Abstract
The effect of interleukin-18 (IL-18) on metastasis of highly metastatic LM8 mouse osteosarcoma cells was investigated using nude mice treated with anti-asialo GM1 serum to exclude anti-tumor actions of IL-18 through activation of T and natural killer cells. Injection of LM8 cells which do not express IL-18 receptor β into a tail vain resulted in the formation of pulmonary and hepatic metastatic foci. Daily injection of mice with IL-18 starting the fifth day from the cell injection had no significant effect on the number of metastatic foci, while five daily injections of IL-18 before and after the cell injection resulted in marked decreases. Culture of LM8 cells with IL-18 for 5 days before the injection into mice produced no significant effect on the number of pulmonary and hepatic metastatic foci. In contrast, pretreatment of mice with IL-18 for 5 days before the cell injection markedly decreased metastatic foci. The retention of LM8 cells in the lung 24 h after their injection was also reduced by the pretreatment of mice with IL-18. Serum obtained from mice pretreated with IL-18 for 5 days suppressed mobility of LM8 cells but IL-18 itself did not. These results suggest that IL-18 inhibits metastasis of LM8 cells partly by inducing a factor(s) in the host which suppresses cell mobility.
Keywords: Osteosarcoma, Interleukin-18, Metastasis, Migration
Introduction
Osteosarcoma is the most frequently occurring primary malignant bone tumor excluding multiple myeloma, found most commonly in people between 10 and 30 years of age [10]. Recent advances in adjuvant chemotherapy after surgery have improved the rate of disease-free survival of patients with osteosarcomas [18, 27]. However, patients ultimately suffer from recurrence with metastasis mostly in the lung [13, 27], which invariably affects patient’s survival [28]. Therefore, the suppression of pulmonary metastasis is one of the most important issues in the treatment of osteosarcomas.
Interleukin-18 (IL-18), previously called interferon-γ (IFN-γ)-inducing factor [17, 26], exerts an anti-tumor action through activation of natural killer cells (NK cells) or cytotoxic T lymphocytes [12, 17, 25, 30], suppression of angiogenesis [2] and induction of Fas-ligand on tumor cells expressing Fas [24]. We have shown that IL-18 inhibits the growth of osteosarcoma cells by activating T lymphocytes, suggesting the usefulness of IL-18 as a therapy agent for osteosarcoma [25]. In this study, we examined the effect of IL-18 on pulmonary metastasis of osteosarcoma using an experimental metastasis model in which highly metastatic LM8 mouse osteosarcoma cells were injected into a tail vein of T cell-deficient nude mice treated with anti-asialo GM1 serum to abolish the activity of NK cells. We found that IL-18 inhibited metastasis of osteosarcoma cells partly by inducing a factor(s) in the host which suppressed mobility of LM8 cells.
Materials and methods
Materials
Anti-asialo GM1 rabbit serum was purchased from Wako Pure Chemicals (Osaka, Japan), 5-[125I]iodo-2′-deoxyuridine (2,000 Ci/mmol) and methyl-[3H]thymidine (5 Ci/mmol) from Amersham Pharmacia Biotech UK (Little Chalfont, Buckinghamshire, UK), a PKH2 green fluorescent cell linker kit and a leukocyte acid phosphatase kit from Sigma (St. Louis, MO), a mouse IFN-γ enzyme-linked immunosorbent assay (ELISA) kit from Techne (Minneapolis, MN), a nitrite/nitrate assay kit from R&D Systems (Minneapolis, MN) and fetal calf serum (FCS) from Hyclone (Logan, UT). Recombinant murine IL-18 was generously provided by GlaxoSmithkline Pharma (King of Prussia, PA).
Mice
Male CD1-Foxn1 nu mice (nude mice) of 5 weeks of age were purchased from Japan Charles River (Atsugi, Kanagawa, Japan). Mice were maintained in a pathogen-free environment at 25°C under controlled lighting (12 h light/12 h darkness), and allowed free access to water and food pellets. All experiments with mice were approved by the Animal Care Committee of Hyogo College of Medicine, and the mice were given the most human care.
Cells
The murine osteosarcoma cell line, LM8, which is highly metastatic to the lung, was established from Dunn osteosarcoma [1]. LM8 cells were maintained in Eagle’s minimum essential medium (EMEM) supplemented with 10% FCS in a humidified atmosphere of 5% CO2 in air at 37°C. When cells were subconfluent, they were harvested by treatment with 0.25% trypsin and 0.01% EDTA in Ca2+- and Mg2+-free Dulbecco’s phosphate-buffered saline, PH 7.4 (PBS). Highly metastatic mouse B16 melanoma cells and Lewis lung carcinoma cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FCS [29]. The mouse MS1 pancreatic islet endothelial cell line was purchased from ATCC (Manassas, VA) and maintained in DMEM supplemented with 5% FCS. FCS was heated at 56°C for 30 min to inactivate complements before use.
Reverse-transcriptase polymerase chain reaction
Total RNA of LM8 cells or 5E3 cells [25] was extracted using a Trizol solution (Gibco Laboratories; Gland Island, NY). First-strand cDNA was synthesized from total RNA (5 μg) with Superscript II reverse transcriptase (Life Technologies; Grand Island, NY) and random primers, and subjected to PCR amplification with EX Taq polymerase (Takara Shuzo; Kyoto, Japan) and specific PCR primers. The PCR primers used were sense (5′-CGTGACAAGCAGAGATGTTG-3′, 755–774) and antisense (5′-ATGTTGTCGTCTCCTTCCTG-3′, 1163–1182) primers for the mouse IL-18 receptor α gene, sense (5′-ATGCTCTGTTTGGGCTGGGT-3′, 437–456) and antisense (5′-CTGTCTTGATACAACAGGCCA-3′, 843–863) primers for the mouse IL-18 receptor β gene, and sense (5′-AGGTATCCTGACCCTGAAGTAC-3′, 266–287) and antisense (5′-TCTTCATGAGGTAGTCTGTCAG-3′, 633–654) primers for the mouse β-actin gene. The PCR conditions were 94°C for 30 s, 58°C for 30 s, and 72°C for 1 min for 35 or 40 cycles. The amplified fragments were resolved by electrophoresis in 1% agarose gel and detected by ethidium bromide staining. As positive controls for expression of IL-18 receptor α and β mRNAs, total RNA extracted form 5E3 cells were used [25].
Growth of LM8 cells in vitro
LM8 cells seeded at a density of 1×104 cells/35 mm-dish were cultured in 3 ml of EMEM containing 10% FCS for 7 days with medium change on day 4. The number of viable cells was determined by the Trypan blue exclusion test.
