Abstract
The Mycobacterium avium complex consists of epidemiologically distinct subsets. The classification of these subsets is complicated by a number of factors, including the ambiguous results obtained with phenotypic and genetic assays and the recent appreciation that human and avian strains appear to be distinct. In previous work, sequencing based on a 441-bp portion of the hsp65 gene has proven to efficiently classify isolates within the Mycobacterium genus but provides low resolution for distinguishing among members of the M. avium complex. Therefore, in this study, we have targeted the more variable 3′ region of the hsp65 gene to determine whether it can effectively discriminate M. avium complex isolates at the levels of species and subspecies. Primers designed for this target consistently generated amplicons for all organisms classified as M. avium complex. Sequences obtained indicate that M. intracellulare is genetically divergent from M. avium organisms, and distinct sequevars were obtained for M. avium subsets, including M. avium subsp. avium (bird type), M. avium subsp. hominissuis, and M. avium subsp. paratuberculosis. In addition, sequence differences served to distinguish bovine from ovine strains of M. avium subsp. paratuberculosis. A unique profile for M. avium subsp. silvaticum was not obtained. These results indicate that sequencing the 3′ region of the hsp65 gene can simply and unambiguously distinguish species and subspecies of the M. avium complex.
Members of the Mycobacterium avium complex (MAC) are ubiquitous in the environment and have a wide source range, causing disease in various mammals and birds, opportunistic infections in immunocompromised patients, and pulmonary disease in both immunocompromised and immunocompetent patients. Different subsets of the MAC preferentially infect different ranges of mammalian hosts (19). Second only to the Mycobacterium tuberculosis complex, members of the MAC are the mycobacteria most frequently isolated from humans in developed countries. Traditionally, MAC has been described as a single entity comprising M. avium and M. intracellulare, although their separation into distinct species from each other was first shown by DNA-DNA hybridization studies more than two decades ago (1). The development and use of various biochemical and molecular tools have delineated the MAC into further groupings, depending on the approach used, which has led to an understanding that it consists of a heterogeneous group of strains with varying epidemiologic implications. In 1990, M. avium was divided into three subspecies: M. avium subsp. avium, M. avium subsp. paratuberculosis, and M. avium subsp. silvaticum (34). More recently, a fourth subspecies, M. avium subsp. hominissuis, has been described to reflect the distinction of human and porcine isolates from bird-type strains (23).
Classification of M. avium complex isolates was conventionally based on a range of laboratory phenotypes, including serotype analysis, but over the last 15 years there has been an increasing use of molecular tests. One phenotypic assay widely employed examines the dependence on mycobactin J for in vitro growth of M. avium subsp. paratuberculosis (34). However, mycobactin dependency was also ascribed to the wood pigeon mycobacteria, later named M. avium subsp. silvaticum, although for this organism, the requirement appears to pertain only to primary isolation and not subsequent in vitro culture (39). The inability to grow on egg media and stimulation of growth by pyruvate have been described as characteristics of M. avium subsp. silvaticum (34). The difficulty in assigning species identification by such phenotypic methods has fostered efforts to characterize MAC isolates by molecular tests. Methods such as restriction enzyme analysis (30), detection of species-specific insertion elements (5, 13, 17), and sequencing of certain targets (12, 30, 31) have been applied to identify the various entities of MAC. For a variety of reasons, not all molecular tools lend themselves to all subsets; for instance, sequencing of the 16S rRNA gene and the 16S-23S internal transcribed spacer sequence (ITS1) is not sufficiently discriminatory to differentiate between some of these organisms. Among the most widely used methods has been the testing of species-specific insertion sequences. Despite the advantages of using these generally high-copy-number elements, there are at least 10 different insertion sequences found or characterized in MAC (8), some of them related, and cross-hybridization with PCR primers or probes used in restriction fragment length polymorphism (RFLP) procedures can potentially be misleading (14, 16). In the case of M. avium subsp. paratuberculosis, which can be very difficult to grow and may at times only be detected by molecular methods in liquid culture, an alternate target to complement IS900 PCR may prove useful to confirm the presence of M. avium subsp. paratuberculosis organisms.
The groEL2 gene, also known as the 65-kDa heat shock protein gene or hsp65 gene, has been a very useful tool in mycobacterial diagnostics for over a decade; this method was first implemented by Telenti et al. as a PCR-restriction enzyme analysis (PRA) method and employs a 441-bp fragment of the >1,600-bp complete gene (33). Overlapping PRA patterns among species and interlaboratory inconsistency in fragment size interpretation are drawbacks of the method, but as sequencing facilities are becoming more accessible, sequencing of this 441-bp fragment may increasingly be used instead of PRA as a means of molecular identification for Mycobacterium species. Sequencing the 441-bp fragment is able to differentiate among the great majority of 111 species of mycobacteria, with the exception of some species such as members of the M. tuberculosis complex and subspecies of the MAC (22). Evaluation of the MAC by hsp65 sequencing or PRA has revealed a number of subsets, with the greatest variety among M. intracellulare strains, along with two common groups for M. avium strains (31). As the 441-bp fragment was chosen to target a region of high conservation across the mycobacterial genus, we postulated that sequencing of the remainder of this gene might reveal greater variability, consistent with a PRA-based comparison of M. avium subsets including M. avium subsp. paratuberculosis by use of a 960-bp fragment of hsp65 (10). An in silico analysis of the groEL2 gene in the M. avium subsp. paratuberculosis K-10 genome sequence (MAP3936; GenBank no. AE016958) compared to the corresponding sequence segment of the M. avium 104 genome available from TIGR (http://www.tigr.org/) revealed the presence of a total of 11 single-nucleotide polymorphisms (SNPs), none in the 441-bp region, prompting us to further investigate the feasibility of using a different part of hsp65 as a genotyping method for the systematic identification of the various MAC subsets, with a focus on those of the M. avium species.
MATERIALS AND METHODS
Mycobacterial strains and identification.
