Abstract
Background: Replication-competent, tumor specific herpes simplex virus NV1066 expresses green fluorescent protein (GFP) in infected cancer cells. We sought to determine the feasibility of GFP-guided imaging technology in the intraoperative detection of small tumor nodules.
Methods: Human cancer cell lines were infected with NV1066 at multiplicities of infection of 0.01, 0.1 and 1. Cancer cell specific infectivity, vector spread and GFP signal intensity were measured by flow cytometry and time-lapse digital imaging (in vitro); and by use of a stereomicroscope and endoscope equipped with a fluorescent filter (in vivo).
Results: NV1066 infected all cancer cell lines and expressed GFP at all MOIs. GFP signal was significantly higher than the autofluorescence of normal cells. One single dose of NV1066 spread within and across body cavities and selectively infected tumor nodules sparing normal tissue. Tumor nodules undetectable by conventional thoracoscopy and laparoscopy were identified by GFP fluorescence.
Conclusion: Virally-directed fluorescent imaging (VFI) is a real-time novel molecular imaging technology that has the potential to enhance the intraoperative detection of endoluminal or endocavitary tumor nodules.
Keywords: Fluorescent detection, Oncolytic virus, Gene therapy, Fluorescent laparoscopy, Fluorescent thoracoscopy, Herpes simplex virus
Introduction
Despite significant improvements in preoperative tumor staging, peritoneal and pleural disease is first detected during surgery in more than 20 to 30% of patients(1-3). It is important to accurately identify the extent of disease to determine which patients are eligible for curative resection and those better served by neoadjuvant therapy. Diagnostic laparoscopy and thoracoscopy (hereafter referred to as endoscopy) have improved our ability to identify the extent of disease, particularly in the areas of peritoneal, pleural and other metastatic spread(1;3-5). Studies suggest that staging laparoscopy is valuable for esophageal, gastric, pancreatic and hepatobiliary cancer(2;3;6;7) as well as for intraabdominal lymphomas. Furthermore, endoscopic oncological surgery has proven to be feasible, safe and oncologically sound(8;9). Laparoscopic resection of small malignant liver lesions and laparoscopy-assisted colectomy have been shown to be both safe and effective(1;10). Similarly thoracoscopy has become the standard of care in wedge resection of small lung cancer nodules and in lymph node excision(5). Thus, minimally-invasive endoscopy has emerged as sensitive and specific methods of comprehensive abdominal and chest exploration for surgical staging and the resection of cancers.
Despite the improved assessment by diagnostic endoscopy, a significant amount of tumor burden may not be visible using current techniques(11;12). In addition, intraoperative confirmation of suspected lesions requires histopathological examination which is time consuming and has limitations. Intraoperative tumor specific imaging may improve detection of small tumor nodules and allow surgeons to precisely biopsy or excise those lesions with improved sensitivity. This paradigm requires an appropriate tumor marker that can facilitate visualization of tumor cells separate from normal cells.
Herpes simplex virus (HSV) mediated oncolysis and gene therapy have emerged as promising treatment modalities against cancer. A number of studies from our group and others have determined that these viruses are highly specific for tumor cells while sparing normal cells(13-21). Oncolytic HSV therapy has shown to be effective against multiple tumor types including lung, esophageal, gastric, colorectal, gallbladder, hepatic, pancreatic, bladder cancer and mesothelioma(13-22). Given the specificity of herpes viral replication for tumor cells, we sought to determine whether NV1066, a HSV-1 mutant virus used in this study can delineate tumor tissue from normal tissue. NV1066 carries a transgene for an enhanced green fluorescent protein (GFP)(14), which is constitutively expressed 4 to 6 hours following viral entry into cells. Previous studies from our laboratory have shown that NV1066-induced GFP expression can be used as a marker of viral infection, and thus to identify cancer(17;23). In this study we further seek to determine whether NV1066-induced GFP expression can be used to diagnose small metastatic deposits of pleural and peritoneal cancer invisible to the naked eye in an in vivo murine model and the sensitivity of such detection method in improving diagnostic accuracy.
Materials and Methods
Herpes Simplex Virus, NV1066: NV1066 is a novel, attenuated, replication-competent oncolytic herpes virus described in detail previously(14). Single copies of the viral genes ICP0, γ134.5, and ICP4 were deleted to attenuate the virus and a marker gene for enhanced green fluorescent protein is inserted. The resultant virus is one with high specificity for the infection of cancer cells and it constitutively expresses the GFP.
Cell Lines: Human cancer cell lines from eight different primary organs (lung, esophagus, stomach, colon, liver, gallbladder, pancreas and pleural cancer) were studied. VAMT mesothelioma cells were a kind gift of Dr. Francis Sirotnak (Memorial Sloan-Kettering Cancer Center, New York) and were maintained in RPMI (Roswell Park Memorial Institute, Buffalo, NY) with 10% fetal calf serum(FCS). HCT-8 colon cancer cells were grown in RPMI with 10% FCS. OCUM-2MD3 human gastric cancer cells were a gift of Dr. Masakazu Yashiro (Osaka City University Medical School, Japan) and were maintained in DMEM with high glucose, 2 mM L-glutamine and 0.5 mM Napyruvate. The remaining human cancer cell lines and Vero cells were obtained from the American Type Culture Collection (ATCC®, Rockville, MD) or other independent investigators, grown in appropriate media, and maintained in a humidified incubator supplied with 5% CO2 at 37°C. All media was supplemented with penicillin and streptomycin.
In vitro Imaging: A Zeiss LSM 510 confocal laser scanning microscope (Carl Zeiss, Inc., Oberkochen, Germany) and the MetaMorph Imaging System (Downingtown, PA) were used to visualize GFP-expressing cancer cells. Imaging was performed in both bright-field and fluorescent modes. Viable cells were identified by Hoechst staining and examined under a DAPI filter. GFP expression was identified after placement of both excitation and emission filters.