Injection of IL-18 and anti-asialo GM1 serum
IL-18 (2 μg) was dissolved in 0.2 ml of PBS containing 0.1% mouse serum and injected daily into a mouse intraperitoneally. Anti-asialo GM1 serum (20 μl) was dissolved in 0.1 ml of PBS and injected into a mouse intraperitoneally every 5 days throughout the experimental period. The dose of anti-asialo GM1 serum used has been reported to deplete the NK cell activity almost completely [30].
Growth of LM8 cells inoculated into mice subcutaneously
LM8 cells were suspended in 0.2 ml serum-free EMEM, and injected subcutaneously into the interscapular space of nude mice (1×107 cells/mouse) injected with anti-asialo GM1 serum 5 days before the cell inoculation and once every 5 days thereafter. The tumor volume was determined every 2 or 3 days after the cell inoculation according to the formula; tumor volume (mm3) = S 2 × L × 0.5, where L and S indicate the size in millimeters of the longest and shortest part, respectively.
Metastasis of LM8 cells to the lung and liver
To produce pulmonary and hepatic metastasis of LM8 cells, 2×105 LM8 cells suspended in 0.2 ml of PBS were injected into a tail vein of each nude mouse. Mice were killed 19 or 21 days later, and the lung and liver were removed and weighed. Metastatic foci on the lung and liver were counted under a dissection microscope.
Retention of LM8 cells in the lung 24 h after their injection
LM8 cells were labeled with 125I by culturing in EMEM containing 10% FCS and 5-[125I]iodo-2′-deoxyuridine (0.5 μCi/ml) for 24 h. IL-18 was injected into mice daily for 5 days prior to the injection of 125I-labeled LM8 cells (2×105 cells/0.2 ml PBS), and anti-asialo GM1 serum was injected 5 days prior to and on the day of the injection of LM8 cells. Mice were killed 24 h after the injection of LM8 cells, and the lungs were removed and fixed in 10% phosphate (0.01 M)-buffered (PH 7.4) formalin. The fixed lungs were washed with water daily for 3 days, and analyzed for the retained radioactivity with an autowell γ-counter system ARC-300 (Aloka; Tokyo, Japan). The radioactivity obtained was expressed as a percentage of the injected radioactivity and used as the retention of LM8 cells in the whole lungs.
Distribution of LM8 cells in the lung 24 h after their injection
Fluorescent LM8 cells were prepared with a PKH2 green fluorescent cell linker kit according to manufacturer’s instructions, and injected into each nude mouse (2×105 cells/0.2 ml PBS/mouse). The mice were killed 24 h later, and the lungs were fixed in 10% phosphate (0.01 M)-buffered (PH 7.4) formalin and embedded in paraffin. Sections of 5 μm thickness were prepared and examined for fluorescence using a fluorescence microscope equipped with a C488 dual scan CCD camera (Hamamatsu Photonics; Hamamatsu, Shizuoka, Japan) and an Argus software (Hamamatsu Photonics).
Assay of IFN-γ and nitrite plus nitrate
IFN-γ in serum was determined using a mouse IFN-γ ELISA kit. The amount of nitrite plus nitrate in serum was assayed using a nitrite/nitrate assay kit.
The wound assay for estimation of cell motility
Nude mice were injected with IL-18 daily for 5 days, with anti-asialo GM1 serum injected on the day of the first injection of IL-18. Twenty-four hours after the last injection of IL-18, sera were obtained and stored at -80°C. For the wound assay [4], LM8 cell (2×104 cells), B16 melanoma cells (1×105 cells) or Lewis lung carcinoma cells (2×105 cells) were seeded on a well of a 96-well plate and cultured in 100 μl of EMEM containing 10% FCS until cells became confluent. A line of scratch was made in the culture with a plastic tip, cell debris formed was removed by washing with EMEM, and the cells were further cultured in 75 μl of DMEM containing 50% mouse serum in the presence or absence of IL-18 (100 ng/ml) for 24 h. The width of a scratch wound was recorded using a phase contrast microscope equipped with a digital CCD camera (Hamamatsu Photonics) and an Aquacosmos software (Hamamatsu Photonics) before and after the culture, and differences in the scratch width at three different points were averaged and used as a cell mobility distance. In addition to the measurement of cell motility, the effect of serum on the growth of LM8 cells was examined. LM8 cells (2×104 cells) were seeded on a well of a 96-well plate and cultured in 100 μl of EMEM containing 10% FCS for 24 h. The cells were washed with EMEM and cultured in medium composed of 80 μl of EMEM containing 50% mouse serum and methyl-[3H]thymidine (0.5 μCi) for 24 h. The cells in each well were washed with EMEM three times, detached by incubation with 0.25% trypsin and harvested on an Unifilter (GF/F) (PerkinElmer; Boston, MA) using a Filtermate Harvester (PerkinElmer). Thereafter, the radioactivity was counted using a microplate scintillation counter (PerkinElmer).
Cell attachment assay
MS1 mouse endothelial cells were seeded at 1×105 cells/well in a 96-well plate and cultured in 100 μl of DMEM containing 5% FCS in the presence or absence of IL-18 (100 ng/ml) until confluency. The cells were washed with DMEM three times, and 1×104 fluorescent LM8 cells (see above) suspended in 75 μl of DMEM containing 50% mouse serum prepared for the wound assay, were added to each well and incubated for 3 h. MS1 and LM8 cells were washed with PBS two times and fixed in 10% phosphate (0.01 M)-buffered (PH 7.4) formalin, and fluorescent LM8 cells in each well were counted using a fluorescence microscope equipped with a C488 dual scan CCD camera (Hamamatsu Photonics) and an Argus software (Hamamatsu Photonics).
Statistical analysis
Data were expressed as mean ± SE. Data of three groups were analyzed by the Bonferroni multiple comparison test. Data of two groups were analyzed by the Mann–Whitney’s U-test. A P value of less than 0.05 was considered significant.
Results
Effect of IL-18 on the growth of LM8 cells in vitro and in vivo
LM8 cells expressed mRNA of the IL-18 receptor α, but not of the IL-18 receptor β, suggesting that IL-18 has no direct action on LM8 cells (Fig. 1). This is supported by the results that IL-18 at 10 or 100 ng/ml did not affect the growth of LM8 cells in vitro (Fig. 2).
Fig. 1.