Strains or DNAs were obtained from several hosts and geographical locations and differed in numerous features (Table 1). Some of these have been previously characterized in other studies (23, 28, 29), with the basic findings as follows. All strains of M. avium subsp. paratuberculosis were identified on the basis of mycobactin J dependence and the presence of the IS900 element. Additionally, these isolates were classified as exhibiting a cattle or sheep genotype on the basis of a M. avium subsp. paratuberculosis-specific PCR that distinguished between these host-associated types (4). All other strains were identified as M. avium or M. intracellulare by Accuprobe and/or sequencing of the 16S rRNA gene or the ITS1. Strains of the bird type were identified on the basis of presenting with a three-band bird-type RFLP pattern (23, 26) due to nonstringent hybridization to two copies of IS1311 and one copy of IS1245 (3, 13). All strains of the M. avium subspecies (i.e., excluding M. intracellulare) were also tested for the IS901 element (17) by use of in-house primers IS901L (5′-GCGCTGAGTTCCTCGTAAT-3′) and IS901R (5′-CTTCGATCTTGAGGCTGGA-3′). Attempts were made to span a wide range of isolates for best representation; these isolates included the following: M. avium subsp. paratuberculosis strains of the bovine type (n = 10), M. avium subsp. paratuberculosis strains of the ovine type (n = 5), M. avium subsp. silvaticum (n = 3), M. avium subsp. avium strains known to have a bird-type IS1245 RFLP pattern or considered a bird-type reference strain (n = 8), M. avium subsp. hominissuis strains from humans (n = 27), miscellaneous M. avium strains from animals and cigarette or reference strains (n = 8), M. intracellulare strains (n = 10), and M. chimaera strains (n = 2) for a total of 73 strains.
TABLE 1.
Strains and associated molecular characteristics evaluated in this studya
| Speciesh | Strain | Host | Isolate origin | IS901 | ITS1 | Hsp65 codeb | Additional comment |
|---|---|---|---|---|---|---|---|
| M. avium subsp. avium | ATCC 25291T | Chicken | + | Mav-A | 4 | Bird type RFLPc | |
| M. avium subsp. avium | ATCC 35713 | Chicken | + | Mav-A | 4 | PPD-avian | |
| M. avium subsp. avium | R13 | Crane | The Netherlands | + | Mav-A | 4 | Bird type RFLP |
| M. avium subsp. avium | D71076 | Owl | The Netherlands | + | Mav-A | 4 | Bird type RFLP |
| M. avium subsp. avium | IWGMT 26 | Reference | + | Mav-A | 4 | Bird type RFLP | |
| M. avium subsp. avium | IWGMT 17 | Reference | + | Mav-A | 4 | Bird type RFLP | |
| M. avium subsp. avium | 222 | Sheep | + | Mav-A | 4 | Bird type RFLP | |
| M. avium subsp. avium | IWGMT 55 | Reference | + | Mav-C | 4 | Bird type RFLP | |
| M. avium subsp. silvaticum | ATCC 49884T | Wood pigeon | France | + | Mav-A | 4 | |
| M. avium subsp. silvaticum | 9801574 | Wood pigeon | Belgium | + | Mav-A | 4 | Silvaticum type RFLP |
| M. avium subsp. silvaticum | 9800851 | Bird | Belgium | + | Mav-A | 4 | Silvaticum type RFLP |
| M. avium subsp. hominissuis | ATCC 49601 | Human | United States | − | Mav-A | 1 | AIDS patient |
| M. avium subsp. hominissuis | 1464bd | Deer | New Zealand | − | Mav-A | 1 | |
| M. avium subsp. hominissuis | 9601596 | Human | The Netherlands | − | Mav-A | 1 | |
| M. avium subsp. hominissuis | ATCC 700897 | Human | United States | − | Mav-B | 1 | AIDS patient; macrolide susceptible |
| M. avium subsp. hominissuis | ATCC 700898 | Human | United States | − | Mav-B | 1 | AIDS patient; macrolide resistant |
| M. avium subsp. hominissuis | 103 | Human | United States | − | Mav-B | 1 | AIDS patient |
| M. avium subsp. hominissuis | 104 | Human | United States | − | Mav-B | 1 | AIDS patient; sequenced genome (The Institute for Genomic Research) |
| M. avium subsp. hominissuis | 105 | Human | United States | − | Mav-B | 1 | AIDS patient |
| M. avium subsp. hominissuis | IWGMT 28 | Reference | − | Mav-D | 1 | Multiband RFLP | |
| M. avium subsp. hominissuis | 9500606 | Human | − | Mav-B | 1 | COPDg patient; multiband RFLP | |
| M. avium subsp. hominissuis | 9601034 | Human | − | Mav-B | 1 | COPD patient; multiband RFLP | |
| M. avium subsp. hominissuis | FCC 1803 | Bilby | Australia | − | Mav-B | 1 | |
| M. avium subsp. hominissuis | 9700642 | Pig | − | Mav-B | 1 | Multiband RFLP | |
| M. avium subsp. hominissuis | FCC 268 | Australia | − | Mav-B | 1 | ||
| M. avium subsp. hominissuis | 2000-337 | Human | United States | − | Mav-A | 2 | |
| M. avium subsp. hominissuis | 9801022 | Cigarette | − | Mav-A | 2 | ||
| M. avium subsp. hominissuis | 102 | Human | United States | − | Mav-B | 2 | AIDS patient |
| M. avium subsp. hominissuis | 108 | Human | United States | − | Mav-B | 2 | AIDS patient |
| M. avium subsp. hominissuis | 9702462 | Human | − | Mav-B | 2 | Multiband RFLP; HIV+ | |
| M. avium subsp. hominissuis | 74301 | Human | Canada | − | Mav-B | 2 | |
| M. avium subsp. hominissuis | 76102 | Human | Canada | − | Mav-B | 2 | |
| M. avium subsp. hominissuis | 976 | Human | Canada | − | Mav-B | 2 | |
| M. avium subsp. hominissuis | 98838 | Human | Canada | − | Mav-B | 2 | |
| M. avium subsp. hominissuis | 63787 | Human | Canada | − | Mav-B | 2 | |
| M. avium subsp. hominissuis | 62490 | Human | Canada | − | Mav-B | 2 | |
| M. avium subsp. hominissuis | 395 | Human | Canada | − | Mav-B | 2 | |
