Abstract
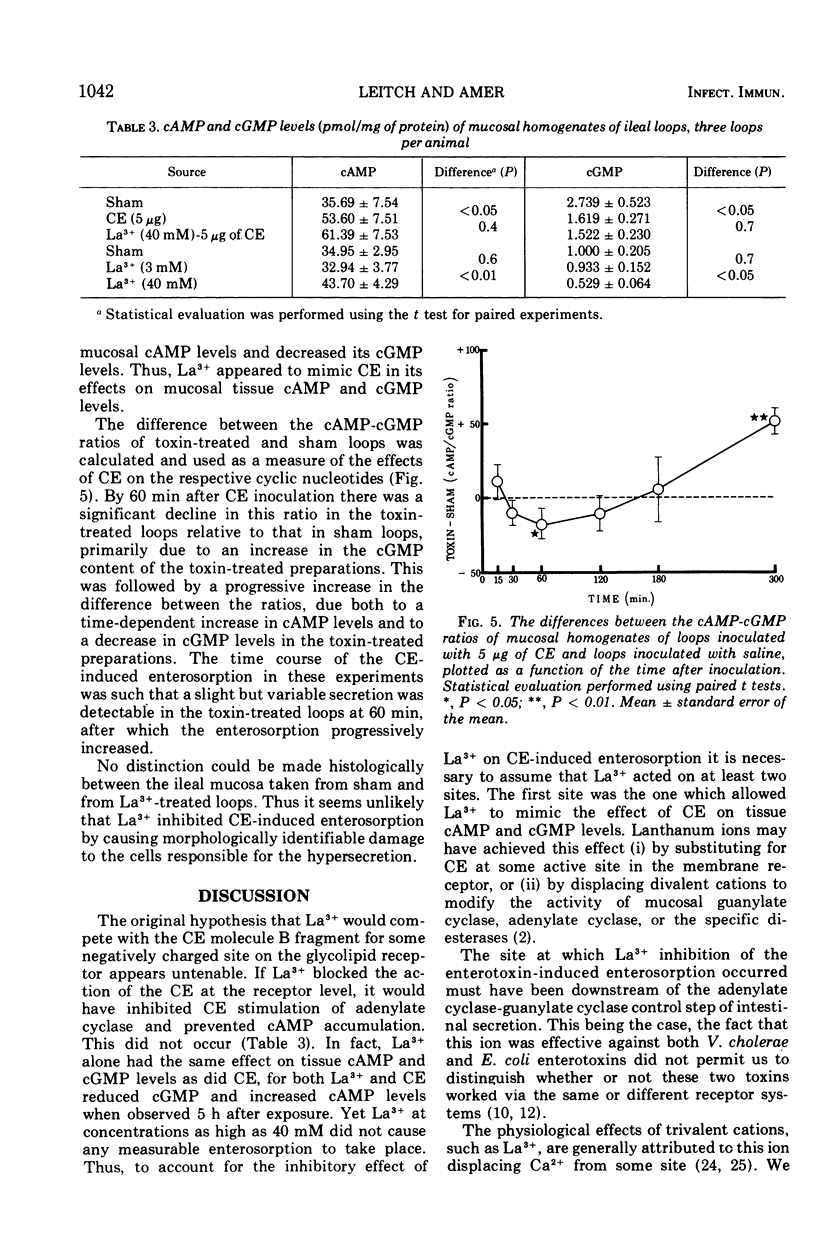
Several trivalent cations, including lanthanum (La3+), inhibited the secretion (enterosorption) induced by the enterotoxins of Vibrio cholerae and Escherichia coli in the rabbit ileum in vivo. High concentrations (greater than 10 mM) of La3+ were required to inhibit cholera enterotoxin (CE)-induced enterosorption, probably because of the adsorption of the La3+ often potentiated the CE-induced enterosorption. If luminal La3+ exposure followed CE exposure, some recovery of the enterosorptive response was observed. The longer the lag between the CE exposure and the La3+ exposure, the greater was the recovery of the enterosorptive response. Lanthanum inhibited HCO3- secretion more than Cl- secretion. By altering the luminal fluid pH at the time of La3+ exposure, it was found that La3+ was adsorbed to negatively charged luminal sites, having an apparent pK between 2.5 and 3.0. Although La3+ antagonized the enterosorptive response to CE, it mimicked rather than antagonized the cyclic adenosine 3',5'-monophosphate elevation and cyclic guanosine 3',5'-monophosphate depression induced by the toxin. It is therefore concluded that the La3+ inhibition of the CE-induced enterosorption must have occurred at a site following the generation of the cyclic nucleotides. Cholera enterotoxin caused complex time-dependent changes in the mucosal cyclic adenosine 3',5'-monophosphate and cyclic guanosine 3',5'-monophosphate levels, as revealed by studying tissue cyclic adenosine 3',5'-monophosphate/cyclic guanosine 3',5'-monophosphate ratios. The possible roles these two cyclic nucleotides may play in the pathogenesis of the cholera diarrhea are discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amer M. S. Cyclic guanosine 3',5'-monophosphate and gallbladder contraction. Gastroenterology. 1974 Aug;67(2):333–337. [PubMed] [Google Scholar]
- Amer M. S., McKinney G. R. Possibilities for drug development based on the cyclic AMP system. Life Sci. 1973 Oct 1;13(7):753–767. doi: 10.1016/0024-3205(73)90066-0. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Gangliosides and membrane receptors for cholera toxin. Biochemistry. 1973 Aug 28;12(18):3558–3566. doi: 10.1021/bi00742a032. [DOI] [PubMed] [Google Scholar]
- Field M. Intestinal secretion. Gastroenterology. 1974 May;66(5):1063–1084. [PubMed] [Google Scholar]
- Finkelstein R. A., Boesman M., Neoh S. H., LaRue M. K., Delaney R. Dissociation and recombination of the subunits of the cholera enterotoxin (choleragen). J Immunol. 1974 Jul;113(1):145–150. [PubMed] [Google Scholar]
- Forstner G., Shih M., Lukie B. Cyclic AMP and intestinal glycoprotein synthesis: the effect of -adrenergic agents, theophylline, and dibutyryl cyclic AMP. Can J Physiol Pharmacol. 1973 Feb;51(2):122–129. doi: 10.1139/y73-016. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg N. D., O'Dea R. F., Haddox M. K. Cyclic GMP. Adv Cyclic Nucleotide Res. 1973;3:155–223. [PubMed] [Google Scholar]