Flow cytometry for GFP: VAMT and HCT-8 cells were plated in 6-well plates with 2 ml of media and infected with NV1066 at MOIs (multiplicity of infection, ratio of viral particles to tumor cells) of 0.01, 0.1, or 1 in 100 μl of phosphate buffered saline (PBS). Untreated cells served as negative controls. Cells were harvested with 0.25% trypsin in 0.02% EDTA, combined with the supernatant fraction, centrifuged, washed in PBS, and resuspended in 100 μl of PBS. Five μl of 7-amino-actinomycin (BD Pharmingen, San Diego, CA) was added as an exclusion dye for cell viability. Data for GFP expression from 104 cells was acquired on a FACS Calibur machine equipped with Cell Quest software (Becton Dickinson, San Jose, CA). Results are reported as percentage of live cells expressing GFP. Each experiment was repeated a minimum of three times.
Correlation of GFP expression to HSV infection: Cells were plated and infected with NV1066 at MOIs of 0.01, 0.1, and 1 and harvested 6, 12, and 24 hours after infection. GFP-expressing cells were separated from non-GFP-expressing cells by fluorescence-activated cell sorting (MoFlo; Dako Cytomation, Fort Collins, CO) and fixed on slides for immunohistochemistry. Uninfected cells served as negative controls. Slides were stained by the improved biotin-streptavidin amplified method (BioGenex Super Sensitive Detection System, Biogenex, San Ramon, CA) using a polyclonal antibody to HSV-1 and examined using a Zeiss Axiovert 200 microscope (Carl Zeiss, Inc.) for the presence of HSV and GFP. Overlay images were created of the same field viewed under routine bright-field microscopy and GFP modes. Each condition was performed in triplicate.
Viral Titering: Cells were grown in 24-well flat-bottom plates and were infected with NV1066 at MOIs of 0.01, 0.1, and 1. Culture supernatants were collected each day for 7 days postinfection. Viruses in supernatants were enumerated in Vero cells using a standard viral plaque assay. All samples were tested in triplicate, with the experiment repeated at least three times.
Cytotoxicity assay: Cells were grown in 24-well flat-bottom plates and subsequently infected with NV1066 at MOIs of 0.01, 0.1 and 1 and incubated at 37°C for 1 to 7 days. Each day after infection, cells were washed in PBS and lysed with a 1.35% Triton-X solution (% volume/PBS) to release intracellular lactate dehydrogenase (LDH). The cytotoxic effect was determined by comparing release of intracellular LDH from virally-infected tumor cells to release in untreated, control cells grown under identical conditions by standard Cytotox 96 nonradioactive cytotoxicity assay (Promega, Madison, WI)(17;19;23). Results were expressed as the surviving fraction compared to untreated control cells. All samples were tested in triplicate with the experiment repeated a minimum of three times.
Animal experiments: Animal procedures were approved by the Memorial Sloan-Kettering Institutional Animal Care and Use Committee (New York, NY). Six-week-old male athymic mice (National Cancer Institute, Bethesda, MD) used for experiments were provided food and water ad libitum. Inhalational isoflurane was used for all experimental procedures.
Pleural carcinomatosis model: 2 x 107 VAMT cells were injected percutaneously into the pleural cavity of athymic mice in 100 μl of PBS. Ten days following tumor cell injection, mice were divided into two groups and treated with either 1 x 107 plaque forming units (pfu) of NV1066 in 50 μl PBS (n = 10) or 50 μl of PBS alone (n = 10) via percutaneous injection into the pleural cavity. Three days after NV1066 or PBS inoculation, animals were sacrificed with the heart, lungs, and mediastinum removed en bloc. For both PBS and NV1066 treated groups of animals, chest wall nodules were counted after removal of organs both under bright-field and fluorescent microscopy with a GFP filter.
Abdominal carcinomatosis model: Mice injected intraperitoneally with 2 x 107 OCUM gastric cancer cells in 250 μl PBS developed disseminated peritoneal cancer ten days after injection. Ten days following tumor cell injection, mice were divided into two groups and treated with either 1 x 107 pfu of NV1066 (n = 10) or PBS alone (n = 10) via percutaneous injection into the peritoneal cavity. Three days after NV1066 or PBS inoculation, animals were sacrificed. For both PBS and NV1066 treated groups of animals, abdominal and peritoneal nodules were counted both under bright-field and fluorescent microscopy with a GFP filter.
In vivo imaging: One x 107 VAMT mesothelioma cells were injected into the pleural cavities and HCT-8 colon and OCUM-2MD3 gastric cancer cells were injected into the peritoneal cavity as described previously. Animals (n = 5) were treated with 1 x 107 pfu of intrapleural or intraperitoneal NV1066 as described above. For imaging of micrometastatic disease, a cohort of mice was treated two days after cell injection. For imaging of gross pleural disease, a separate cohort of mice was treated ten days after cell injection. Mice were examined 72 hours later in both cases, using a fluorescent thoracoscopic/laparoscopic system and by fluorescent stereomicroscopy. The fluorescent thoracoscopic/laparoscopic system was developed in collaboration with Olympus America, Inc. (Scientific Equipment Division, Melville, NY) to allow for the detection of GFP as well as routine bright-field imaging. The GFP images were taken with minimal background illumination to illustrate the surrounding organs.
Confirmation of viral specificity to tumor by real-time polymerase chain reaction (PCR): Organs and tumors from animals with established carcinomatosis treated with either PBS or with 1.0 x 107 pfu NV1066 were harvested and snap-frozen in liquid nitrogen. Genomic DNA was isolated (Wizard Genomic DNA Isolation Kit, Promega) and standard curves were made by spiking uninfected samples with known quantities of NV1066 prior to extraction. Real-time quantitative PCR was performed to quantitate herpes ICP0 immediate-early gene.