| M. avium subsp. hominissuis | 9701650 | Human | The Netherlands | − | Mav-A | 3 | Multiband RFLP; HIV+ |
| M. avium subsp. hominissuis | 9701648 | Human | The Netherlands | − | Mav-A | 3 | Multiband RFLP; HIV+ |
| M. avium subsp. hominissuis | 28132 | Human | Canada | − | Mav-B | 3 | |
| M. avium subsp. hominissuis | 9700752 | Human | − | Mav-B | 3 | Multiband RFLP; HIV+ | |
| M. avium subsp. hominissuis | 9601934 | Human | − | Mav-B | 3 | Multiband RFLP; HIV+ | |
| M. avium subsp. hominissuis | 95/4920-679 | Cow | New Zealand | + | Mav-A | 4 | |
| M. avium subsp. hominissuis | 2000-333 | Human | United States | − | Mav-G | 7 | |
| M. avium subsp. hominissuis | HMC36 | Human | United States | − | Mav-A | 8 | |
| M. avium subsp. hominissuis | 822 | African buffalo | Kruger animal park | − | Mav-B | 9 | |
| M. avium subsp. paratuberculosis | K-10 | Cow | United States | − | Mav-A | 5 | Cow typed |
| M. avium subsp. paratuberculosis | MAP 17 | Bison | Canada | − | Mav-A | 5 | Cow type |
| M. avium subsp. paratuberculosis | 6024 | Cow | New Zealand | − | Mav-A | 5 | Cow type; RFLP M. avium subsp. paratuberculosis C1e |
| M. avium subsp. paratuberculosis | 1997-1088 | Cow | Czech Republic | − | Mav-A | 5 | Cow type |
| M. avium subsp. paratuberculosis | ATCC 19698T | Cow | − | Mav-A | 5 | Cow type | |
| M. avium subsp. paratuberculosis | ATCC 43015 | Human | United States | − | Mav-A | 5 | Cow type; strain Linda |
| M. avium subsp. paratuberculosis | ATCC 43544 | Human | United States | − | Mav-A | 5 | Cow type; strain Ben |
| M. avium subsp. paratuberculosis | 1997-1779 | Rabbit | United Kingdom | − | Mav-A | 5 | Cow type |
| M. avium subsp. paratuberculosis | 7296 | Deer | New Zealand | − | Mav-A | 5 | Cow type; RFLP M. avium subsp. paratuberculosis C8 |
| M. avium subsp. paratuberculosis | 6354 | Deer | New Zealand | − | Mav-A | 5 | Cow type; RFLP M. avium subsp. paratuberculosis C1 |
| M. avium subsp. paratuberculosis | LN20 | Pig | Canada | − | Mav-A | 6 | Sheep type |
| M. avium subsp. paratuberculosis | 3579 | Deer | New Zealand | − | Mav-A | 6 | Sheep type; RFLP M. avium subsp. paratuberculosis S1 |
| M. avium subsp. paratuberculosis | 575A | Sheep | South Africa | − | Mav-A | 6 | Sheep type; RFLP M. avium subsp. paratuberculosis S2 |
| M. avium subsp. paratuberculosis | P465 | Sheep | Iceland | − | Mav-A | 6 | Sheep type; RFLP M. avium subsp. paratuberculosis S4 |
| M. avium subsp. paratuberculosis | 85-14 | Sheep | Canada | − | Mav-A | 6 | Sheep type; RFLP M. avium subsp. paratuberculosis S6 |
| M. intracellulare | MB055329 | Human | Canada | − | MAC-U | 1 | Unique species per 16S rRNA data (refer to text) |
| M. intracellulare | ATCC 13950T | Human | ND | Min-A | 10 | ||
| M. intracellulare | ATCC 35761T | Human | ND | Min-A | 10 | Source of PPD-B | |
| M. intracellulare | 74925 | Human | Canada | ND | Min-A | 10 | |
| M. intracellulare | FCC 1804 | Potoroo | Australia | ND | Min-A | 11 | |
| M. intracellulare | FCC 375 | Numbat | Australia | NDi | Min-A | 11 | |
| M. intracellulare | 68959 | Human | Canada | ND | Min-A | 11 | |
| M. intracellulare | 96006 | Human | Canada | ND | Min-A | 12 | |
| M. intracellulare | 90331 | Human | Canada | ND | Min-A | 14 | |
| M. intracellulare | FCC389 | Bovine | Australia | ND | Uniquef | 13 | |
| M. chimaera | 74023 | Human | Canada | ND | MAC-A | 13 | |
| M. chimaera | MI-JC | Human | Canada | ND | MAC-A | 13 |
The species designation given was based on previous knowledge of strain characteristics.
IS1245/IS1311 RFLP pattern types were determined for M. avium subsp. avium, M. avium subsp. silvaticum, and M. avium subsp. hominissuis isolates (37).
M. avium subsp. paratuberculosis cow type or sheep type determined by PCR as described by Collins et al. (4).
IS900 RFLP pattern types was determined for M. avium subsp. paratuberculosis isolates (6).
ITS1 sequevar: 1 bp from Min-B.
COPD, chronic obstructive pulmonary disease.
Strains that did not show characteristics that indicated that they belonged to other groups were ascribed to M. avium subsp. hominissuis.
ND, not done.
Primer design.
Primers to amplify the complete or near-complete hsp65 gene were designed with the help of the Primer3 web site (27) by use of the complete hsp65 gene sequence (1,626 bp) and approximately 200 bp upstream and downstream from the genome sequence of M. avium subsp. paratuberculosis K10 (MAP3936). The resulting primers spanned position 12 of the gene coding sequence (primer MAChsp65F: 5′-AATTGCGTACGACGAAGAGG-3′) to 6 bases downstream of the terminator codon (primer MAChsp65R: 5′-ACGGACTCAGAAGTCCATGC-3′), amplifying a 1,621-bp fragment. Another primer was designed to amplify only the 3′ portion of the gene to obtain a 1,059-bp PCR product beginning at position 574 (primer MAChspF_574: 5′-CGGTTCGACAAGGGTTACAT-3′, paired with primer MAChsp65R), encompassing all of the 11 SNPs between M. avium 104 and M. avium subsp. paratuberculosis K-10. The nature of the gene did not permit the design of universal mycobacterial primers for amplification of the near-complete gene when aligned against the M. tuberculosis and M. marinum-ulcerans complexes, M. leprae, or M. smegmatis. However, the goal was to characterize members of the M. avium complex and therefore this was deemed satisfactory.
PCR and sequencing of the ITS1 and hsp65.