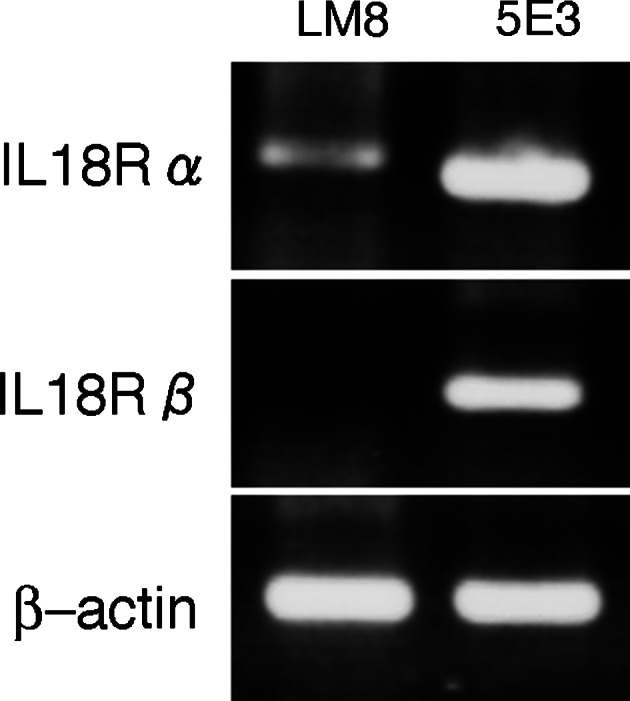
Reverse-transcriptase polymerase chain reaction (RT-PCR) analaysis of mRNAs of interleukin-18 (IL-18) receptor subunits (IL-18 R α and β) in LM8 cells. 5E3 cells were used as a positive control for the expression of mRNAs of IL-18 R α and β
Fig. 2.
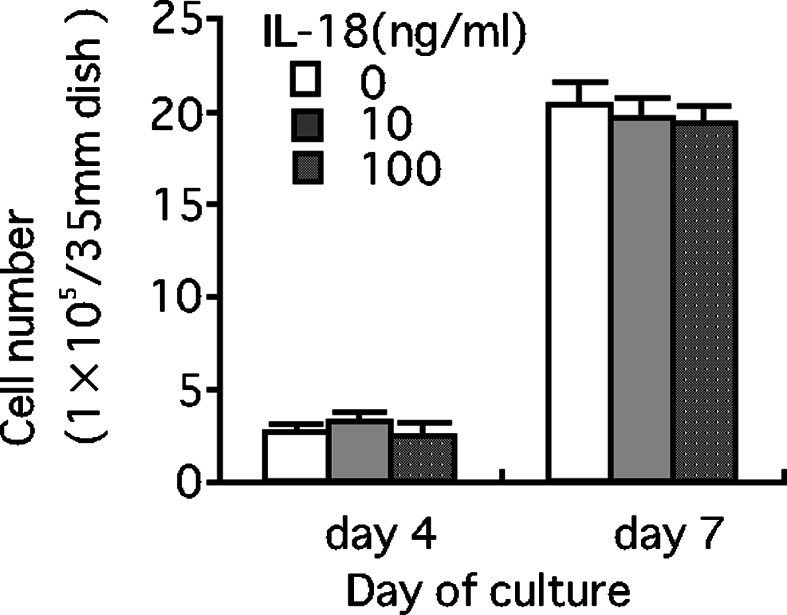
Effect of IL-18 on the growth of LM8 cells in vitro. LM8 cells (1×104 cells/35 mm-dish) were cultured in Eagle’s minimum essential medium (EMEM) containing 10% fetal calf serum (FCS) for 4 or 7 days in the presence or absence of IL-18 (10 or 100 ng/ml). Each bar represents a mean + SE of four dishes
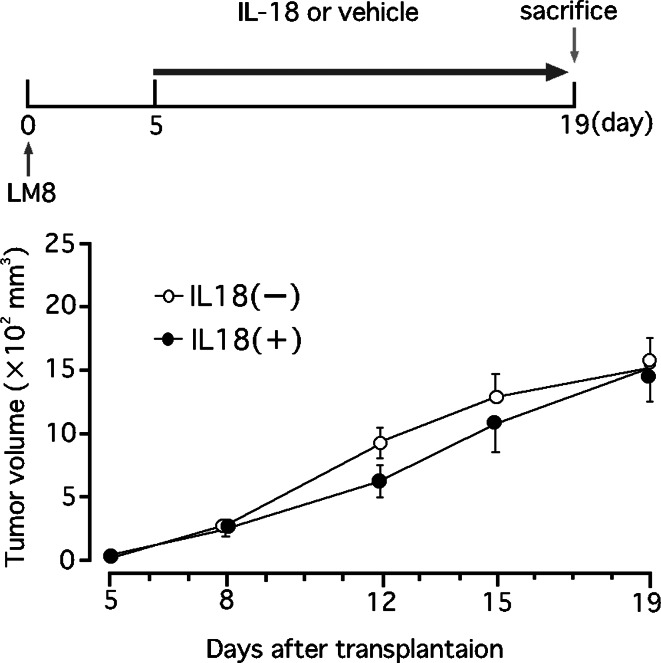
In vivo, IL-18 has been shown to exhibit indirect anti-tumor activity by mechanisms including activation of NK cells and cytotoxic T lymphocytes [12, 17, 25, 30]. In this study, daily injections of IL-18 (2 μg/mouse/day) did not affect the growth of LM8 cells in nude mice deficient in T lymphocytes in which NK cells were inactivated by injection of anti-asialo GM1 serum (Fig. 3).
Fig. 3.
