev.biochem.69.1.497. [DOI] [PubMed] [Google Scholar]; (e) Kool ET. Annu Rev Biochem. 2002;71:191–219. doi: 10.1146/annurev.biochem.71.110601.135453. [DOI] [PubMed] [Google Scholar]
- 3.Kim TW, Delaney JC, Essigmann JM, Kool ET. Proc Natl Acad Sci USA. 2005;102:15803–15808. doi: 10.1073/pnas.0505113102. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biedermann M, Hartung H, Dolling W, Verjus P. Acta Cryst. 1998;C54(4):507–509. [Google Scholar]
- 5.Connolly BA. Methods Enzymol. 1992;211:36–53. doi: 10.1016/0076-6879(92)11005-4. [DOI] [PubMed] [Google Scholar]
- 6.(a) Coleman RS, Siedlecki JM. J Am Chem Soc. 1992;114:9229–9230. [Google Scholar]; (b) Xu YZ. Progr Nat Sci. 2000;10:401–413. [Google Scholar]
- 7.(a) Liu JQ, Taylor JS. Nucleic Acids Res. 1998;26:3300–3304. doi: 10.1093/nar/26.13.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lavrik OI, Zakharenko AL, Prasad R, Vlasov VA, Bogachev VS, Favre A. Mol Biol. 1998;32:510–517. [Google Scholar]; (c) Favre A, Saintome C, Fourrey JL, Clivio P, Laugaa P. J Photochem Photobiol B. 1998;42:109–124. doi: 10.1016/s1011-1344(97)00116-4. [DOI] [PubMed] [Google Scholar]; (d) Massey A, Xu YZ, Karran P. Current Biol. 2001;11:1142–1146. doi: 10.1016/s0960-9822(01)00272-x. [DOI] [PubMed] [Google Scholar]; (e) Chepanoske CL, Lukianova OA, Lombard M, Golinelli-Cohen MP, David SS. Biochemistry. 2004;43:651–662. doi: 10.1021/bi035537e. [DOI] [PubMed] [Google Scholar]
- 8.(a) Connolly BA, Newman P. Nucleic Acids Res. 1989;17:4957–4974. doi: 10.1093/nar/17.13.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Massey A, Xu YZ, Karran P. DNA Repair. 2002;1:275–286. doi: 10.1016/s1568-7864(02)00004-6. [DOI] [PubMed] [Google Scholar]
- 9.Gao J, Liu H, Kool ET. J Am Chem Soc. 2004;126:11826–11831. doi: 10.1021/ja048499a. [DOI] [PubMed] [Google Scholar]
- 10.(a) Rajur SB, McLaughlin LW. Tetrahedron Lett. 1992;33:6081–6084. [Google Scholar]; (b) Kuimelis RG, Nambiar KP. Nucleic Acids Res. 1994;22:1429–1436. doi: 10.1093/nar/22.8.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Hofer B, Köster H. Nucleic Acids Res. 1981;9:753–767. doi: 10.1093/nar/9.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sismour AM, Benner SA. Nucleic Acids Res. 2005;33:5640–5646. doi: 10.1093/nar/gki873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao TVS, Haber MT, Sayer JM, Jerina DM. Bioorg Med Chem Lett. 2000;10:907–910. doi: 10.1016/s0960-894x(00)00123-2. [DOI] [PubMed] [Google Scholar]
- 13.Krynetski E, Evans WE. Oncogene. 2003;22:7403–7413. doi: 10.1038/sj.onc.1206944. [DOI] [PubMed] [Google Scholar]
- 14.(a) Ling YH, Nelson JA, Cheng YC, Anderson RS, Beattie KL. Mol Pharm. 1991;40:508–514. [PubMed] [Google Scholar]; (b) Rappaport HP. Biochemistry. 1993;32:3047–3057. doi: 10.1021/bi00063a016. [DOI] [PubMed] [Google Scholar]; (c) Somerville L, Krynetski EY, Krynetskaia NF, Beger RD, Zhang W, Marhefka CA, Evans WE, Kriwacki RW. J Biol Chem. 2003;278:1005–1011. doi: 10.1074/jbc.M204243200. [DOI] [PubMed] [Google Scholar]
- 15.Strerath M, Cramer J, Restle T, Marx A. J Am Chem Soc. 2002;124:11230–11231. doi: 10.1021/ja027060k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details of DNA synthesis and characterization, thermal denaturation, and enzyme kinetics (10 pages). See any masthead page for Internet access information.