Amplification of the ITS1 or the hsp65 gene was performed in a 50 μl final reaction volume consisting of 5 to 50 ng of DNA template, 2.5 mM MgCl2, 1× PCR buffer (Invitrogen, Carlsbad, CA), 5 μl of 50% acetamide, 0.2 mM deoxynucleoside triphosphates, 0.5 μM of each primer, and 1 U of Taq DNA polymerase (Invitrogen). Primers used for amplification and sequencing of the hsp65 gene were as described above. Primers used for amplification of the ITS1 fragment were Ec16S.1390F (5′-TTGTACAACACCGCCCGTC-3′) and MB23S.44nR (5′-TCTCGATGCCAAGGCATC-3′) (12). PCR was performed using Applied Biosystems Gene Amp PCR system 2700 and the following conditions: 94°C for 3 min; 35 cycles with intervals of 94°C (45 s), 55°C (45 s), and 72°C (1 min); and 72°C for 10 min followed by holding of the reaction mixture at 4°C. A 5-μl volume of the PCR product was run on a 1.5% agarose gel containing ethidium bromide to check for successful amplification of the desired product. The remaining PCR product was submitted to a core sequencing facility (McGill University and Génome Québec Innovation Centre) for sequencing on a 3730XL DNA Analyzer system. The same primers used for PCR served for the sequencing of forward and reverse fragments. The resulting chromatograms were manually edited to ensure sequence accuracy and added to the alignment component of MEGA 3.1 (15). Sequence comparisons of ITS1 were performed by BLAST analysis using sequences in NCBI (http://www.ncbi.nlm.nih.gov/) with referral to published data (12, 23). Sequence comparisons of the 3′ portion of the hsp65 gene were performed in-house using only the strains tested in this study, since availability of data spanning this region in public databases is limited. Comparative analyses spanning the 441-bp region were performed by BLAST analysis in NCBI. Phylogeny reconstruction of all sequence alignments was performed in MEGA 3.1 using the neighbor-joining method.
Nucleotide accession numbers.
Sequences of the nearly complete hsp65 gene representing each sequevar recognized in this study (codes 1 to 14) were deposited in GenBank under accession numbers DQ284765 to DQ284778.
RESULTS
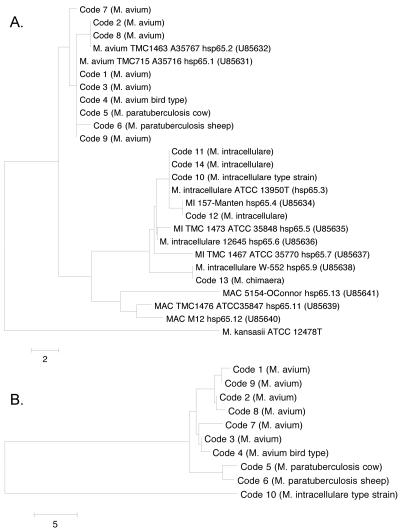
In all, 14 different hsp65 sequevars were identified among the M. avium complex isolates tested: 9 for the M. avium strains and a further 5 for the M. intracellulare and M. chimaera isolates (Table 2 and Table 3). These 14 sequevars, hereafter called hsp65 codes 1 to 14, were resolved into eight variants in examinations of the 441-bp region of the gene alone (Fig. 1). The 441-bp fragment provided adequate variability in distinguishing between the species M. avium, M. intracellulare, and M. chimaera. However, within a species, M. avium in particular, the use of the 3′ region of hsp65 provided much greater discrimination, at once differentiating subspecies and even M. avium subsp. paratuberculosis host-associated types. Results are described below in detail as a function of the groups to which isolates had been previously assigned.
TABLE 2.
SNPs among the M. avium strains in comparison with prototype strain M. avium 104 (MAA104)a
| Representative strain or feature (hsp65 code no.) | Nucleotide at indicated base pair positionb
|
n | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 240 | 276 | 324 | 612 | 633 | 645 | 861 | 1092 | 1128 | 1136* | 1218 | 1269 | 1272 | 1296 | 1350 | 1435* | 1468* | 1488 | 1536 | ||
| MAA104 (1) | C | G | C | C | C | C | G | G | C | C | A | G | C | C | G | A | G | G | A | |
| 76021 (2) | · | · | T | · | · | · | · | · | · | · | G | · | · | · | · | · | · | · | G | 3 |
| 28132 (3) | · | · | · | · | · | · | · | · | G | · | G | C | G | · | · | · | · | · | G | 5 |
| MAA bird (4) | · | · | · | · | · | T | · | · | G | · | G | C | G | · | · | · | · | · | G | 6 |
| MAP cow (5) | · | · | · | G | G | · | T | · | G | · | G | T | G | A | · | · | A | C | G | 11 |
| MAP sheep (6) | T | · | · | G | G | · | T | · | G | · | G | C | G | · | · | G | A | C | G | 12 |
| 2000-333 (7) | · | C | · | · | · | · | · | A | G | · | · | C | G | · | A | · | · | · | G | 7 |
| HMC36 (8) | · | · | T | · | · | · | · | · | · | T | G | · | · | · | · | · | · | · | G | 4 |
| 822 (9) | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | G | 1 |
Numbers represent base pair positions on the complete hsp65 gene; n = number of SNPs from the prototype strain MAA 104. Positions between bp 145 and 585 are represented within the 441-bp region used by Telenti et al (33). Positions 612 and above are located in the 3′ region amplified by primers MAChspF_574 and MAChsp65R.
Asterisks denote nonsynonymous positions.
TABLE 3.
SNPs among M. intracellulare and related strains in comparison with ATCC 13950T (M. intracellulare type)
| Representative strain or feature (hsp65 code no.) | Nucleotide at indicated base pair positiona
|
No. of SNPs from M. intracellulare ATCC 13950T | No. of SNPs from M. avium str. 104 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 93 | 102 | 132 | 192 | 249 | 279 | 285 | 459 | 1371 | 1423* | 1467 | |||
| MI type (10) | C | T | T | G | C | G | T | C | G | C | C | 65 | |
| FCC1804 (11) | · | · | · | · | · | · | · | · | · | · | T | 1 | 66 |
| 96006 (12) | · | · | · | · | · | · | · | T | · | · | · | 1 | 66 |
| MI_JCb (13) | T | C | C | T | T | T | C | · | · | T | · | 8 | 71 |
| 90331 (14) | · | · | · | · | · | · | · | · | A | · | T | 2 | 67 |
Asterisk denotes a nonsynonymous position.
Identified as M. chimaera based on the ITS1 results (MAC-A).
FIG. 1.
(A) Phylogenetic analyses of hsp65 sequence data spanning 360 bp of the 441-bp fragment with previously described sequevars (GenBank accession numbers in parentheses) (31), with M. kansasii ATCC 12478T used as the outgroup. This region corresponds to hsp65 positions 190 to 549. (B) Phylogenetic analysis of the 3′ region of hsp65, from positions 612 to 1588, for the M. avium complex subsets shown in panel A. For the 3′ region, M. intracellulare ATCC 13950T (code 10) is provided as the outgroup to emphasize the distinctions among codes 1 through 9. The bar scale represents the number of base pair variations.