Effect of IL-18 on the growth of LM8 cells in nude mice. Mice were injected with anti-asialo GM1 serum (20 μl/mouse) and, 5 days later, inoculated with LM8 cells (1×107 cells) subcutaneously. Injection of anti-asialo GM1 serum continued every 5 days. Starting the fifth day from the cell inoculation, mice received daily injection of IL-18 (2 μg/mouse) or vehicle. The upper figure shows the experimental design and the lower one the results. Each point represents a mean ± SE of eight mice
Effect of IL-18 on the pulmonary and hepatic metastases of LM8 cells
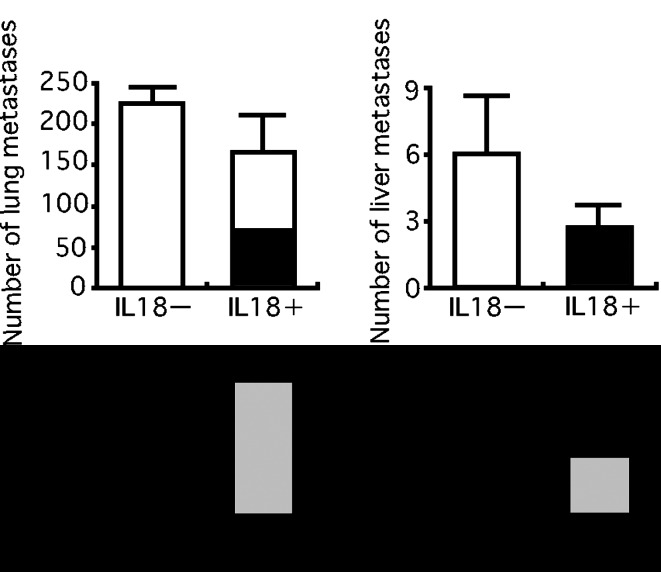
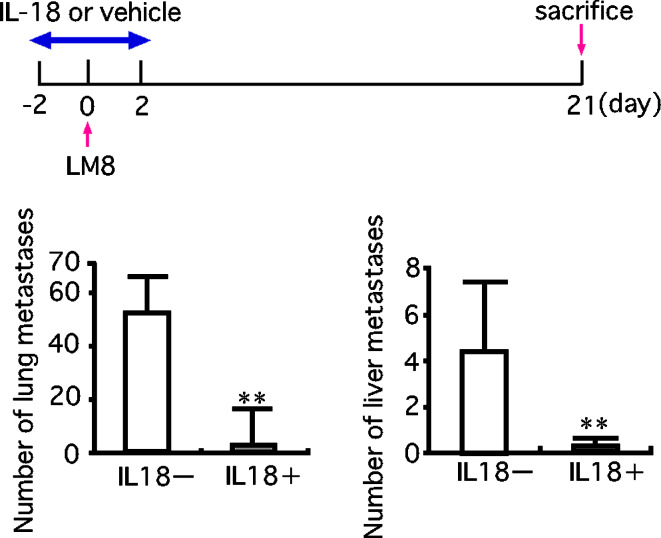
We examined the possibility that IL-18 may affect metastasis of LM8 cells in nude mice treated with anti-asialo GM1 serum. An injection of LM8 cells into a tail vein of nude mice treated with anti-asialo GM1 serum produced pulmonary and hepatic metastases, with a much larger number of foci in the lung than in the liver. In agreement of the result shown in Fig. 3, daily injections of IL-18 (2 μg/mouse/day) starting the fifth day from the injection of LM8 cells, i.e., after the lodging of LM8 cells in the lung and liver, produced no significant effect on the number of pulmonary and hepatic metastatic foci (Fig. 4). However, five daily injections of IL-18 starting 2 days before the injection of LM8 cells and ending 2 days after resulted in marked decreases in metastatic foci, suggesting that IL-18 inhibited the lodging of LM8 cells in the lung and liver (Fig. 5).
Fig. 4.
Effect of IL-18 on the growth of LM8 cells metastasized to the lung and liver. LM8 cells (2×105 cells) were injected into a tail vein of nude mice. Anti-asialo GM1 serum and IL-18 or vehicles were injected as described in the legend of Fig. 3. On the 19th day from the injection of LM8 cells, metastatic foci on the lung and liver were counted. The upper figure shows the experimental design and the lower one the results. Each bar represents a mean + SE of ten mice
Fig. 5.
Effect of IL-18 on pulmonary and hepatic metastases of LM8 cells. LM8 cells (2×105 cells) were injected into a tail vein of nude mice. These mice were daily injected with IL-18 (2 μg/mouse) or vehicle from 2 days before the cell injection to 2 days after (total 5 days). Anti-asialo GM1 serum (20 μl/mouse) was injected every 5 days starting 5 days before the injection of LM8 cells. On the 21st day from the injection of LM8 cells, metastatic foci on the lung and liver were counted. The upper figure shows the experimental design and the lower one the results. Each bar represents a mean + SE of ten mice. **P<0.01, significant difference from the values of mice injected with vehicle only
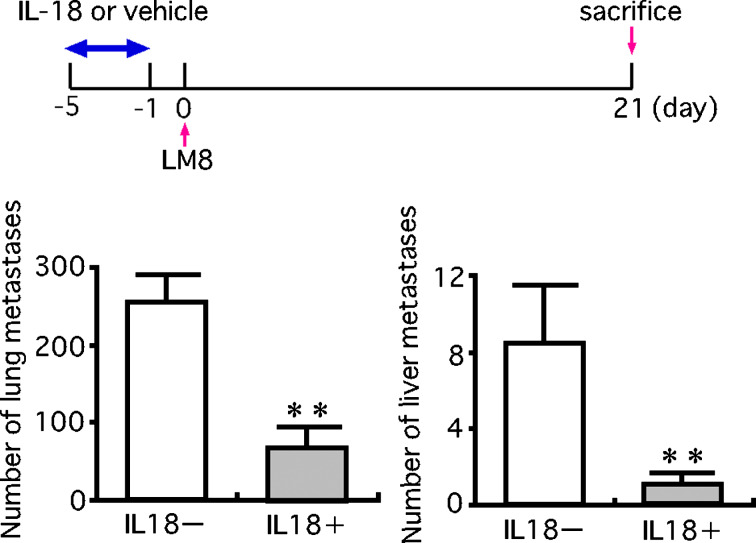
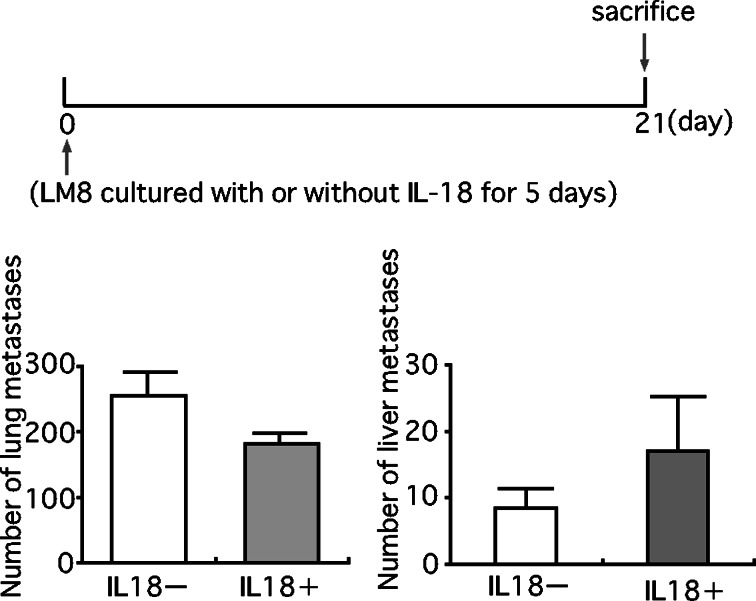
Pretreatment of mice with IL-18 for 5 days before the injection of LM8 cells also markedly decreased the number of pulmonary and hepatic metastatic foci (Fig. 6). In contrast, culture of LM8 cells with IL-18 (100 ng/ml) for 5 days prior to the injection into mice resulted in no significant change in the number of pulmonary and hepatic metastatic foci (Fig. 7). These results suggested that the inhibition of metastasis of LM8 cells by IL-18 was not due to direct action on LM8 cells but to indirect action on host cells.
