Abstract
The presence of circulating antineuronal antibodies has been associated with paraneoplastic neurological syndromes (PNS). Ri antibodies are often associated with lung or breast cancer, but the prevalence of such antibodies in large cancer materials is largely unknown. We used a highly sensitive immunoprecipitation assay to study the level of Ri antibodies in blood samples from 200 patients with small cell lung cancer (SCLC), 253 patients with breast cancer and 557 patients with ovarian cancer. Two hundred blood donors and six Ri positive PNS patients served as controls. The recombinant antigen used in the immunoprecipitation assay was radiolabeled by a coupled in vitro transcription and translation (ITT) technique, enabling low levels of antibodies to be detected. None of the blood donors contained Ri antibodies, whereas all of the sera from the PNS patients were positive. Ri antibodies were present in 4.5% of the patients with SCLC, 0.8% of the patients with breast cancer and in 0.2% of the patients with ovarian cancer. Retesting of the Ri positive samples with immunofluorescense and immune blot showed that the immunoprecipitation technique was more sensitive than the other immune assays. Ri antibodies were not associated with PNS in the patients with breast or ovarian cancer. Neurological data were not available for the SCLC patients, but in these, Ri antibodies were not associated with survival.
Keywords: Immunoprecipitation, Onconeural antigens, Ri antibodies, Paraneoplastic neurological syndromes
Introduction
Paraneoplastic neurological syndromes (PNS) are rare and occur as a remote effect of cancer. The various syndromes are usually associated with different antibodies, resulting from an autoimmune response directed at neuron-specific antigens ectopically expressed by the tumour cells [1]. The antibodies cross-react with antigens present in the nervous system, and may thereby cause a neurological syndrome. Some onconeural antibodies are known to be pathogenic, such as antibodies to voltage-gated calcium channels of the neuromuscular junction [2], but most of the onconeural antibodies, such as anti-Hu, anti-Yo and anti-Ri, are so far only considered as specific markers of an underlying cancer.
Ri antibodies are directed against the RNA binding protein Nova-1 (neuro-oncological ventral antigen-1). Nova-1 is a neuron-specific protein involved in splicing of pre-messenger RNA. The protein is considered as a target antigen in paraneoplastic opsoclonus-myoclonus, a disease characterized by loss of inhibitory control of motor neurons in the spinal cord and brainstem [3]. Nova-1 is the first neuron-specific alternative splicing factor described in mammals, but the relationship between neurological disease and regulation of alternative splicing remains to be understood [4].
Ri antibodies have usually been associated with carcinoma of lung and breast [5], but other cancers reported with anti-Ri include ovary [6], fallopian tube [7], bladder [3] and stomach [8]. The prevalence of Ri antibodies in these cancers is largely unknown.
Onconeural antibodies in serum, plasma or cerebrospinal fluid are usually assayed by the combination of immunohistochemistry against neuronal tissue and immune blotting against neuronal tissue or cloned antigens. Recently, we developed an in vitro transcription and translation (ITT) immunoprecipitation technique for the detection of Hu antibodies, and the technique was found to be more sensitive than the conventional immune assays [9]. The aim of the present study was to elaborate a similar immunoprecipitation technique to search for Ri antibodies in large populations of patients with breast, ovarian or small cell lung cancer (SCLC).
Materials and methods
Patients
We analyzed sera from 200 patients with SCLC, 253 patients with breast cancer and 99 patients with ovarian cancer for the presence of Ri antibodies. All patients had either a histologic or cytologic cancer diagnosis. We also tested EDTA-blood from another 458 patients with ovarian cancer. For comparison, sera from 100 blood donors and EDTA-blood from another 100 blood donors were analyzed. All samples were analyzed by ITT immunoprecipitation, and positive samples were retested by immunofluorescense, dot blot and Western blot against recombinant Nova-1. Neurological data of the Ri-positive breast and ovarian cancer patients were studied in retrospect, and the clinical observation time was >5 years. Neurological information was not obtainable for the 200 patients with SCLC. These patients have previously been tested for anti-Hu and anti-VGCC, and survival data were available [10]. Survival was determined from start of treatment (chemotherapy) to death or last visit. Follow-up time for long-term survivors was at least 9 years. We also included 6 patients with PNS with Ri antibodies previously determined by immunofluorescence and dot blot.