Results
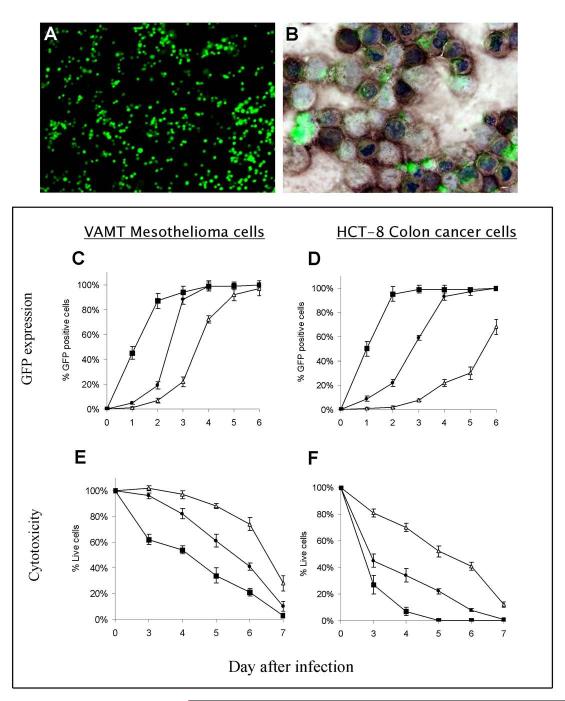
NV1066 infects and expresses GFP in a wide range of cancer cell lines: In vitro, NV1066 infected all cancer cell lines at all MOIs. NV1066-infected cancer cell expressed GFP within 1 to 2 hours. The expressed GFP is intracellular and can be detected by fluorescent microscopy (Figure 1A). Twenty-four hours after infection at an MOI of 1, greater than 50% of the cancer cells were infected and expressed GFP. Even a low MOI of 0.01 was capable of infecting and expressing GFP in all cell lines by day 6. At the higher MOI of 1, most of the cells in the sample were infected at an earlier time point and expressed strong GFP fluorescence. Infected cancer cells have 31 - 744 -fold higher mean intensity compared to background autofluorescence.
Figure 1.
NV1066 infects and express GFP in cancer cells. NV1066 infected cancer cells express GFP as identified by fluorescent microscopy. Shown in Figure A are the GFP expressing cancer cells 24 hours following infection at an MOI of 1. Shown in Figure B is the overlay of GFP expression with HSV immunohistochemistry to confirm GFP expression is due to HSV infection (GFP, green color; HSV antibody staining, brown color; nuclear Hoechst staining, blue color). VAMT malignant mesothelioma cells and HCT-8 colon cancer cells were infected with NV1066 at an MOI of 0.01 (open triangles) or 0.1 (filled circles) or 1 (filled squares). Cells were harvested at day 1, 2, 3, 4, 5 and 6 and analyzed by flow cytometry for GFP expression. GFP expressing cells were represented as percentage of control of uninfected cells in Figures C (VAMT) and D (HCT-8). Lactate dehydrogenase cytotoxicity assay was performed to assess viable cells on day 3, 4, 5, 6 and 7. Results for the treated group were expressed as cell survival compared to untreated control cells grown under identical conditions in Figures E (VAMT) and F (HCT-8). GFP: green fluorescent protein, MOI: multiplicity of infection; HSV: herpes simplex virus
GFP expression is due to NV1066 Infection: GFP expression corresponds to immunohistochemistry proven NV1066 infection (Figure 1B). After cultured cells were infected with NV1066 at MOIs of 0.01, 0.1, and 1, they were fluorescence-activated cell-sorted into GFP-expressing and non-GFP-expressing populations at various time points. One hundred percent of GFP-expressing cells were positive for HSV by immunohistochemistry. Of the cells sorted by flow cytometry that did not express GFP, none were positive for HSV by immunohistochemistry.
Dose-dependent increase in intensity and percentage of GFP expressing cells over time: The percentage of GFP expressing cells was quantified by flow cytometry following infection with NV1066 in vitro. Infected cells demonstrate a progressive increase in GFP expression over time at MOIs from 0.01 to 1 (Figure 1C & D). Each MOI ultimately induces GFP expression in nearly 100% of cells, demonstrating spread of virus throughout the cell culture well. Greater initial viral inoculation results in a more robust expression of GFP. For example, at an MOI of 1, nearly 50% of the cells express GFP 1 day after infection, while 50% of those infected at MOI of 0.1 express GFP 3 to 4 days after infection (p < .001 vs. untreated control cells, t-test).
NV1066 replicates in cancer cells: NV1066 replicates effectively in cancer cells following infection at MOIs of 0.1 and 1.0. Viral titers increase steadily following infection. In VAMT cells infected at an MOI of 0.1, on the day of the peak viral burst (day 6), there is a 330 –fold increase in viral titer compared to the initial viral dose. Treatment of cells with lower MOIs led to higher peak viral titers 4 to 6 days following infection (p < .001, t-test). Peak viral titers using the lower MOI demonstrated an approximate 127 to 5300-fold increase over the initial dose of virus in all cell lines tested. This phenomenon occurs following in vitro treatment with oncolytic HSV strains, as early cytotoxic death in cell populations treated with higher MOIs limits the time and number of viable cells for productive cellular replication of virus.
NV1066 kills infected cancer cells: NV1066 infection is cytotoxic to all cancer cell lines tested at all MOIs. NV1066 progressively killed cancer cells in vitro at all MOIs. Although cell kill was initially more pronounced at the higher MOI of 1.0, an MOI of 0.01 also demonstrated significant cytotoxic effects over time. By day 7, in vitro cell kill at MOIs of 0.01, 0.1 and 1.0 is 72 ± 6%, 90 ± 4% and 97 ± 3% respectively (Figure 1E, VAMT, p < .01, t-test). NV1066 kills HCT-8 cells over the range of MOIs tested (Figure 1F, HCT-8). By day 7, in vitro cell kill at MOIs of 0.01, 0.1 and 1.0 is 88 ± 2%, 99 ± 1% and 100% respectively (p < .01, t-test). Similar cell kill was observed in all other cancer cells. At all three MOIs, cytotoxicity increases progressively over the 7 day period, resulting in near complete tumor cell lysis for MOIs of 1, 0.1, and 0.01 by day 4 - 6, 6 - 7, and 6 - 9 respectively.
Time course of in vivo GFP expression: Several previous studies from our laboratory have demonstrated the ability of NV1066 to replicate in in vivo in murine flank tumors (15). Viral titers increase over time as measured by real-time PCR for viral genomic DNA, with a large replication burst evident between 24 to 48 hours. In flank tumor studies established with VAMT and HCT-8 cells, GFP expression can be seen robustly 48 to 72 hours after infection with NV1066 (data not shown). Therefore, in the present study, we decided to use time points of 72 hours or later to assess for GFP expression in the pleural and peritoneal carcinomatosis model, as these time points were more likely to allow for viral replication and robust GFP expression even when initial infective dose is low.