Fig. 6.
Effect of pretreatment of mice with IL-18 on the pulmonary and hepatic metastases of LM8 cells. LM8 cells (2×105 cells) were injected into a tail vein of nude mice. These mice were daily injected with IL-18 (2 μg/mouse) or vehicle from 5 to 1 day before the injection of LM8 cells (total 5 days). Anti-asialo GM1 serum (20 μl/mouse) was injected every 5 days starting 5 days before the injection of LM8 cells. On the 21st day from the injection of LM8 cells, metastatic foci on the lung and liver were counted. The upper figure shows the experimental design and the lower one the results. Each bar represents a mean + SE of ten mice. **P<0.01, significant difference from the values of mice injected with vehicle only
Fig. 7.
Effect of treatment of LM8 cells with IL-18 in vitro on their pulmonary and hepatic metastasis. LM8 cells (2×105 cells) which had been cultured in medium with or without IL-18 (100 ng/ml) for 5 days, were injected into a tail vein of nude mice. Anti-asialo GM1 serum (20 μl/mouse) was injected every 5 days starting 5 days before the injection of LM8 cells. On the 21st day from the injection of LM8 cells, metastatic foci on the lung and liver were counted. The upper figure shows the experimental design and the lower one the result. Each bar represents a mean + SE of ten mice
It has been reported that treatment of normal mice with IL-18 elevates serum levels of IFN-γ [23] and that IFN-γ inhibits metastasis of osteosarcoma cells [5]. In this study, we found no increase in serum IFN-γ levels in anti-asialo GM1 serum-treated nude mice which were administered with IL-18 for 5 days.
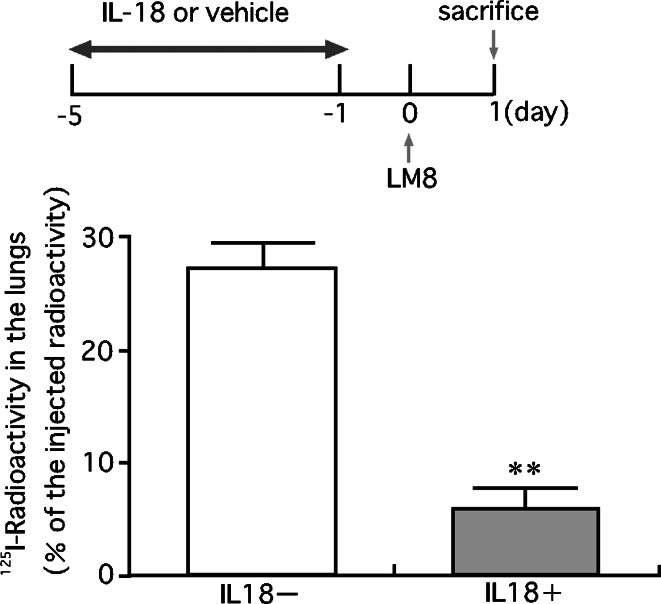
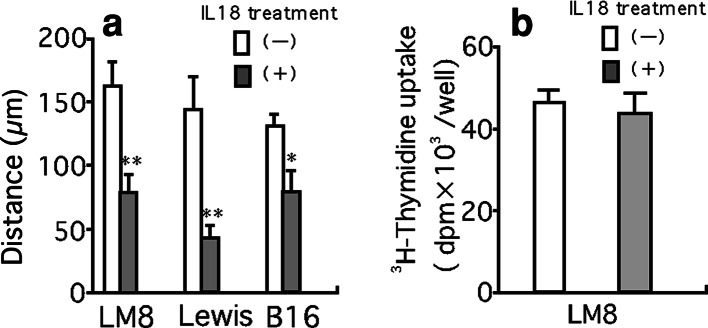
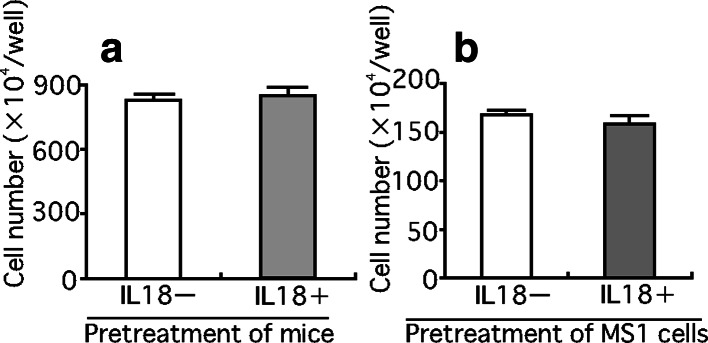
To explore mechanisms of inhibition of LM8 metastasis by pretreatment with IL-18, we examined the effect of IL-18 on the retention of LM8 cells in the lung (Fig. 8). Pretreatment of mice with IL-18 for 5 days resulted in a marked decrease in the number of LM8 cells retained in the lung 24 h after the injection of LM8 cells (Fig. 8), with most LM8 cells injected located outside pulmonary capillaries (Fig. 9). These results suggested that pretreatment of mice with IL-18 prevented the process by which LM8 cells attach to endothelial cells and/or migrate out from the capillary. We then examined whether IL-18 increases vascular dilation by increasing production of nitric oxide. Nude mice were injected with anti-asialo GM1 serum (20 μl) and IL-18 (2 μg/mouse) or the vehicle, followed by four daily injections of IL-18 or the vehicle. Sera were obtained 24 h after the last injection and assayed for nitrite plus nitrate. No significant differences were found between mice treated with IL-18 and the vehicle only [32.3±1.0 vs 29.9±1.1 pg/ml (means ± SE, n=5)]. Next, we examined the effect of serum of mice treated with IL-18 on motility of LM8 cells using the wound assay. Serum from IL-18-treated mice suppressed the motility of LM8 cells (Fig. 10a) while no effect was observed with serum from control mice with or without addition of IL-18 (100 ng/ml) (data not shown). Serum from mice treated with IL-18 also suppressed the motility of highly metastatic mouse B16 melanoma cells and Lewis lung carcinoma cells (Fig. 10a). On the other hand, serum from mice treated with IL-18 showed no significant effect on the proliferation of LM8 cells (Fig. 10b) and their attachment to mouse MS1 endothelial cells (Fig. 11a). Treatment of mouse MS1 endothelial cells with IL-18 (100 ng/ml) for 24 h did not affect attachment of LM8 cells to MS1 cells (Fig. 11b), although MS1 cells expressed both IL-18 receptor α and β (data not shown).
Fig. 8.