ITT immunoprecipitation
We developed an immunoprecipitation assay based on ITT for the detection of Ri antibodies in patient sera or EDTA-blood. mRNA was extracted from rat cerebellum (Qiagen, Hilden, Germany) and reverse transcribed to cDNA (Amersham Pharmacia Biotech, Buckinghamshire, England). The Nova-1 coding sequence was PCR-amplified from the cDNA and directionally cloned into an expression vector (pIVEX2.3, Roche Diagnostics, Mannheim, Germany). The Nova-1 insert was verified by DNA sequencing using a capillary electrophoresis method (Applied Biosystems, Foster City, CA) (Accession no AY262017). The plasmid was electroporated into Eschericia coli XL1-Blue MRF’ using a BioRad gene pulser (BioRad, Hercules, CA) at 12.5 kV/cm and 25 μF. Bacteria containing the plasmid were grown in Luria Bertani medium containing ampicillin, and plasmid DNA was purified using the Qiagen plasmid midi kit (Qiagen). ITT was performed using the TNT coupled reticulocyte lysate system (Promega, Madison, WI), and the Nova-1 protein 35S labeled by including 35S-met in the reaction (35S methionine, Amersham Biosciences, Uppsala, Sweden). MultiSceen 96-well filtration plates (MABV N12; Millipore, Bedford, MA) were used for immunoprecipitation experiments. Each well was pretreated as previously described [9]. 35S labeled Nova-1 protein and patient sera or EDTA-blood diluted 1:10 in incubation buffer (20 mmol/l Tris–HCl, 150 mmol/l NaCl, 0.1% BSA 0.15% Tween-20 and 0.001% azide, pH 8.0) were incubated at 4°C overnight. The following day, 50 μl of a 50% (vol/vol) slurry of resuspended Protein-A Sepharose (Pharmacia, Stockholm, Sweden) in incubation buffer was added to each well of the MultiScreen plates followed by the addition of the immune complexes. The plates were then incubated on a shaking platform for 45 min at 6°C, washed, and left to dry overnight. Finally, 20 μl of scintillation fluid (Microscint; Packard Sciences, Groningen, the Netherlands) was added to each well, and the amount of radiolabeled immunoprecipitate was measured in a beta counter (Topcount NXT microplate scintillation and luminescence counter; Packard).
Each patient serum or EDTA-blood was run in triplicate and the mean value of these three results was used. The results were expressed as a Ri index: (cpmsample−cpmnegative control)/(cpmpositive control−cpmnegative control)×1,000. Serum from a Ri positive PNS patient and a pool of 100 blood donors were used as positive and negative controls, respectively. The overall signal-to-noise ratio, calculated as mean value of cpmpositive control/cpmnegative control, was 20. The cut off value for the ITT index was calculated as the mean value of the blood donors +3SD, and determined to be 77 for the serum samples and 250 for the EDTA-blood samples. Positive results were reproduced by three consecutive ITT immunoprecipitation experiments.
Immunofluorescence and dot blot
Formalin fixed sections of rat cerebellar and brainstem tissue were incubated in a moist chamber at 4°C overnight with sera or EDTA-blood from the patients with Ri antibodies (determined by ITT immunoprecipitation) diluted 1:100 and 1:500 in phosphate buffered saline (PBS). The slides were subsequently washed in PBS and incubated with fluorescein isothiocyanate labeled rabbit anti-human immunogloblulin antibodies (F0200; DAKO, Glostrup, Denmark) diluted 1:50 in PBS for 1 h at room temperature. After washing, the slides were mounted and examined by immunofluorescence microscopy.