In vivo imaging: Following administration of NV1066 in vivo, GFP expression was easily visualized by fluorescent microscopy and endoscopy in the pleural and peritoneal carcinomatosis model (Figures 2 and 3). The pleural and peritoneal cavities were examined in both bright-field and GFP modes. The thoracoscopic / laparoscopic device can be easily changed between the two modes without pausing the procedure. Figures 2 and 3 demonstrate clearly that GFP expression was visualized in all NV1066 treated animals and is localized to tumor deposits, sparing normal tissues. Green fluorescence could be used to identify tumor deposits, localizing even microscopic collections of tumor cells (< 1 mm) not apparent under bright-field endoscopy (Figure 2H, 2J, 2L, 3D, 3F, 3H and 3J). Non-tumor bearing thoracic and abdominal organs did not fluoresce in vivo when examined through the GFP filter and were easily distinguished from infected tumor deposits. The presence of tumor cells was confirmed by histology. Topographic maps of GFP expression were generated from the digital images obtained with the fluorescent microscopic or endoscopic systems. This technology enables quantification of in vivo fluorescent intensity. The sample images obtained by laparoscopy demonstrate similar findings of selective infection and fluorescence of tumor in different quadrants of the abdomen.
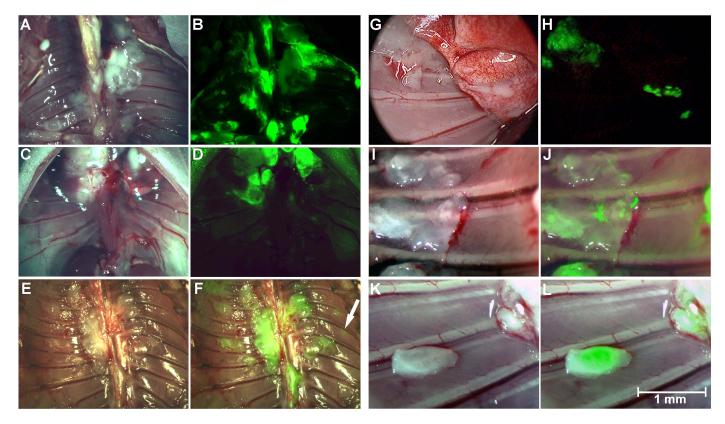
Figure 2.
Thoracoscopic identification of pleural carcinomatosis by GFP expression. Using fluorescent thoracoscopy, the pleural cavities of mice with macroscopic tumor deposits were examined in bright-field (A & E, parietal pleura; G, parietal and visceral pleura) and GFP (B, D, F and H) modes. Diaphragmatic pleural deposits (C) can be readily identified by the fluorescent laparoscopy (D). Following intrapleural administration, NV1066-induced GFP expression localized to tumor deposits, sparing normal tissues as identified by fluorescent thoracoscopy (J and L, bright-field: I and K). GFP: green fluorescent protein
Figure 3.
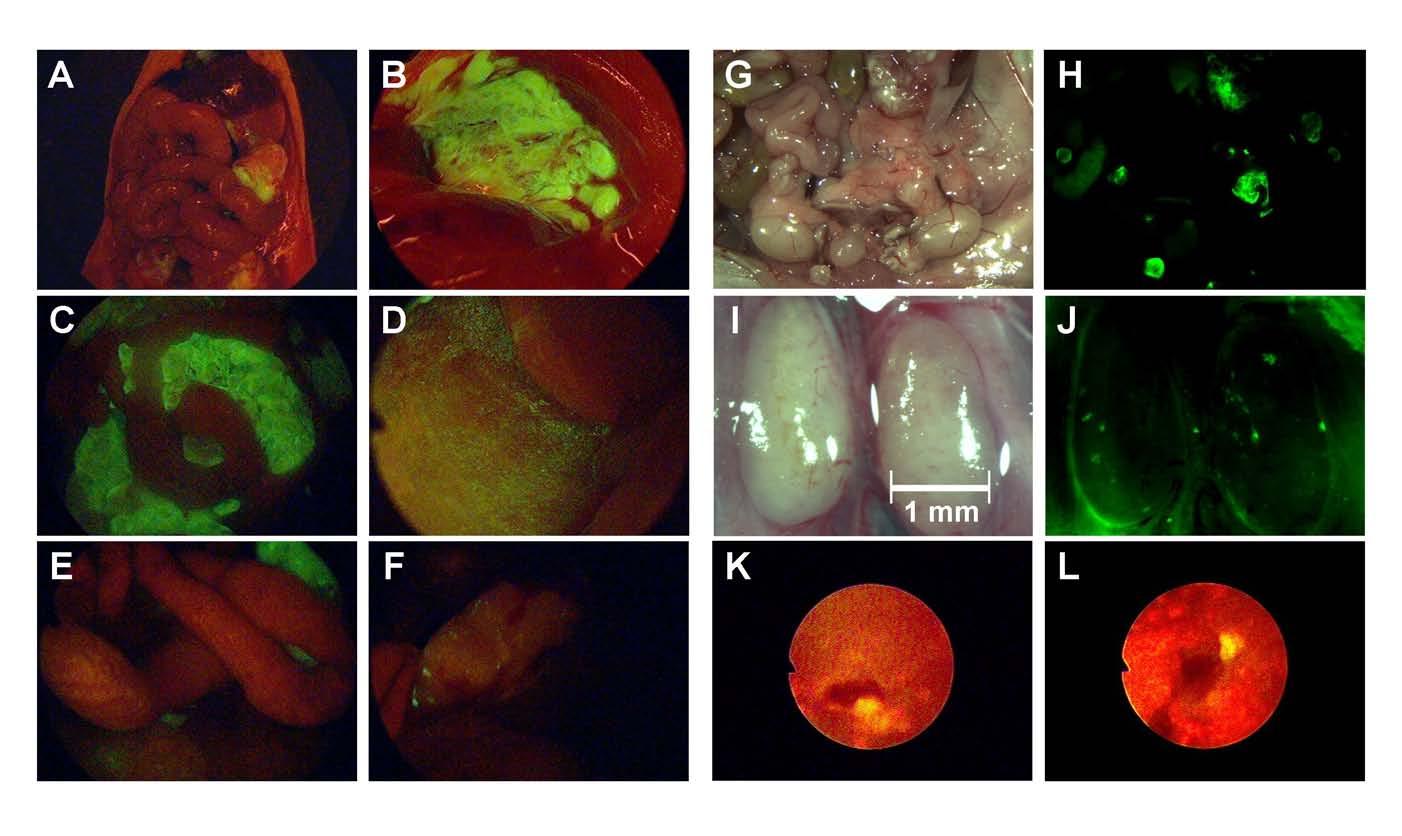
Laparoscopic identification of peritoneal carcinomatosis by GFP expression. Intraperitoneal administration of NV1066 facilitates visualization of intraabdominal macroscopic and microscopic carcinomatosis. Peritoneal macroscopic carcinomatosis is visualized by fluorescent laparoscopy (A and C, intraperitoneal deposits; B, subdiaphragmatic deposits). Macroscopically undetectable peritoneal nodules can be accurately visualized by fluorescent laparoscopy (D, overlay of bright-field and GFP). Similarly, disseminated tumor nodules can be clearly identified by GFP expression (G, bright-field; H, GFP mode). Microscopic nodal deposits that cannot be visualized by bright-field (I) can be readily identified by GFP expression (J). Fluorescent upper gastrointestinal endoscopy identified cancerous lesions (<1 mm.) (K and L). GFP: green fluorescent protein