Effect of pretreatment of mice with IL-18 on the retention of LM8 cells in the lung. LM8 cells (2×105 cells) labeled with 125I by culturing them with [125I]iodo-2′-deoxyuridine, were injected into a tail vein of nude mice. Anti-asialo GM1 serum and IL-18 or vehicles were injected as described in the legend of Fig. 6. All mice were killed 24 h after the injection of LM8 cells, and the radioactivity retained in the whole lung was counted. The upper figure shows the experimental design and the lower one the results. Each bar represents a mean + SE of ten mice. **P<0.01, significant difference from the values of mice injected with vehicle only
Fig. 9.
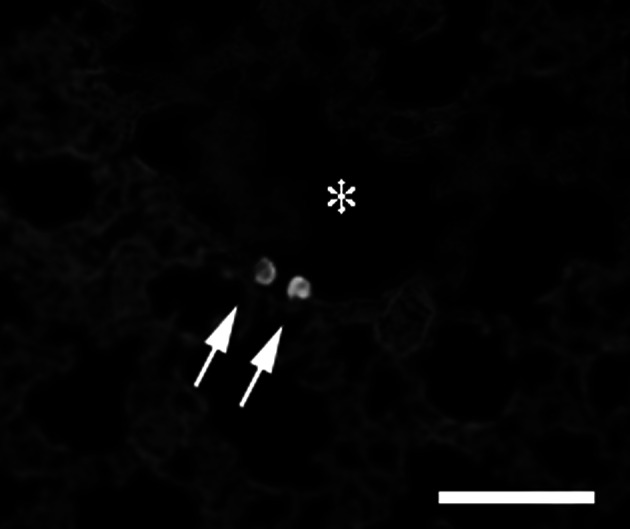
Distribution of LM8 cells in the lung. LM8 cells (2×105 cells) labeled with a fluorescent dye were injected into a tail vein of nude mice and, 24 h later, the lungs were removed, cut into sections and examined under a fluorescence microscope . Fluorescent LM8 cells were located in an alveolar cavity, not in the lumen of capillaries. The asterisk shows an alveolar cavity and the arrows show LM8 cells in it. Bar: 50 μm
Fig. 10.
Effect of serum obtained from mice treated with IL-18 on the motility of LM8, B16 melanoma and Lewis lung carcinoma cells, and the proliferation of LM8 cells. Nude mice were treated with anti-asialo GM1 serum and IL-18 or vehicle as described in Fig. 6, and sera of these mice were obtained 24 h after the last injection of IL-18 or vehicle. The effect of serum (50% in medium) of mice treated with IL-18 on the motility of LM8, Lewis lung carcinoma and B16 melanoma cells (a), and on the proliferation of LM8 cells (b) were estimated by the wound assay and 3H-thymidine uptake, respectively. Each bar represents a mean + SE of ten wells. *P<0.05, **P<0.01, significant difference from the values of mice treated with vehicle only
Fig. 11.
Effect of serum obtained from mice treated with IL-18 on the attachment of LM8 cells to MS1 endothelial cells and effect of treatment of mouse MS1 endothelial cells with IL-18 on the attachment of LM8 cells. a Effect of serum on LM8–MS1 interaction. LM8 cells were incubated with MS1 endothelial cells in the presence of 50% serum from mice which were treated with IL-18 or vehicle as described in Fig. 10. b Effect of treatment of MS1 endothelial cells with IL-18 on the attachment to LM8 cells. MS1 cells were cultured in the presence or absence of IL-18 (100 ng/ml) for 24 h, and incubated with LM8 cells for 3 h in the presence of 50% serum from mice treated with vehicle. Each bar represents a mean + SE of ten wells
Discussion
In this study, we showed that IL-18 inhibited pulmonary and hepatic metastases of LM8 cells in nude mice. This inhibitory effect of IL-18 was not due to the inhibition of cell growth, but to the inhibition of the lodging of LM8 cells in the lung and liver. Kuhara et al. [16] have reported that increases in IL-18 production in the intestinal epithelium induced by oral administration of bovine lactoferrin cause suppression of metastasis of colon 26 carcinoma cells in Balb/c mice and that this inhibitory effect of IL-18 is ascribed to the enhancement of the cytotoxic activity of NK or T lymphocytes. In our study, IL-18 inhibited metastasis of LM8 cells in nude mice treated with anti-asialo GM1 serum to inhibit the cytotoxic activity of NK cells, indicating that the mechanism of the inhibition of metastasis of LM8 cells by IL-18 is different from that of colon 26 carcinoma cells.
Regarding the mechanism by which IL-18 inhibited metastasis, our results showed that serum from mice pretreated with IL-18 inhibited motility of LM8 cells in vitro, suggesting that IL-18 induced a factor(s) which suppressed migration of LM8 cells from the capillary. This serum factor inhibited motility of not only LM8 cells but also B16 melanoma cells and Lewis lung carcinoma cells, indicating that it is not specific to osteosarcoma.
Glasgow and Kern [5] have reported that seven daily injections of IFN-γ from the day of an intravenous injection of mouse osteosarcoma cells reduce pulmonary metastasis. IL-18 has been reported to elevate serum IFN-γ levels in normal mice [23]. However, in this study we did not observe increases in serum IFN-γ levels in nude mice treated with anti-asialo GM1 serum. Therefore, it is unlikely that the inhibitory effect of IL-18 on metastasis of LM8 cells is mediated by IFN-γ.
Interleukin-18 has been reported to increase nitric oxide production to induce vascular dilation in normal mice in vivo, suggesting that IL-18 decreases retention of LM8 cells inside capillaries [9]. However, we did not observe increases in nitrite plus nitrate levels in anti-asialo GM1 serum-treated nude mice which were administered with IL-18, ruling out the involvement of nitric oxide in the inhibition of metastasis of LM8 cells.
LM8 cells highly express vascular endothelial growth factor mRNA which might facilitate neovascularization at the site of metastasis, resulting in rapid growth of LM8 cells [1]. Consistent with this, Mori et al. [21] have reported that TNP-470 having anti-angiogenic activity as well as cytostatic activity toward LM8 cells, markedly inhibits pulmonary metastasis of LM8 cells when administered daily from the day of an intravenous injection of LM8 cells. IL-18 also has been reported to have an anti-angiogenic activity against murine T241 fibrosarcoma cells [2]. However, in this study, we observed that daily injections of IL-18 after a subcutaneous inoculation and intravenous injection of LM8 cells did not affect the growth of LM8 cells at the site of the inoculation (Fig. 3) and pulmonary and hepatic metastasis of LM8 cells (Fig. 4). These results suggest that IL-18 exerts no significant effect on angiogenesis of LM8 cells at the site of metastasis.