Dot blot was performed with a commercial kit (Anti-Onconeural Antigens, Milenia Biotec GmbH, Bad Neuheim, Germany) containing the recombinant proteins HuD, Yo (cdr2), Ri (Nova-1) and amphiphysin. Another commercial dot blot kit (Ravo Diagnostika GmbH, Freiburg, Germany) was used to detect additional CRMP5 or Ma2 antibodies. The kits were used according to the manufacturers’ instructions, and only clearly defined bands were considered positive.
Recombinant protein and Western blot analysis
Recombinant Nova-1 protein was obtained by subcloning the gene into a pET100 vector using TOPO technology (Invitrogen, Carlsbad, CA). The plasmid was transformed into OneShot Rosetta (DE3)pLysS (Novagen, Madison, WI), and protein expression was induced with 1 μM IPTG when cell density reached OD600=0.5. Bacteria were harvested after 4 h, and the cell pellet was dissolved in B-PER Bacterial Protein Extraction Reagent according to manufacturer’s instructions (Pierce, Rockford, IL). The His-tagged protein was purified using a Ni Sepharose affinity column (HiTrap Chelating HP 1 ml, Amersham Pharmacia Biotech). Fractions containing Ri protein were combined and dialysed against PBS using a Slide-A-Lyzer 10 K dialysis cassette (Pierce). Final protein concentration was measured to 410 μg/ml.
Immunoblots of recombinant protein (2 μg) were tested with patient sera or EDTA-blood (diluted 1:100 to 1:500 in PBS containing 0.5% dry milk) using a secondary HRP-conjugated rabbit anti-human IgG antibody diluted 1:500 in PBS (P0214; DAKO) and developed with 4-chloro-1-naphtol (Sigma, St. Louis, MO) and H2O2 in PBS.
Statistical analysis
Kaplan–Meier survival curves were used to describe the survival after SCLC diagnosis and the log-rank test was used to compare survival of Ri positive and Ri negative patients. The analysis was performed with SPSS version 11 (SPSS Inc, Chicago, IL) and S-plus (version 6.0 professional release 1, Insightful Corp, Seattle, WA).
Results
None of the 200 blood donors were positive by ITT immunoprecipitation. The six Ri positive PNS patients used as positive controls had Ri indexes varying from 362 to 1,000 as shown in Fig. 1, and were all positive by the three other immune assays (dot blot, Western blot with recombinant protein and immunofluorescence).
Fig. 1.
Positive Ri indexes for patients with small cell lung cancer (SCLC) (9/200), ovarian cancer (1/557), breast cancer (2/253) and control group with paraneoplastic neurological syndromes (PNS) (6/6)
Ri antibodies were detected in samples from 9 of 200 (4.5%) patients with SCLC, 2 of 253 (0.8%) patients with breast cancer and 1 of 557 (0.2%) patients with ovarian cancer by ITT immunoprecipitation (Fig. 1). Ri antibodies were detected in all of the samples except for one patient, by at least one of the three other immune assays (Table 1). The Ri index was low (120) in the one sample in which the presence of Ri antibodies could not be confirmed by any of the other immune assays. However, in another sample with similar Ri index (119), Ri antibodies could be detected also by the other immune assays. The immunofluorescence studies were negative in four of the samples.
Table 1.
Ri antibodies detected by ITT imunoprecipitation (Ri index), indirect immuno-fluorescence, dot blot and Western blot
| Sample | Ri index | Immuno-fluorescence 1:500/1:100 | Dot blot a, b | Western blot 1:500/1:100 | Other onconeural antibodies |
|---|---|---|---|---|---|
| SCLC | 144 | −/+ | − | +/+ | − |
| SCLC | 316 | +/+ | + | +/+ | Hua, b |
| SCLC | 292 | +/+ | + | −/+ | − |
| SCLC | 207 | −/+ | − | +/+ | − |
| SCLC | 184 | −/− | − | −/+ | − |
| SCLC | 119 | +/+ | − | −/+ | Hua |
| SCLC | 120 | −/− | − | −/− | − |
| SCLC | 400 | −/− | − | +/+ | − |
| SCLC | 150 | +/+ | − | −/+ | Hua, Ma2b |
| Breast ca. | 129 | −/+ | − | −/+ | − |
| Breast ca. | 382 | −/− | + | +/+ | − |
| Ovarian ca. | 728 | −/+ | + | +/+ | − |
The results are indicated as numbers for the Ri index (mean value of three experiments) and as positive or negative for the other assays. Other onconeural antibodies were also present in some of the samples
SCLC small cell lung cancer
a Milenia dot blot kit
b RAVO dot blot kit
In 3 of the 12 Ri positive samples, also other well-characterized onconeural antibodies were detected by dot blot. Three of the Ri positive patients harbored Hu antibodies (25%). In one Ri positive SCLC sample, both anti-Hu and anti-Ma2 were detected (Table 1).