M. avium subsp. avium group.
M. avium strains known to be bird type all contained a hsp65 sequevar (code 4) that was unique compared to the rest of the MAC. This group included the type strain M. avium ATCC 25291T. Additionally, these isolates all tested positive for the IS901 element by PCR. These isolates were generally uniform by ITS1 sequencing and contained the Mav-A ITS1 allele, with the exception of IWGMT 55 (also known as strain 6194) (26), which had a Mav-C allele as previously described (12). Additionally, one cattle isolate (95/4920-679) for which limited information was available also shared the characteristics of M. avium subsp. avium in that it was code 4 and IS901 positive by PCR.
M. avium subsp. silvaticum group.
In common with the so-called bird strains in our panel, three strains previously identified as M. avium subsp. silvaticum, including the type strain ATCC 49884, were positive for IS901 and had a code 4 hsp65 sequevar. Each also presented with a Mav-A ITS1 sequence. These results challenge the designation M. avium subsp. silvaticum, as it does not appear to be clearly distinct from M. avium subsp. avium.
M. avium subsp. hominissuis group.
The remaining strains of M. avium, most of which were assumed to be of the subspecies hominissuis, constituted a heterogeneous group with six different hsp65 sequevars that were consistently distinct from those seen for M. avium subsp. avium and M. avium subsp. paratuberculosis. The most divergent strain, representing code 7, had four to eight SNPs compared to the other five sequevars; its genetic distinction from the others was supported by ITS1 sequencing, and this was the only isolate tested that had the Mav-G ITS1 sequevar. Of the other five sequevars, M. avium 104 was represented in one group (code 1), while the remaining four groups (codes 2, 3, 8, and 9) had one to five base variations compared to strain 104 (Table 2). hsp65 sequevar code 3 differed from the bird type (code 4) by one base only, at position 645, but all code 3 isolates tested were IS901 negative.
M. avium subsp. paratuberculosis group.
Among the 15 strains of M. avium subsp. paratuberculosis tested, only two hsp65 sequevars were detected and these divided perfectly between the bovine and ovine forms. In comparison to M. avium subsp. avium 104 strain, M. avium subsp. paratuberculosis isolates identified as the bovine type by PCR shared the same 11 SNPs as strain M. avium subsp. paratuberculosis K-10. M. avium subsp. paratuberculosis isolates identified as sheep type had 12 SNPs with respect to M. avium subsp. avium 104; as these were not all the same SNPs seen in bovine type M. avium subsp. paratuberculosis, we uncovered 3 SNPs that consistently differed between the two M. avium subsp. paratuberculosis groups. Most M. avium subsp. paratuberculosis-associated SNPs were synonymous, but one nonsynonymous SNP distinguished M. avium subsp. paratuberculosis from non-M. avium subsp. paratuberculosis isolates and another nonsynonymous SNP distinguished bovine from ovine M. avium subsp. paratuberculosis.
M. intracellulare group.
Strains of M. intracellulare were occasionally more difficult to amplify, likely due to the high level of sequence variation between it and M. avium (>65 SNPs throughout the complete gene; 96% identity [present study]) from which primers were designed. Despite this, five sequevars were identified in our small selection of M. intracellulare (identified by a Min-A ITS1 sequevar) and M. chimaera (identified by a MAC-A ITS1 sequevar) strains. The separation of M. intracellulare from the rest of the MAC is clearly evident here (Fig. 1A) and either challenges the common practice of uniting M. intracellulare and M. avium into one entity or argues for the inclusion of M. chimaera in the complex. An interesting observation made among the strains of M. chimaera and M. intracellulare was that unlike the M. avium and M. avium subsp. paratuberculosis groups, the majority of SNPs were situated within the first 500 bases of the gene, including seven of the eight differences between M. chimaera and M. intracellulare. M. intracellulare strain 96006 (hsp65 code 12), with only one SNP compared to M. intracellulare ATCC 13950T at bp 459, cannot be distinguished using only sequence from the 3′ end of the gene. In the 441-bp fragment region, each of our M. intracellulare sequevars corresponded to previously documented sequences of hsp65.3, hsp65.4, and hsp65.9 (Fig. 1A). M. chimaera is represented by the hsp65.9 sequence but has not been documented as such, since M. chimaera has only recently been described (35).
Miscellaneous.
One isolate, MB055329, presented with an initially confounding genotype. It had a MAC-U ITS1 and a 16S rRNA gene sequence identity with strain HSC 1852 (AY184225). HSC 1852 shows four SNPs from M. avium ATCC 25291T and five SNPs from M. intracellulare ATCC 13950T in the entire 16S rRNA gene. Similarity search results against the RIDOM database (http://www.ridom-rdna.de/) indicated 100% identity with reference strain M. intracellulare S350 (not the type strain). However, in our study the hsp65 gene sequence of strain MB055329 had perfect sequence identity with that of M. avium 104 (code 4). A recent publication by Lebrun et al. aimed at providing clarification of the identity of MAC strains with an ambiguous result, particularly those which were identified as “MAIS” but which could not be assigned to the species M. avium, M. intracellulare, or M. scrofulaceum, by use of an ITS1-based oligonucleotide system (18). In that study, the lack of correlation between the three sequence targets (16S rRNA, ITS1, and hsp65) was striking among subsets with the MAC-U ITS1 sequevar: the hsp65 sequence suggested an identification of M. avium, while the 16S rRNA data indicated M. intracellulare, as seen for our isolate MB055329. Strains represented by this novel 16S rRNA gene sequence and sharing similar genotypic characteristics observed for our isolate are currently being evaluated for their validation as a new species (M. J. Garcia, personal communication).
DISCUSSION
The definition of what constitutes the M. avium complex is often reflective of the annotation given in the pre-molecular era, when M. avium and M. intracellulare and related strains were virtually indistinguishable by conventional phenotypic testing. From genetic and, more recently, genomic analysis, the taxonomy and the epidemiology of the M. avium complex are being reevaluated using different approaches and to achieve different goals. While it may still be practical to continue grouping M. avium and M. intracellulare together in a clinical setting, future epidemiological or functional studies will likely benefit from genotypic characterization to give confidence in knowing what exactly is being studied. As well, the taxonomic reclassification of M. paratuberculosis as a subspecies of the M. avium complex in 1990 has not been unanimously embraced because of the marked differences in laboratory and epidemiology characteristics of M. avium subsp. paratuberculosis versus the rest of the M. avium complex. Nevertheless, the high level of gene homology between these organisms suggests that these differences may be due to genetic or genomic events that occur among MAC species (24, 28, 29) rather than to the clear emergence of a new species lineage. Our data, using sequences from a single housekeeping gene, provide a simple approach to characterizing MAC isolates and addressing some of these questions.