NV1066-induced GFP expression augments pleural and peritoneal cancer nodule detection: Injection of cancer cells into the pleural cavity led to disseminated tumor growth on the parietal and visceral pleura, the diaphragm, and the mediastinum. All areas of gross tumor seen under bright-field fluoresce when examined under the GFP filter (Figure 2). Internal organs were removed and the parietal pleura was examined with the thoracoscope and stereomicroscope. An average of 27 ± 11 nodules were counted per mice. Fluorescent thoracoscopy increased the yield of nodule identification that were not visualized by conventional bright-field thoracoscopy by 24%. Microscopic tumor nodules (≤ 1 mm), inconspicuous in the bright-field mode, were identified exclusively as a result of their characteristic bright green fluorescence following NV1066 infection (Figure 2J and L). Similar findings were confirmed for the detection of intraperitoneal nodules by fluorescent laparoscopy (Figure 3D, F and L). Small foci of tumor, not initially obvious by examination of the abdomen under direct bright light, expressed GFP, facilitating detection (Figure 3 D, E, F, H and J). Determination of the number of intraperitoneal metastases under bright-field and fluorescent light by three independent observers demonstrated that 32% of the total number of metastasis seen with fluorescent imaging was missed by routine bright-field inspection. Fluorescence laparoscopy facilitated micronodular detection most commonly in the mesentery (Figure 3E), the intestinal serosal surface (Figure 3F), the pelvis (Figure 3G) and in retroperitoneal lymph nodes (Figure 3J). Similarly fluorescence endoscopy facilitated the identification of microscopic tumor deposits in the stomach during upper gastrointestinal endoscopy in mice (Figure 3K and L). A randomized number of these tumors were resected and histologically confirmed to be carcinomas while tissue samples thought to be normal were tumor free. In both the thoracic and abdominal cavities, non-tumor bearing mice were negative for GFP expression.
Viral specificity for tumor: Following pleural administration of NV1066, strong GFP expression was noted in tumor nodules when examining serial pathological sections. All sections that expressed GFP were found to have tumor cell infiltrates corresponding to areas of expression (Figure 4A). H&E staining confirmed that GFP expression localized to foci of cancer (Figure 4B). Staining for polyclonal HSV-1 antibody also corresponded to areas of GFP expression by histology. No viral staining was evident in tissues that did not express GFP in all samples sectioned. Similar immunohistochemical studies confirmed viral specificity to tumor in the peritoneal carcinomatosis model.
Figure 4.
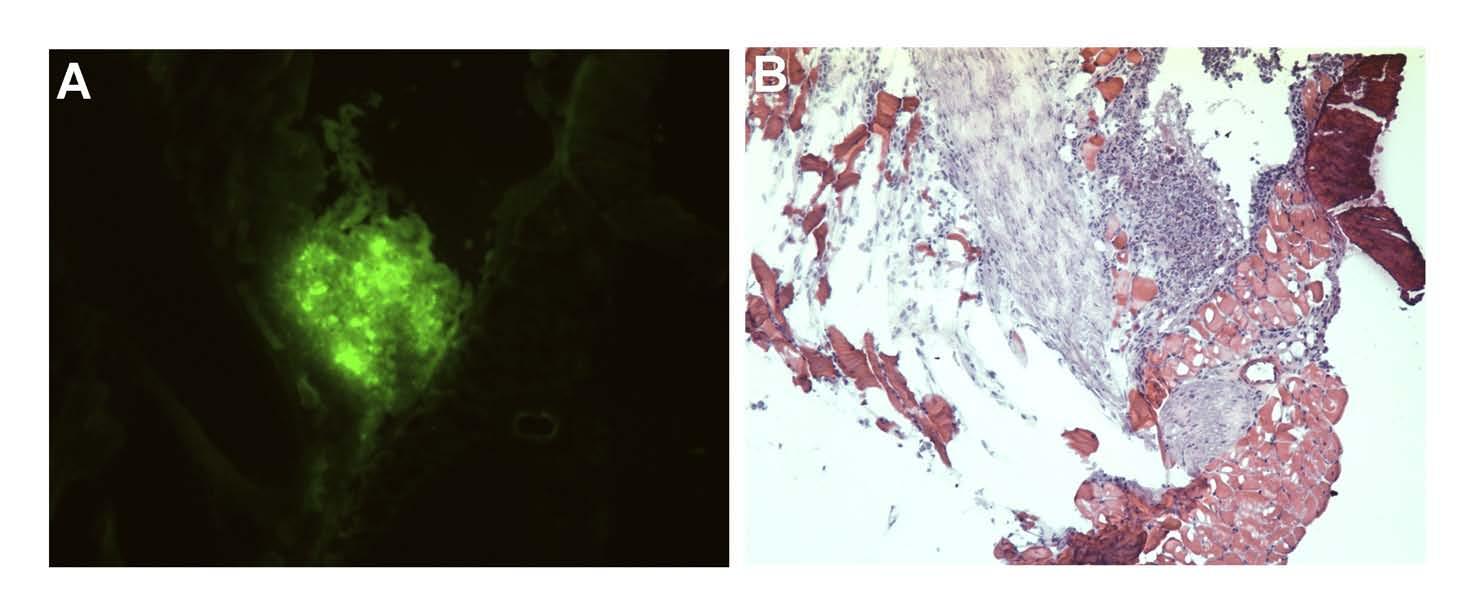
Histopathological confirmation that NV1066 selectively infects and expresses GFP in tumor deposits, sparing normal tissue: Tissue specimens were selected by GFP expression under fluorescent microscopy. Following sectioning, specimens were examined under fluorescent microscopy (A), and then H & E stained (B) for identification of tumor cells. All sections that expressed GFP had tumor cell infiltrates. H & E: Hematoxylin and Eosin staining, GFP: green fluorescent protein
Real-Time PCR for HSV-1: Peritoneal and pleural nodules as identified by GFP were isolated as tumor nodules. Solid organs from both cavities and serum were analyzed along with tumor nodules for the presence of viral gene by PCR. No evidence of viral presence was noted in normal organs and serum three days after viral inoculation. The amplification in tumor tissue from 7-47 fold confirmed viral presence and replication, which is dependent on the time period after viral injection.