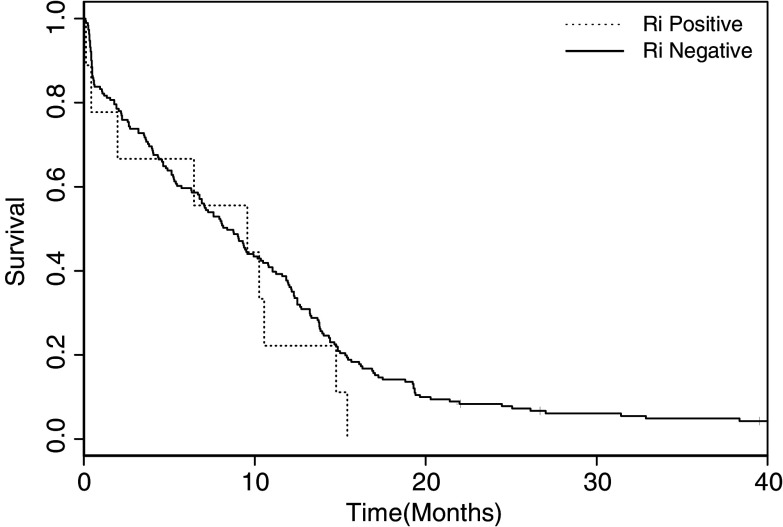
The two patients with breast cancer and the one patient with ovarian cancer and Ri antibodies did not have any signs of neurological syndromes. We did not have any information regarding the neurological status on the SCLC patients positive for anti-Ri. Figure 2 shows Kaplan–Meier survival curves for Ri positive SCLC patients compared with Ri negative patients. There was no association between the presence of Ri antibodies and survival (P=0.35).
Fig. 2.
Kaplan–Meier survival curves for Ri positive SCLC patients compared with Ri negative SCLC patients. There was no correlation between the presence of Ri antibodies and survival (P=0.35)
Discussion
We found that Ri antibodies were present in 4.5% of patients with SCLC, and thereby far more prevalent than in breast (0.8%) or ovarian cancer (0.2%). This may reflect differences in antigen presentation as well as immune response in the various cancer types. Ri antibodies have previously been detected in sera from 7 of 181 (3.9%) ovarian cancer patients using immunofluorescence and Western blot of human cerebral cortex neurons [6]. The reason for the higher prevalence of Ri antibodies in this study may be that the results were not confirmed by immune blot with recombinant Nova-1 protein. Previous studies have also examined the prevalence of Ri antibodies by first selecting for neurological symptoms. In a study of PNS in 92 women with breast or gynecologic cancer, 63 (68%) of the patients harbored onconeural antibodies, and of these 3 (5.4%) had anti-Ri [11]. In another study of 75,000 sera submitted for diagnostic purpose due to neurological symptoms, Ri antibodies were found in 34 patients (0.04%). Cancer was diagnosed in 24 of the 34 (86%) patients and lung and breast cancer were most prevalently associated with anti-Ri [5].