The discriminatory power of the 3′ region of the hsp65 gene is evident in comparisons with analyses resulting from the commonly used 441-bp portion. The nine 3′ hsp65 codes among the M. avium subspecies described here are represented by only four 441-bp sequevars. Codes 1 and 2 represented sequevars within the 441-bp fragment already documented in the literature: hsp65.1 and hsp65.2 (31). However, five and two of the nine M. avium subsets could be grouped in the hsp65.1 and hsp65.2 categories, respectively (Fig. 1A). It is not unwise to assume that due to the existence of other 441-bp sequevars not detected in our panel of isolates, a greater number of 3′ hsp65 variants among M. avium could surface in future studies.
Clear genotypic and host-associated distinctions made in previous studies are confirmed and further resolved with hsp65-based sequence analysis. This includes M. avium subsp. avium (bird strains), which is identified by a unique and consistent hsp65 sequence, as well as M. avium subsp. paratuberculosis of the bovine type, and M. avium subsp. paratuberculosis of the ovine type. Sequence conservation may be reflective of pathogenic or host-specific mycobacteria such as previously observed for M. tuberculosis, M. leprae, and M. avium subsp. paratuberculosis (11), a hypothesis also supported by our hsp65 data. Furthermore, this tool may prove useful in the characterization of newly recognized, host-related, and epidemiologically important subsets, for example, the bison type M. avium subsp. paratuberculosis group characterized by a distinct IS1311 allele from cow and sheep types and found among United States bison isolates (40) and Indian goats and sheep isolates (I. Sevilla, S. V. Singh, J. M. Garrido, G. Aduriz, S. Rodríguez, M. V. Geijo, R. J. Whittington, V. Saunders, R. H. Whitlock, R. A. Juste, Abstr. 8th Int. Coll. Paratuberculosis, abstr. 115, 2005).
Not only did our hsp65 data serve to subdivide classification by 16S rRNA gene sequencing, we were able to find an association for the presence of the IS901 insertion elements with bird-derived M. avium (code 4), as previously documented (2, 25). Importantly, this group is associated with virulence in this host (3, 8, 25) and found in lesion-associated MAC isolates from deer, cattle, and pigs (3).
Molecular diagnostics of the M. avium complex have often included amplification of species or subspecies-specific insertion elements, particularly with M. avium subsp. paratuberculosis. A benefit of using a sequence-based assay to brand such organisms is the elimination of confusion surrounding insertion element specificity or lack thereof. We found that the presence of insertion elements was in fact unequivocally associated with specific hsp65 sequevars (IS901 with code 4 and IS900 with codes 5 and 6) and therefore confirms that the limitations in relying on insertion sequence elements for diagnostics is not in the specificity of the element but in test performance itself, where nonspecific detection of similar insertion elements in other species can occur (7, 9, 32).
The taxonomic validation of M. avium subsp. silvaticum remains uncertain. The molecular data presented here strongly suggest that M. avium subsp. silvaticum strains are simply M. avium subsp avium. Strains identified as M. avium subsp. silvaticum have previously been described as having a three-band IS1245/IS1311 RFLP pattern that is in some cases identical to the typical three-band bird pattern and in other cases shows a one-band difference (8). The single-band difference likely represents an IS1311 band (based on observations from references 14, 8, and 3), and therefore it appears that bird-type and silvaticum-type isolates have the same single-band IS1245 profile. Very few strains of M. avium subsp. silvaticum are documented in the literature and when documented as such may be the same few used across various studies. The rarity of these may perhaps be due to their dependence on mycobactin J for initial isolation; however, the reliability of this characteristic is unclear, as mycobactin J is reportedly not required for subculture. Further studies of isolates named M. avium subsp. silvaticum are warranted to determine whether this designation is truly valid. PCR amplification and sequencing of the 3′ region of hsp65 should provide a more reliable means of classifying such isolates.
The use of the designation M. avium subsp. hominissuis for M. avium strains that do not belong in the three groups defined above (M. avium bird type and M. paratuberculosis bovine and ovine types) is becoming the new norm since its proposal by Mijs et al. in 2002 (23). This change in MAC taxonomy needs to be taken into consideration, particularly when performing human-related virulence or functional studies, since the type strain and other reference strains of M. avium are bird type and may not be representative of human isolates. Assuming a greater interest in human disease, choosing strain M. avium 104 from a human immunodeficiency virus (HIV) patient for the first M. avium genome sequencing project instead of the type strain ATCC 25291 was a sensible approach. In addition, this new designation appears to comprise a heterogeneous group with at least six hsp65 sequevars, perhaps because human isolates simply reflect what is found in their environment (38). This possibility is also supported by the observation that porcine isolates have shown a link to their environment (20, 21) and share features similar to those of human isolates (2, 13).
A small subset of M. intracellulare strains were evaluated in this study; this subset is by no means representative of the species as a whole. Nevertheless, four hsp65 sequevars were recognized among only eight strains with a Min-A ITS1 (M. intracellulare sensu stricto), suggesting that this sequence-based tool may serve well in defining subsets of epidemiological implications.
In conclusion, we have identified a higher discriminatory power in analyzing the 3′ region of the hsp65 gene. Based on this marker alone, several epidemiologically important members of the MAC can be identified and classified into distinct lineages such as M. avium subsp. paratuberculosis cow, M. avium subsp. paratuberculosis sheep, and M. avium subsp. avium (bird type). For the time being, we propose that all other M. avium can be assigned to the hominissuis subspecies. This method can be used alone or as a complement to other approaches such as RFLP, PCR of insertion elements, or PCR for large sequence polymorphisms (28, 29). This target could also prove useful in other mycobacterial subsets and has previously been demonstrated for strains of the M. marinum and M. ulcerans complex (36).
Acknowledgments
We are grateful to Gerard Cangelosi, Anita Michel, and Petra de Haas for the provision of strains or DNA.