- Holmgren J. Comparison of the tissue receptors for Vibrio cholerae and Escherichia coli enterotoxins by means of gangliosides and natural cholera toxoid. Infect Immun. 1973 Dec;8(6):851–859. doi: 10.1128/iai.8.6.851-859.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Lönnroth I., Svennerholm L. Tissue receptor for cholera exotoxin: postulated structure from studies with GM1 ganglioside and related glycolipids. Infect Immun. 1973 Aug;8(2):208–214. doi: 10.1128/iai.8.2.208-214.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor H. S., Tao P., Wisdom C. Action of Escherichia coli enterotoxin: adenylate cyclase behavior of intestinal epithelial cells in culture. Infect Immun. 1974 Jun;9(6):1003–1010. doi: 10.1128/iai.9.6.1003-1010.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberg D. V., Field M., Gershon E., Henderson A. Effects of prostaglandins and cholera enterotoxin on intestinal mucosal cyclic AMP accumulation. Evidence against an essential role for prostaglandins in the action of toxin. J Clin Invest. 1974 Mar;53(3):941–949. doi: 10.1172/JCI107635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leitch G. J., Glinsukon T. Intestinal mucosal epithelial cell electrophoretic mobility and brush border chemistry during experimental cholera. Exp Mol Pathol. 1969 Oct;11(2):153–162. doi: 10.1016/0014-4800(69)90004-5. [DOI] [PubMed] [Google Scholar]
- Leitch G. J., Iwert M. E., Burrows W. Experimental cholera in the rabbit ligated ileal loop: toxin-induced water and ion movement. J Infect Dis. 1966 Jun;116(3):303–312. doi: 10.1093/infdis/116.3.303. [DOI] [PubMed] [Google Scholar]
- Lucid S. W., Cox A. C. The effect of cholera toxin on the phosphorylation of protein in epithelial cells and their brush borders. Biochem Biophys Res Commun. 1972 Dec 4;49(5):1183–1186. doi: 10.1016/0006-291x(72)90593-1. [DOI] [PubMed] [Google Scholar]
- Moritz M., Womelsdorf A. H. Rabbit cholera: inhibitory effect of tenuazonic acid on cholera-induced secretion of water and electrolytes. Gastroenterology. 1973 Aug;65(2):259–264. [PubMed] [Google Scholar]
- Parkinson D. K., Ebel H., DiBona D. R., Sharp G. W. Localization of the action of cholera toxin on adenyl cyclase in mucosal epithelial cells of rabbit intestine. J Clin Invest. 1972 Sep;51(9):2292–2298. doi: 10.1172/JCI107039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer D. E., Lust W. D., Sircar B., Goldberg N. D. Elevated concentration of adenosine 3':5'-cyclic monophosphate in intestinal mucosa after treatment with cholera toxin. Proc Natl Acad Sci U S A. 1970 Oct;67(2):851–856. doi: 10.1073/pnas.67.2.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. J., Schmidt D. E., Robison G. A. Cyclic adenosine monophosphate in brain areas: microwave irradiation as a means of tissue fixation. Science. 1971 Sep 17;173(4002):1142–1143. doi: 10.1126/science.173.4002.1142. [DOI] [PubMed] [Google Scholar]
- Smith N. W., Sack R. B. Immunologic cross-reactions of enterotoxins from Escherichia coli and Vibrio cholerae. J Infect Dis. 1973 Feb;127(2):164–170. doi: 10.1093/infdis/127.2.164. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Van Heyningen W. E., Carpenter C. C., Pierce N. F., Greenough W. B., 3rd Deactivation of cholera toxin by ganglioside. J Infect Dis. 1971 Oct;124(4):415–418. doi: 10.1093/infdis/124.4.415. [DOI] [PubMed] [Google Scholar]
- Wietzerbin J., Lange Y., Gary-Bobo C. M. Lanthanum inhibition of the action of oxytocin on the water permeability of the frog urinary bladder: effect on the serosal and the apical membrane. J Membr Biol. 1974;17(1):27–40. doi: 10.1007/BF01870170. [DOI] [PubMed] [Google Scholar]