In the present study we used a highly sensitive immunoprecipitation technique to determine the prevalence and level of Ri antibodies. The assay has previously been found to be more sensitive than immunofluoresence and dot blot for the detection of Hu antibodies [9]. The increased sensitivity of immunoprecipitation may partly be due to the use of radiodetection, which enables low levels of antibodies to be detected. The use of mammalian reticulocyte lysate in the ITT reaction makes the synthesis very similar to in vivo protein expression. Combined with the fact that the antibody–antigen interaction takes place in the liquid phase, where the three dimensional structure of the protein is retained, ensures a high specificity as well as sensitivity. Furthermore, the lower dilutions of serum or EDTA-blood used in this assay may also be of importance.
Recombinant Nova-1 was also used as antigen in dot blot and Western blot assays. The dot blot assay was least sensitive, as Ri antibodies were only detected in four of the samples. We used commercial dot blot kits from two different manufacturers. The results were correlative for anti-Ri, but not for anti-Hu. Four samples were negative in the immunofluorescense assay. Furthermore, the specificity of this assay is lower, as the staining pattern of Ri and Hu antibodies is similar in sections of central nervous tissue.
We found that the presence of Ri antibodies were associated with other onconeural antibodies, especially anti-Hu. Recently, it has been shown that Hu antibodies coexist in 23%, and CRMP5 antibodies in 12% of 17 patients with Ri antibodies, whereas Yo antibodies were not present with anti-Ri [12]. In this study the onconeural antibodies were studied in patients with suspected paraneoplastic disorders, and histologically proven cancer was found in 57% of the Ri patients. Our study preselected patients with cancer, and since Hu antibodies are prevalent in SCLC [10], this probably explains the high coexistence of Ri and Hu antibodies. Of interest is that one of the patients with SCLC harbored three different onconeural antibodies which indicates that SCLC may express several different neural antigens.
We found that the two Ri positive patients with breast cancer and the one Ri positive patient with ovarian cancer had no signs of PNS, even with an observation time of >5 years. This is in concordance with a previous study on Ri positive ovarian cancer patients where none of the patients developed PNS during a follow up of 2 years [6]. The results therefore indicate that the presence of Ri antibodies are associated with cancer, but not necessarily with PNS.
Except for the one ovarian cancer patient, the levels of Ri antibodies in the SCLC and breast cancer patients were generally lower than for the six patients with PNS. The high Ri index in the ovarian cancer sample may partly be due to examination of EDTA blood. The possibility that the amount of onconeural antibodies required to cause neurological disease needs to be above a certain ‘threshold’ level might explain why the Ri positive patients in our study did not have PNS. Whether the presence of low-level amounts of Ri antibodies reflects general tumor immunity rather than neurological disease is presently unclear. To further evaluate the possible role of such antibodies, long term serological as well as clinical studies should be performed.
The paraneoplastic antigens cdr2 and HuD are widely expressed in ovarian and breast cancer [13] and SCLC [14], respectively, allowing apoptotic tumor cells to trigger tumor immunity. Although this has not been examined for other paraneoplastic antigens such as Nova-1, it might apply as a general mechanism regarding ectopically expression of neuronal proteins.
We did not have any data regarding the neurological status of the 200 SCLC patients, and could therefore not correlate the presence of Ri antibodies to PNS. However, there was no association between the presence of such antibodies and survival of SCLC. This is in line with recent results that Hu or VGCC antibodies do not correlate with survival of SCLC [10]. Ri antibodies have been found to inhibit Nova-1 RNA interactions [15], but whether such inhibition may influence cancer or neurological disease is not known.
Acknowledgements
We thank Mette Haugen, Kibret Mazengia and Cecilie Totland for technical help. This study was supported by grants from the University of Bergen and Helse Vest.