Morel et al. [20] have shown that IL-18 enhances expression of vascular cell adhesion molecule-1 (VCAM-1), intercellular cell adhesion molecle-1 (ICAM-1) and endothelial cell (E)-serectin in human dermal microvascular endothelial cells, promoting their adhesion to HL-60 monocyte-like cells. In addition, Carrascal et al. [3] have reported that an increase in endogenous IL-18 promotes hepatic metastasis of mouse B16 melanoma cells by up-regulating VCAM-1 expression in hepatic sinusoidal endothelium (HSE) cells to enhance the adhesion of B16 melanoma cells to HSE. We found that MS1 endothelial cells expressed both VCAM-1 and ICAM-1 but the adhesion of LM8 cells to MS1 endothelial cells were not affected by IL-18. The reason for the discrepancy is not clear at present.
Using highly metastatic cell line (PLA801D) and poorly metastatic cell line (PLA801C), which were derived from the same parent human lung carcinoma cell line (PLA801), Jiang et al. [14] suggested that IL-18 secreted in medium stimulates motility of lung carcinoma cells and their invasion through their receptor. In contrast, we found that IL-18 did not affect motility of LM8 cells which may be due to the lack of expression of the IL-18 receptor.
Interleukin-18 exerts anti-tumor actions on a variety of tumor cells [2, 12, 17, 24, 25, 30] and inhibits metastasis as shown by our studies and those of Kuhara et al. [16]. However, there are some reports [8, 15] showing that serum IL-18 levels are elevated in patients with various tumors and high serum levels of IL-18 are related to metastasis and poor prognosis. An increase in serum IL-18 level associated with tumor progression seems to reflect enhanced host immune surveillance against tumors but not promotion of tumor development by IL-18.
Some reports [6, 19] have shown that osteosarcomas produce factors which promote differentiation and activation of osteoclasts to induce osteolysis, leading to the release of growth factors in the bone matrix and complications such as bone fracture, bone pain and hypercalcemia [7]. Therefore, in treatment of osteosarcomas, it is important to inhibit osteoclastic bone resorption as well as tumor growth and metastasis. We have previously shown that IL-18 inhibits osteoclastic bone resorption in vivo [11, 22] and growth of mouse osteosarcoma cells in vivo by augmenting the killing activity of T lymphocytes [25]. These results, together with the present results showing inhibition of metastasis of osteosarcoma cells, suggest that it is worthwhile investigating further the usefulness of IL-18 as a therapy agent for osteosarcoma.
Acknowledgements
We thank Ms. Hiromi Takeda, Ms. Michiko Kakihana, and Ms. Ayako Kuhara for their technical support. This work was in part supported by a Hitec Research Center grant, the fund of Cancer Research from Hyogo Prefecture Health Promotion Association, a grant for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology, and Grant-in-Aid for Graduate Students, Hyogo College of Medicine.
Abbreviations
- IL-18
Interleukin-18
- NK cells
Natural killer cells
- IFN-γ
Interferon-γ
- FCS
fetal calf serum
- EMEM
Eagle’s minimum essential medium
- DMEM
Dulbecco’s modified Eagle’s medium
- RT-PCR
Reverse-transcriptase polymerase chain reaction
- ELISA
Enzyme-linked immunosorbent assay
- HSE
Hepatic sinusoidal endothelium
- VCAM
Vascular cell adhesion molecule
- ICAM
Intercellular cell adhesion molecule
- E-secretin
Endothelial cell serectin
References
- 1.Asai T, Ueda T, Itoh K, Yoshioka K, Aoki Y, Mori S, Yoshikawa H. Establishment and characterization of a murine osteosarcoma cell line (LM8) with high metastatic potential to the lung. Int J Cancer. 1998;76:418–422. doi: 10.1002/(SICI)1097-0215(19980504)76:3<418::AID-IJC21>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Cao R, Farnebo J, Kurimoto M, Cao Y. Interleukin-18 acts as angiogenesis and tumor suppressor. FASEB J. 1999;13:2195–2202. doi: 10.1096/fasebj.13.15.2195. [DOI] [PubMed] [Google Scholar]
- 3.Carrascal MT, Mendoza L, Valcarcel M, Salado C, Egilegor E, Telleria N, Vidal-Vanaclocha F, Dinarello CA. Interleukin-18 binding protein reduces B16 melanoma hepatic metastasis by neutralizing adhesiveness and growth factors of sinusoidal endothelium. Cancer Res. 2003;63:491–497. [PubMed] [Google Scholar]
- 4.Giancotti FG, Ruoslahti E. Elevated levels of the alpha 5 beta 1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell. 1990;60:849–859. doi: 10.1016/0092-8674(90)90098-Y. [DOI] [PubMed] [Google Scholar]
- 5.Glasgow LA, Kern ER. Effect of interferon administration on pulmonary osteogenic sarcomas in an experimental murine model. J Natl Cancer Inst. 1981;67:207–212. [PubMed] [Google Scholar]
- 6.Good CR, O’Keefe RJ, Puzas JE, Schwarz EM, Rosier RN. Immunohistochemical study of receptor activator of nuclear factor kappa-B ligand (RANK-L) in human osteolytic bone tumors. J Surg Oncol. 2002;79:174–179. doi: 10.1002/jso.10067. [DOI] [PubMed] [Google Scholar]
- 7.Guise TA, Mundy GR. Cancer and bone. Endocr Rev. 1998;19:18–54. doi: 10.1210/er.19.1.18. [DOI] [PubMed] [Google Scholar]
- 8.Gunel N, Coskun U, Sancak B, Hasdemir O, Sare M, Bayram O, Celenkoglu G, Ozkan S. Prognostic value of serum IL-18 and nitric oxide activity in breast cancer patients at operable stage. Am J Clin Oncol. 2003;26:416–421. doi: 10.1097/00000421-200308000-00023. [DOI] [PubMed] [Google Scholar]
- 9.Hosohara K, Ueda H, Kashiwamura S, Yano T, Ogura T, Marukawa S, Okamura H. Interleukin-18 induces acute biphasic reduction in the levels of circulating leukocytes in mice. Clin Diagn Lab Immunol. 2002;9:777–783. doi: 10.1128/CDLI.9.4.777-783.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huvos A. Bone tumors: diagnostic treatment, and prognosis. Philadelphia, PA: W.B. Saunders; 1991. [Google Scholar]