Discussion
Accurate preoperative staging is essential in planing therapeutic strategies in patients with malignant disease. Despite advances in preoperative imaging, precise assessment of the local, regional and distant disease is inadequate. Endoscopy has emerged as a highly sensitive and specific approach to comprehensively examine the thoracic and abdominal cavities. Early identification of unresectable disease protects patients from the increased morbidity and mortality of a major surgery. Several studies documented the beneficial role of preoperative minimally-invasive endoscopy in detecting advanced tumor. In one such study, D'Angelica et. al.(6) published a prospective study of 401 cases of hepatobiliary malignancy in which complete laparoscopic examinations were carried out in 291 patients (73%). Unresectable disease was discovered by laparoscopy in 84 patients (20.9%). In patients in whom unresectable disease was identified by laparoscopy the hospital stay was 3 days and the postoperative morbidity was 9% while patients who were not identified as unresectable until the time of laparotomy had a hospital stay of 8 days with a 28% postoperative morbidity rate. While these benefits are significant, it is important to note that 21% of patients thought to be resectable after laparoscopic evaluation were found to be unresectable during laparotomy.
Endoscopic visualization is hindered by poor illumination, light reflection off surfaces, low contrast, and high scatter. Other techniques are being developed to improve endoscopic tumor identification. For instance, fluorescent techniques have been shown to enhance tumor visualization(24;25). Using the exogenously administered photosensitizer, 5-aminolevulinic acid (ALA), laparoscopic visualization of peritoneal tumor deposits in an animal model was improved by 17%-35%, and that of pleural nodules by 30% compared to conventional bright-field laparoscopy alone. Although ALA specifically accumulates in tumor cells, its use is limited by a narrow time window for visualization and by the possibility of skin phototoxicity. Similarly, intraoperative ultrasound is limited by the inability of ultrasound waves to penetrate air as well as impaired detail and spatial definition. Intraoperative magnetic resonance imaging is being explored but it is expensive, increases operative time and can be compromised by contrast leak or other signal changes around the resection bed.
Oncolytic HSV strains are replication-competent viruses that selectively infect and lyse tumor cells, sparing normal tissue, and have been shown to be effective against a number of malignancies in experimental models. The HSV strain used in this study, NV1066, carries a marker gene for GFP. The integration of GFP into HSV allows for a combined therapeutic and diagnostic approach to cancer. While GFP and its derivative proteins have been used extensively as reporters and / or markers in numerous biologic studies(26-28), their use to detect macroscopically invisible tumor nodules in vivo is an emerging technology. Several researchers attempted to deliver GFP in vivo by use of viral vectors. However, such recombinant viral vectors infect a wide variety of dividing and nondividing cells and thus are not specific to tumor cells. In this study, we were able to exploit the tumor selective nature of HSV in inducing GFP specifically in tumor cells while sparing normal tissue. As these viruses replicate and propagate among tumor cells, even an initial small dose can infect, replicate and then express GFP throughout the tumor. In a murine flank tumor model, fluorescence increased over time with viral replication so that infected tumors were clearly distinguishable from non-infected tumors by 48 to 72 hours following infection. We extended this observation to in vivo studies in body cavities in a murine model. In our study, fluorescence guided technique significantly improved the detection of macroscopically unidentifiable tumor nodules by 20%-30%. Moreover, viral infection is isolated to tumor cells, which is confirmed by RT-PCR and immunohistochemistry. Such tumor specific expression of GFP produced by viral infection has a similar potential to improve detection of tumor deposits by use of a wide variety of minimally-invasive endoscopic procedures such as fluorescent laryngoscopy, thoracoscopy, bronchoscopy, mediastinoscopy, upper and lower gastrointestinal endoscopy, laparoscopy, cystoscopy and arthroscopy.
Another advantage of NV1066 administration is that it is both diagnostic and therapeutic. In vitro, by day 7, most cancer cells were killed irrespective of cell line or the infecting dose. Previous studies from our laboratory have shown similar results using in vivo models(23;29). The therapeutic benefit is added without any additional risk of toxicity. This data suggests that unresectable tumor foci can be lysed by oncolytic HSV.
NV1066 induced GFP expression clearly delineated the tumor margins as shown in endoscopic pictures (Figure 2 and 3), which is also confirmed histologically (Figure 4). In vitro data presented in other publications from our laboratory suggests that as few as 104 clustered cells infected and expressing GFP may be distinguished from autofluorescence, a limit of detection significantly lower than that of other imaging techniques (CT, MR, PET scans cannot detect tumor foci smaller than 3 - 4 mm in size, or approximately 1 x 107 cells). ‘Enhanced’ GFP, eGFP, a functionally better fluorescent variant (Clontech Inc, Mountain View, CA) cloned into NV1066 is a class 2 GFP that significantly enhances the tumor / background signal intensity ratio. Utilizing this protein to imaging advantage is one of the key goals of this study. Following intrapleural and intraperitoneal administration of NV1066, in situ metastatic tumor deposits are clearly distinguished by green fluorescence using fluorescent stereomicroscopy or an endoscopic system. Every fourth lesion was diagnosed simply by adding the optical fluorescence mode to conventional endoscopy. In addition, we performed an objective determination of the fluorescence characteristics by spectrometry. Such spectral information from a lesion is immediately available, allowing a real-time judgment and enables the examiner to take biopsy samples from the areas most likely to yield positive results.