References
- 1.Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349:1543–1554. doi: 10.1056/NEJMra023009. [DOI] [PubMed] [Google Scholar]
- 2.Vincent A. Antibodies to ion channels in paraneoplastic disorders. Brain Pathol. 1999;9:285–291. doi: 10.1111/j.1750-3639.1999.tb00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scaravilli F, Shu FA, Groves M., Thom M. The neuropathology of paraneoplastic syndromes. Brain Pathol. 1999;9:251–260. doi: 10.1111/j.1750-3639.1999.tb00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musunuru K, Darnell RB. Paraneoplastic neurologic disease antigens: RNA-binding proteins and signaling proteins in neuronal degeneration. Annu Rev Neurosci. 2001;24:239–262. doi: 10.1146/annurev.neuro.24.1.239. [DOI] [PubMed] [Google Scholar]
- 5.Pittock SJ, Lucchinetti CF, Lennon VA. Anti-neuronal nuclear autoantibody type 2: paraneoplastic accompaniments. Ann Neurol. 2003;53:580–587. doi: 10.1002/ana.10518. [DOI] [PubMed] [Google Scholar]
- 6.Drlicek M, Bianchi G, Bogliun G, Casati B, Grisold W, Kolig C, Liszka- Setinek U, Marzorati L, Wondrusch E, Cavaletti G. Antibodies of the anti-Yo and anti-Ri type in the absence of paraneoplastic neurological syndromes: a long-term survey of ovarian cancer patients. J Neurol. 1997;244:85–89. doi: 10.1007/s004150050054. [DOI] [PubMed] [Google Scholar]
- 7.Jongen JL, Moll WJ, Sillevis Smitt PA, Vecht CJ, Tijssen CC. Anti-Ri positive opsoclonus-myoclonus-araxia in ovarian duct cancer. J Neurol. 1998;245:691–692. doi: 10.1007/s004150050270. [DOI] [PubMed] [Google Scholar]
- 8.Kikuchi H, Yamada T, Okayama A, Hara H, Taniwaki T, Shigeto H, Sasaki M, Iwaki T, Kira J. Anti-Ri-associated paraneoplastic cerebellar degeneration without opsoclonus in a patient with a neuroendocrine carcinoma of the stomach. Fukuoka Igaku Zasshi. 2000;91:104–109. [PubMed] [Google Scholar]
- 9.Storstein A, Monstad SE, Nakkestad HL, Husebye ES, Vedeler CA. Paraneoplastic antibodies against HuD detected by a sensitive radiobinding assay. J Neurol. 2004;251:197–203. doi: 10.1007/s00415-004-0303-9. [DOI] [PubMed] [Google Scholar]
- 10.Monstad SE, Drivsholm L, Storstein A, Aarseth JH, Haugen M, Lang B, Vincent A, Vedeler CA. Hu and voltage-gated calcium channel (VGCC) antibodies related to the prognosis of small-cell lung cancer. J Clin Oncol. 2004;22:795–800. doi: 10.1200/JCO.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 11.Rojas-Marcos I, Rousseau A, Keime-Guibert F, Rene R, Cartalat-Carel S, Delattre JY, Graus F. Spectrum of paraneoplastic neurolgical disorders in women with breast and gynecologic cancer. Medicine. 2003;82:216–223. doi: 10.1097/00005792-200305000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Pittock SJ, Kryzer TJ, Lennon VA. Paraneoplastic antibodies coexist and predict cancer, not neurological syndrome. Ann Neurol. 2004;56:715–719. doi: 10.1002/ana.20269. [DOI] [PubMed] [Google Scholar]
- 13.Darnell JC, Albert ML, Darnell RB. Cdr2, a target antigen of naturally occuring human tumor immunity, is widely expressed in gynecological tumors. Cancer Res. 2000;60:2136–2139. [PubMed] [Google Scholar]
- 14.Dalmau J, Furneaux HM, Cordon-Cardo C, Posner JB. The expression of the Hu (paraneoplastic encephalomyelitis/sensory neuronopathy) antigen in human normal and tumor tissues. Am J Pathol. 1992;141:881–886. [PMC free article] [PubMed] [Google Scholar]
- 15.Buckanovich RJ, Yang YYL, Darnell RB. The onconeural antigen Nova-1 is a neuron-specific RNA-binding protein, the activity of which is inhibited by paraneoplastic antibodies. J Neurosci. 1996;16:1114–1122. doi: 10.1523/JNEUROSCI.16-03-01114.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]