- 11.Iwasaki T, Yamashita K, Tsujimura T, Kashiwamura S, Tsutsui H, Kaisho T, Sugihara A, Yamada N, Mukai M, Yoneda T, Okamura H, Akedo H, Terada N. Interleukin-18 inhibits osteolytic bone metastasis by human lung cancer cells possibly through suppression of osteoclastic bone-resorption in nude mice. J Immunother. 2002;25:S52–S60. doi: 10.1097/00002371-200203001-00008. [DOI] [PubMed] [Google Scholar]
- 12.Jakub G. Interleukin 18–interferon-γ inducing factor—a novel player in tumor immunotherapy. Cytokine. 2000;12:332–338. doi: 10.1006/cyto.1999.0563. [DOI] [PubMed] [Google Scholar]
- 13.Jeffree GM, Price CH, Sissons HA. The metastatic patterns of osteosarcoma. Cancer. 1975;32:87–107. doi: 10.1038/bjc.1975.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang D, Ying W, Lu Y, Wan J, Zhai Y, Liu W, Zhu Y, Qiu Z, Qian X, He F. Identification of metastasis-associated proteins by proteomic analysis and functional exploration of interleukin-18 in metastasis. Proteomics. 2003;3:724–737. doi: 10.1002/pmic.200300411. [DOI] [PubMed] [Google Scholar]
- 15.Kawabata T, Ichikura T, Majima T, Seki S, Chochi K, Takayama E, Hiraide H, Mochizuki H. Preoperative serum interleukin-18 level as a postoperative prognostic marker in patients with gastric carcinoma. Cancer. 2001;92:2050–2055. doi: 10.1002/1097-0142(20011015)92:8<2050::AID-CNCR1544>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Kuhara T, Iigo M, Itoh T, Ushida Y, Sekine K, Terada N, Okamura H, Tsuda H. Orally administered lactoferrin exerts an antimetastatic effect and enhances production of IL-18 in the intestinal epithelium. Nutr Cancer. 2000;38:192–199. doi: 10.1207/S15327914NC382_8. [DOI] [PubMed] [Google Scholar]
- 17.Lebel-Binay S, Berger A, Zinzindohoue F, Cugnenc P-H, Thiounn N, Fridman WH, Pages F. Interleukin-18: biological properties and clinical implications. Eur Cytokine Netw. 2000;11:15–26. [PubMed] [Google Scholar]
- 18.Meyers PA, Heller G, Healey J, Huvos A, Lane J, Marcove R, Applewhite A, Vlamis V, Rosen G. Chemotherapy for non metastatic osteogenic sarcoma: the Memorial Sloan–Kettering experience. J Clin Oncol. 1992;10:5–15. doi: 10.1200/JCO.1992.10.1.5. [DOI] [PubMed] [Google Scholar]
- 19.Miyamoto N, Higuchi Y, Mori K, Ito M, Tsurudome M, Nishio M, Yamada H, Sudo A, Kato K, Uchida A, Ito Y. Human osteosarcoma-derived cell lines produce soluble factor(s) that induces differentiation of blood monocytes to osteoclast-like cells. Int Immunopharmacol. 2002;2:25–38. doi: 10.1016/S1567-5769(01)00134-5. [DOI] [PubMed] [Google Scholar]
- 20.Morel JCM, Park CC, Woods JM, Koch AE. A novel role for interleukin-18 in adhesion molecule induction through NF kappa B and phosphatidylinositol (PI) 3-kinase-dependent signal transduction pathways. J Biol Chem. 2001;276:37069–37075. doi: 10.1074/jbc.M103574200. [DOI] [PubMed] [Google Scholar]
- 21.Mori S, Ueda T, Kuratsu S, Hosono N, Izawa K, Uchida A. Suppression of pulmonary metastasis by angiogenesis inhibitor TNP-470 in murine osteosarcoma. Int J Cancer. 1995;61:148–152. doi: 10.1002/ijc.2910610125. [DOI] [PubMed] [Google Scholar]
- 22.Nakata A, Tsujimura T, Sugihara A, Okamura H, Iwasaki T, Shinkai K, Iwata N, Kakishita E, Akedo H, Terada N. Inhibition by interleukin 18 of osteolytic bone metastasis by human breast cancer cells. Anticancer Res. 1999;19:4131–4138. [PubMed] [Google Scholar]
- 23.Ogura T, Ueda H, Hosohara K, Tsuji R, Nagata Y, Kashiwamura S, Okamura H. Interleukin-18 stimulates hematopoietic cytokine and growth factor formation and augments circulating granulocytes in mice. Blood. 2001;98:2101–2107. doi: 10.1182/blood.V98.7.2101. [DOI] [PubMed] [Google Scholar]
- 24.Ohtsuki T, Micallef MJ, Kohno K, Tanimoto T, Ikeda M, Kurimoto M. Interleukin 18 enhances Fas ligand expression and induces apoptosis in Fas-expressing human myelomonocytic KG-1 cells. Anticancer Res. 1997;17:3253–3258. [PubMed] [Google Scholar]
- 25.Okamoto T, Yamada N, Tsujimura T, Sugihara A, Nishizawa Y, Ueda H, Kashiwamura S, Tsutsui H, Futani H, Maruo S, Okamura H, Terada N. Inhibition by interleukin-18 of the growth of Dunn osteosarcoma cells. J Interferon Cytokine Res. 2004;24:161–167. doi: 10.1089/107999004322917007. [DOI] [PubMed] [Google Scholar]
- 26.Okamura H, Tsutsui H, Kashiwamura S, Yoshimoto T, Nakanishi K. A novel cytokine that augment both innate and aquired immunity. Adv Immunol. 1998;70:281–312. doi: 10.1016/S0065-2776(08)60389-2. [DOI] [PubMed] [Google Scholar]
- 27.Pratt CB, Champion JE, Fleming ID, Rao B, Kumar APM, Evans WE, Green AA, George S. Adjuvant chemotherapy for osteosarcoma of the extremity; Long-term results of two consecutive prospective protocol studies. Cancer. 1990;65:439–445. doi: 10.1002/1097-0142(19900201)65:3<439::AID-CNCR2820650311>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 28.Saeter G, Hoie J, Stenwig AE, Johansson AK, Hannisdal E, Solheim OP. Systemic relapse of patients with osteogenic sarcoma; Prognostic factors for long term survival. Cancer. 1995;75:1084–1093. doi: 10.1002/1097-0142(19950301)75:5<1084::AID-CNCR2820750506>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto T, Terada N, Nishizawa Y, Tanaka H, Akedo H, Seiyama A, Shiga T, Kosaka H. Effects of N G-nitro-l-arginine and/or l-arginine on experimental pulmonary metastasis in mice. Cancer Lett. 1994;87:115–120. doi: 10.1016/0304-3835(94)90417-0. [DOI] [PubMed] [Google Scholar]
- 30.Yamashita K, Iwasaki T, Tsujimura T, Sugihara A, Yamada N, Ueda H, Okamura H, Futani H, Maruo S, Terada N. Interleukin-18 inhibits lodging and subsequent growth of human multiple myeloma cells in the bone marrow. Oncol Rep. 2002;9:1237–1244. [PubMed] [Google Scholar]