C.Y.T. is supported by a Lloyd-Carr-Harris McGill Major Fellowship and an F. C. Harrison scholarship from the McGill Department of Microbiology and Immunology, M.S. is funded by the Fonds de la Recherche en Sante du Quebec (FRSQ), and M.A.B. is a New Investigator of the Canadian Institutes for Health Research. The work was funded by a grant from the Natural Science and Engineering Research Council (grant GEN2282399).
REFERENCES
- 1.Baess, I. 1983. Deoxyribonucleic acid relationships between different serovars of Mycobacterium avium, Mycobacterium intracellulare and Mycobacterium scrofulaceum. Acta Pathol. Microbiol. Immunol. Scand. Sect. B Microbiol. 91:201-203. [DOI] [PubMed] [Google Scholar]
- 2.Bono, M., T. Jemmi, C. Bernasconi, D. Burki, A. Telenti, and T. Bodmer. 1995. Genotypic characterization of Mycobacterium avium strains recovered from animals and their comparison to human strains. Appl. Environ. Microbiol. 61:371-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins, D. M., S. Cavaignac, and G. W. de Lisle. 1997. Use of four DNA insertion sequences to characterize strains of the Mycobacterium avium complex isolated from animals. Mol. Cell. Probes 11:373-380. [DOI] [PubMed] [Google Scholar]
- 4.Collins, D. M., M. De Zoete, and S. M. Cavaignac. 2002. Mycobacterium avium subsp. paratuberculosis strains from cattle and sheep can be distinguished by a PCR test based on a novel DNA sequence difference. J. Clin. Microbiol. 40:4760-4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins, D. M., D. M. Gabric, and G. W. de Lisle. 1989. Identification of a repetitive DNA sequence specific to Mycobacterium paratuberculosis. FEMS Microbiol. Lett. 51:175-178. [DOI] [PubMed] [Google Scholar]
- 6.Collins, D. M., D. M. Gabric, and G. W. de Lisle. 1990. Identification of two groups of Mycobacterium paratuberculosis strains by restriction endonuclease analysis and DNA hybridization. J. Clin. Microbiol. 28:1591-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cousins, D. V., R. Whittington, I. Marsh, A. Masters, R. J. Evans, and P. Kluver. 1999. Mycobacteria distinct from Mycobacterium avium subsp. paratuberculosis isolated from the faeces of ruminants possess IS900-like sequences detectable by IS900 polymerase chain reaction: implications for diagnosis. Mol. Cell. Probes 13:431-442. [DOI] [PubMed] [Google Scholar]
- 8.Dvorska, L., T. J. Bull, M. Bartos, L. Matlova, P. Svastova, R. T. Weston, J. Kintr, I. Parmova, D. van Soolingen, and I. Pavlik. 2003. A standardised restriction fragment length polymorphism (RFLP) method for typing Mycobacterium avium isolates links IS901 with virulence for birds. J. Microbiol. Methods 55:11-27. [DOI] [PubMed] [Google Scholar]
- 9.Englund, S., G. Bolske, and K. E. Johansson. 2002. An IS900-like sequence found in a Mycobacterium sp. other than Mycobacterium avium subsp. paratuberculosis. FEMS Microbiol. Lett. 209:267-271. [DOI] [PubMed] [Google Scholar]
- 10.Eriks, I. S., K. T. Munck, T. E. Besser, G. H. Cantor, and V. Kapur. 1996. Rapid differentiation of Mycobacterium avium and M. paratuberculosis by PCR and restriction enzyme analysis. J. Clin. Microbiol. 34:734-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frothingham, R. 1999. Evolutionary bottlenecks in the agents of tuberculosis, leprosy, and paratuberculosis. Med. Hypotheses 52:95-99. [DOI] [PubMed] [Google Scholar]
- 12.Frothingham, R., and K. H. Wilson. 1993. Sequence-based differentiation of strains in the Mycobacterium avium complex. J. Bacteriol. 175:2818-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guerrero, C., C. Bernasconi, D. Burki, T. Bodmer, and A. Telenti. 1995. A novel insertion element from Mycobacterium avium, IS1245, is a specific target for analysis of strain relatedness. J. Clin. Microbiol. 33:304-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansen, T. B., B. Djonne, M. R. Jensen, and I. Olsen. 2005. Distribution of IS1311 and IS1245 in Mycobacterium avium subspecies revisited. J. Clin. Microbiol. 43:2500-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 16.Kunze, Z. M., F. Portaels, and J. J. Mcfadden. 1992. Biologically distinct subtypes of Mycobacterium avium differ in possession of insertion sequence IS901. J. Clin. Microbiol. 30:2366-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunze, Z. M., S. Wall, R. Appelberg, M. T. Silva, F. Portaels, and J. J. Mcfadden. 1991. IS901, a new member of a widespread class of atypical insertion sequences, is associated with pathogenicity in Mycobacterium avium. Mol. Microbiol. 5:2265-2272. [DOI] [PubMed] [Google Scholar]
- 18.Lebrun, L., F.-X. Weill, L. Lafendi, F. Houriez, F. Casanova, M. C. Gutierrez, D. Ingrand, P. Lagrange, V. Vincent, and J. L. Herrmann. 2005. Use of the INNO-LiPA-MYCOBACTERIA assay (version 2) for identification of Mycobacterium avium-Mycobacterium intracellulare-Mycobacterium scrofulaceum complex isolates. J. Clin. Microbiol. 43:2567-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackintosh, C. G., G. W. de Lisle, D. M. Collins, and J. F. Griffin. 2004. Mycobacterial diseases of deer. N. Z. Vet. J. 52:163-174. [DOI] [PubMed] [Google Scholar]
- 20.Matlova, L., L. Dvorska, W. Y. Ayele, M. Bartos, T. Amemori, and I. Pavlik. 2005. Distribution of Mycobacterium avium complex isolates in tissue samples of pigs fed peat naturally contaminated with mycobacteria as a supplement. J. Clin. Microbiol. 43:1261-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matlova, L., L. Dvorska, K. Palecek, L. Maurenc, M. Bartos, and I. Pavlik. 2004. Impact of sawdust and wood shavings in bedding on pig tuberculous lesions in lymph nodes, and IS1245 RFLP analysis of Mycobacterium avium subsp. hominissuis of serotypes 6 and 8 isolated from pigs and environment. Vet. Microbiol. 102:227-236. [DOI] [PubMed] [Google Scholar]