Previous studies have found that GFP expression in transfected tumor cells allow for detection of metastases down to the single-cell level in fresh isolated organs or tissues. In subsequent studies, fluorescent localization was found to be more rapid and sensitive than histopathology or immunohistochemistry for identifying tumor deposits. While those studies relied upon stably transfected GFP-expressing cells, we report here a method for identifying tumor cells in vivo with a therapeutic viral vector, a model that may be more readily applied clinically. If macroscopically invisible lesions can be identified during thoracoscopy and laparoscopy, surgeons will be capable of removing disseminated tumors with clear margins. Furthermore, by incorporating an automated detection device that chases GFP fluorescence, future endoscopic, minimally invasive and robotic laser/ablation technology can target microscopic cancer.
In this study, samples of tissue expressing GFP were harvested, sectioned serially, and examined for the presence of virus. All tissue that expressed GFP was noted to stain positively for cancer and virus. This suggests that fluorescent protein expression can be used as a surrogate to identify macroscopically undetectable tumor deposits. Such a method will allow rapid histological examination without any preparation: the unfixed tissue can be cut into thin slices, placed between glass slides, and directly observed with fluorescence microscopy. In comparison with other techniques, such a simple method speeds up the analysis allowing one to multiply the number of tissues analyzed and to precisely obtain clear margins at a molecular level.
In conclusion, our data indicate that locoregional injection of NV1066 into body cavities in combination with highly sensitive fluorescent imaging technology is a potentially useful diagnostic strategy for improved real-time tumor visualization.
Acknowledgments
The authors thank Liza Marsh and Scott Tuorto of the Department of Surgery at Memorial Sloan-Kettering Cancer Center for their editorial assistance. We also thank Brian Horsburgh, Ph.D. and Medigene, Inc. for constructing and providing us with the NV1066 virus. Special thanks to Kan Matsumoto from Olympus America Inc., for design and construction of the fluorescent endoscopic system.
Footnotes
Presentation: Presented at Society of American Gastrointestinal and Endoscopic Surgeons April 13-16 2005 Annual meeting, Florida.
Supported in part by AACR-AstraZeneca Cancer Research and Prevention fellowship (P.S.A), grants RO1 CA 75416 and RO1 CA/DK80982 (Y.F.) from the National Institutes of Health, grant MBC-99366 (Y.F.) from the American Cancer Society, grant BC024118 from the US Army (Y.F.), grant IMG0402501 from the Susan G. Komen Foundation (Y.F. and P.S.A) and grant 032047 from Flight Attendant Medical Research Institute (Y.F. and P.S.A.)
Reference List
- 1.Paraskeva PA, Purkayastha S, Darzi A. Laparoscopy for malignancy: current status. Seminars in Laparoscopic Surgery. 2004;11(1):27–36. doi: 10.1177/107155170401100106. [DOI] [PubMed] [Google Scholar]
- 2.Parra JL, Reddy KR. Diagnostic laparoscopy. Endoscopy. 2004;36(4):289–93. doi: 10.1055/s-2004-814347. [DOI] [PubMed] [Google Scholar]
- 3.Corvera CU, Weber SM, Jarnagin WR. Role of laparoscopy in the evaluation of biliary tract cancer. Surgical Oncology Clinics of North America. 2002;11(4):877–91. doi: 10.1016/s1055-3207(02)00033-9. [DOI] [PubMed] [Google Scholar]
- 4.Asao T, Kuwano H, Mochiki E. Laparoscopic surgery update for gastrointestinal malignancy. Journal of Gastroenterology. 2004;39(4):309–18. doi: 10.1007/s00535-004-1331-z. [DOI] [PubMed] [Google Scholar]
- 5.Saltzman JR. Section III: endoscopic and other staging techniques. Seminars in Thoracic & Cardiovascular Surgery. 2003;15(2):180–6. [PubMed] [Google Scholar]
- 6.D'Angelica M, Fong Y, Weber S, Gonen M, DeMatteo RP, Conlon K, et al. The role of staging laparoscopy in hepatobiliary malignancy: prospective analysis of 401 cases. Annals of Surgical Oncology. 2003;10(2):183–9. doi: 10.1245/aso.2003.03.091. [DOI] [PubMed] [Google Scholar]
- 7.D'Angelica M, Jarnagin W, DeMatteo R, Conlon K, Blumgart LH, Fong Y. Staging laparoscopy for potentially resectable noncolorectal, nonneuroendocrine liver metastases. Annals of Surgical Oncology. 2002;9(2):204–9. doi: 10.1007/BF02557375. [DOI] [PubMed] [Google Scholar]
- 8.Porpiglia F, Fiori C, Terrone C, Bollito E, Fontana D, Scarpa RM. Assessment of surgical margins in renal cell carcinoma after nephron sparing: a comparative study: laparoscopy vs open surgery. Journal Of Urology. 2005;173(4):1098–101. doi: 10.1097/01.ju.0000148360.47191.5e. [DOI] [PubMed] [Google Scholar]
- 9.Mochiki E, Kamiyama Y, Aihara R, Nakabayashi T, Asao T, Kuwano H. Laparoscopic assisted distal gastrectomy for early gastric cancer: Five years' experience. Surgery. 2005;137(3):317–22. doi: 10.1016/j.surg.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Hartley JE, Monson JR. The role of laparoscopy in the multimodality treatment of colorectal cancer. Surgical Clinics Of North America. 2002;82(5):1019–33. doi: 10.1016/s0039-6109(02)00039-7. [DOI] [PubMed] [Google Scholar]
- 11.Lorenz R, Krestin GP, Neufang KF. Diagnosis and differential diagnosis of a peritoneal carcinosis. Conventional techniques, sonography, computed tomography, magnetic resonance tomography. Radiologe. 1990;30(10):477–80. [PubMed] [Google Scholar]
- 12.Lee SY, Lee JH, Hwang NC, Kim YH, Rhee PL, Kim JJ, et al. The role of follow-up endoscopy after total gastrectomy for gastric cancer. European Journal Of Surgical Oncology. 2005;31(3):265–9. doi: 10.1016/j.ejso.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 13.Bennett JJ, Delman KA, Burt BM, Mariotti A, Malhotra S, Zager J, et al. Comparison of safety, delivery, and efficacy of two oncolytic herpes viruses (G207 and NV1020) for peritoneal cancer. Cancer Gene Therapy. 2002;9(11):935–45. doi: 10.1038/sj.cgt.7700510. [DOI] [PubMed] [Google Scholar]
- 14.Wong RJ, Joe JK, Kim SH, Shah JP, Horsburgh B, Fong Y. Oncolytic herpesvirus effectively treats murine squamous cell carcinoma and spreads by natural lymphatics to treat sites of lymphatic metastases. Human Gene Therapy. 2002;13(10):1213–23. doi: 10.1089/104303402320138998. [DOI] [PubMed] [Google Scholar]
- 15.Wong RJ, Kim SH, Joe JK, Shah JP, Johnson PA, Fong Y. Effective treatment of head and neck squamous cell carcinoma by an oncolytic herpes simplex virus. Journal of the American College of Surgeons. 2001;193(1):12–21. doi: 10.1016/s1072-7515(01)00866-3. [DOI] [PubMed] [Google Scholar]
- 16.McAuliffe PF, Jarnagin WR, Johnson P, Delman KA, Federoff H, Fong Y. Effective treatment of pancreatic tumors with two multimutated herpes simplex oncolytic viruses. Journal of Gastrointestinal Surgery. 2000;4(6):580–8. doi: 10.1016/s1091-255x(00)80106-7. [DOI] [PubMed] [Google Scholar]
- 17.Stiles BM, Bhargava A, Adusumilli PS, Stanziale SF, Kim TH, Rusch VW, et al. The replication-competent oncolytic herpes simplex mutant virus NV1066 is effective in the treatment of esophageal cancer. Surgery. 2003;134(2):357–64. doi: 10.1067/msy.2003.244. [DOI] [PubMed] [Google Scholar]
- 18.Varghese S, Rabkin SD. Oncolytic herpes simplex virus vectors for cancer virotherapy. Cancer Gene Ther. 2002;9(12):967–78. doi: 10.1038/sj.cgt.7700537. [DOI] [PubMed] [Google Scholar]
- 19.Stanziale SF, Petrowsky H, Adusumilli PS, Ben Porat L, Gonen M, Fong Y. Infection with oncolytic herpes simplex virus-1 induces apoptosis in neighboring human cancer cells: a potential target to increase anticancer activity. Clinical Cancer Research. 2004;10(9):3225–32. doi: 10.1158/1078-0432.ccr-1083-3. [DOI] [PubMed] [Google Scholar]
- 20.Wong RJ, Chan MK, Yu Z, Ghossein RA, Ngai I, Adusumilli PS, et al. Angiogenesis inhibition by an oncolytic herpes virus expressing interleukin 12. Clinical Cancer Research. 2004;10(13):4509–16. doi: 10.1158/1078-0432.CCR-04-0081. [DOI] [PubMed] [Google Scholar]
- 21.Kim SH, Wong RJ, Kooby DA, Carew JF, Adusumilli PS, Patel SG, et al. Combination of mutated herpes simplex virus type 1 (G207 virus) with radiation for the treatment of squamous cell carcinoma of the head and neck. European Journal Of Cancer. 2005;41(2):313–22. doi: 10.1016/j.ejca.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Adusumilli PS, Stiles BM, Chan MK, Chou TC, Wong RJ, Rusch VW, et al. Radiation therapy potentiates effective oncolytic viral therapy in the treatment of lung cancer. Annals Of Thoracic Surgery. 2005;80(2):409–16. doi: 10.1016/j.athoracsur.2005.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stanziale SF, Stiles BM, Bhargava A, Kerns SA, Kalakonda N, Fong Y. Oncolytic herpes simplex virus-1 mutant expressing green fluorescent protein can detect and treat peritoneal cancer. Hum Gene Ther. 2004;15(6):609–18. doi: 10.1089/104303404323142051. [DOI] [PubMed] [Google Scholar]
- 24.Gahlen J, Stern J, Laubach HH, Pietschmann M, Herfarth C. Improving diagnostic staging laparoscopy using intraperitoneal lavage of delta-aminolevulinic acid (ALA) for laparoscopic fluorescence diagnosis. Surgery. 1999;126(3):469–73. [PubMed] [Google Scholar]
- 25.Gahlen J, Prosst RL, Pietschmann M, Haase T, Rheinwald M, Skopp G, et al. Laparoscopic fluorescence diagnosis for intraabdominal fluorescence targeting of peritoneal carcinosis experimental studies. Ann Surg. 2002;235(2):252–60. doi: 10.1097/00000658-200202000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chishima T, Miyagi Y, Wang X, Yamaoka H, Shimada H, Moossa AR, et al. Cancer invasion and micrometastasis visualized in live tissue by green fluorescent protein expression. Cancer Res. 1997;57(10):2042–7. [PubMed] [Google Scholar]
- 27.Bouvet M, Wang J, Nardin SR, Nassirpour R, Yang M, Baranov E, et al. Real-time optical imaging of primary tumor growth and multiple metastatic events in a pancreatic cancer orthotopic model. Cancer Research. 2002;62(5):1534–40. [PubMed] [Google Scholar]
- 28.Hoffman RM. Orthotopic transplant mouse models with green fluorescent protein-expressing cancer cells to visualize metastasis and angiogenesis. Cancer & Metastasis Reviews. 1998;17(3):271–7. doi: 10.1023/a:1006188412324. [DOI] [PubMed] [Google Scholar]
- 29.Stiles B, Adusumilli P, Bhargava A, Stanziale S, Kim T, Chan M, et al. Minimally-invasive localization of oncolytic herpes simplex viral therapy of micrometastatic pleural cancer Cancer Gene Therapy 2005. epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]