- 22.McNabb, A., D. Eisler, K. Adie, M. Amos, M. Rodrigues, G. Stephens, W. A. Black, and J. Isaac-Renton. 2004. Assessment of partial sequencing of the 65-kilodalton heat shock protein gene (hsp65) for routine identification of Mycobacterium species isolated from clinical sources. J. Clin. Microbiol. 42:3000-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mijs, W., P. de Haas, R. Rossau, L. T. Van der, L. Rigouts, F. Portaels, and D. van Soolingen. 2002. Molecular evidence to support a proposal to reserve the designation Mycobacterium avium subsp. avium for bird-type isolates and “M. avium subsp. hominissuis” for the human/porcine type of M. avium. Int. J. Syst. Evol. Microbiol. 52:1505-1518. [DOI] [PubMed] [Google Scholar]
- 24.Paustian, M. L., V. Kapur, and J. P. Bannantine. 2005. Comparative genomic hybridizations reveal genetic regions within the Mycobacterium avium complex that are divergent from Mycobacterium avium subsp. paratuberculosis isolates. J. Bacteriol. 187:2406-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pavlik, I., P. Svastova, J. Bartl, L. Dvorska, and I. Rychlik. 2000. Relationship between IS901 in the Mycobacterium avium complex strains isolated from birds, animals, humans, and the environment and virulence for poultry. Clin. Diagn. Lab. Immunol. 7:212-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ritacco, V., K. Kremer, T. Van der Laan, J. E. Pijnenburg, P. E. de Haas, and D. van Soolingen. 1998. Use of IS901 and IS1245 in RFLP typing of Mycobacterium avium complex: relatedness among serovar reference strains, human and animal isolates. Int. J. Tuberc. Lung Dis. 2:242-251. [PubMed] [Google Scholar]
- 27.Rozen, S., and H. J. Skaletsky. 2000. Primer3 on the WWW for general users and for biologist programmers, p. 365-386. In S. Krawetz and S. Misener (ed.), Bioinformatics methods and protocols: methods in molecular biology. Humana Press, Totowa, N.J. [DOI] [PubMed]
- 28.Semret, M., D. C. Alexander, C. Y. Turenne, P. de Haas, P. Overduin, D. van Soolingen, D. Cousins, and M. A. Behr. 2005. Genomic polymorphisms for Mycobacterium avium subsp. paratuberculosis diagnostics. J. Clin. Microbiol. 43:3704-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Semret, M., G. Zhai, S. Mostowy, C. Cleto, D. Alexander, G. Cangelosi, D. Cousins, D. M. Collins, D. van Soolingen, and M. A. Behr. 2004. Extensive genomic polymorphism within Mycobacterium avium. J. Bacteriol. 186:6332-6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smole, S. C., F. McAleese, J. Ngampasutadol, C. F. von Reyn, and R. D. Arbeit. 2002. Clinical and epidemiological correlates of genotypes within the Mycobacterium avium complex defined by restriction and sequence analysis of hsp65. J. Clin. Microbiol. 40:3374-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swanson, D. S., V. Kapur, K. Stockbauer, X. Pan, R. Frothingham, and J. M. Musser. 1997. Subspecific differentiation of Mycobacterium avium complex strains by automated sequencing of a region of the gene (hsp65) encoding a 65-kilodalton heat shock protein. Int. J. Syst. Bacteriol. 47:414-419. [DOI] [PubMed] [Google Scholar]
- 32.Tasara, T., L. E. Hoelzle, and R. Stephan. 2005. Development and evaluation of a Mycobacterium avium subspecies paratuberculosis (MAP) specific multiplex PCR assay. Int. J. Food Microbiol. 104:279-287. [DOI] [PubMed] [Google Scholar]
- 33.Telenti, A., F. Marchesi, M. Balz, F. Bally, E. C. Bottger, and T. Bodmer. 1993. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J. Clin. Microbiol. 31:175-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thorel, M. F., M. Krichevsky, and V. V. Levy-Frebault. 1990. Numerical taxonomy of mycobactin-dependent mycobacteria, emended description of Mycobacterium avium, and description of Mycobacterium avium subsp. avium subsp. nov., Mycobacterium avium subsp. paratuberculosis subsp. nov., and Mycobacterium avium subsp. silvaticum subsp. nov. Int. J. Syst. Bacteriol. 40:254-260. [DOI] [PubMed] [Google Scholar]
- 35.Tortoli, E., L. Rindi, M. J. Garcia, P. Chiaradonna, R. Dei, C. Garzelli, R. M. Kroppenstedt, N. Lari, R. Mattei, A. Mariottini, G. Mazzarelli, M. I. Murcia, A. Nanetti, P. Piccoli, and C. Scarparo. 2004. Proposal to elevate the genetic variant MAC-A, included in the Mycobacterium avium complex, to species rank as Mycobacterium chimaera sp. nov. Int. J. Syst. Evol. Microbiol. 54:1277-1285. [DOI] [PubMed] [Google Scholar]
- 36.Ucko, M., A. Colorni, H. Kvitt, A. Diamant, A. Zlotkin, and W. R. Knibb. 2002. Strain variation in Mycobacterium marinum fish isolates. Appl. Environ. Microbiol. 68:5281-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Soolingen, D., J. Bauer, V. Ritacco, S. C. Leao, I. Pavlik, V. Vincent, N. Rastogi, A. Gori, T. Bodmer, C. Garzelli, and M. J. Garcia. 1998. IS1245 restriction fragment length polymorphism typing of Mycobacterium avium isolates: proposal for standardization. J. Clin. Microbiol. 36:3051-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.von Reyn, C. F., J. N. Maslow, T. W. Barber, J. O. Falkinham, III, and R. D. Arbeit. 1994. Persistent colonisation of potable water as a source of Mycobacterium avium infection in AIDS. Lancet 343:1137-1141. [DOI] [PubMed] [Google Scholar]
- 39.Wheeler, W. C., and J. H. Hanks. 1965. Utilization of external growth factors by intracellular microbes: Mycobacterium paratuberculosis and wood pigeon mycobacteria. J. Bacteriol. 89:889-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whittington, R. J., I. B. Marsh, and R. H. Whitlock. 2001. Typing of IS1311 polymorphisms confirms that bison (Bison bison) with paratuberculosis in Montana are infected with a strain of Mycobacterium avium subsp. paratuberculosis distinct from that occurring in cattle and other domesticated livestock. Mol. Cell. Probes 15:139-145. [DOI] [PubMed] [Google Scholar]