Abstract
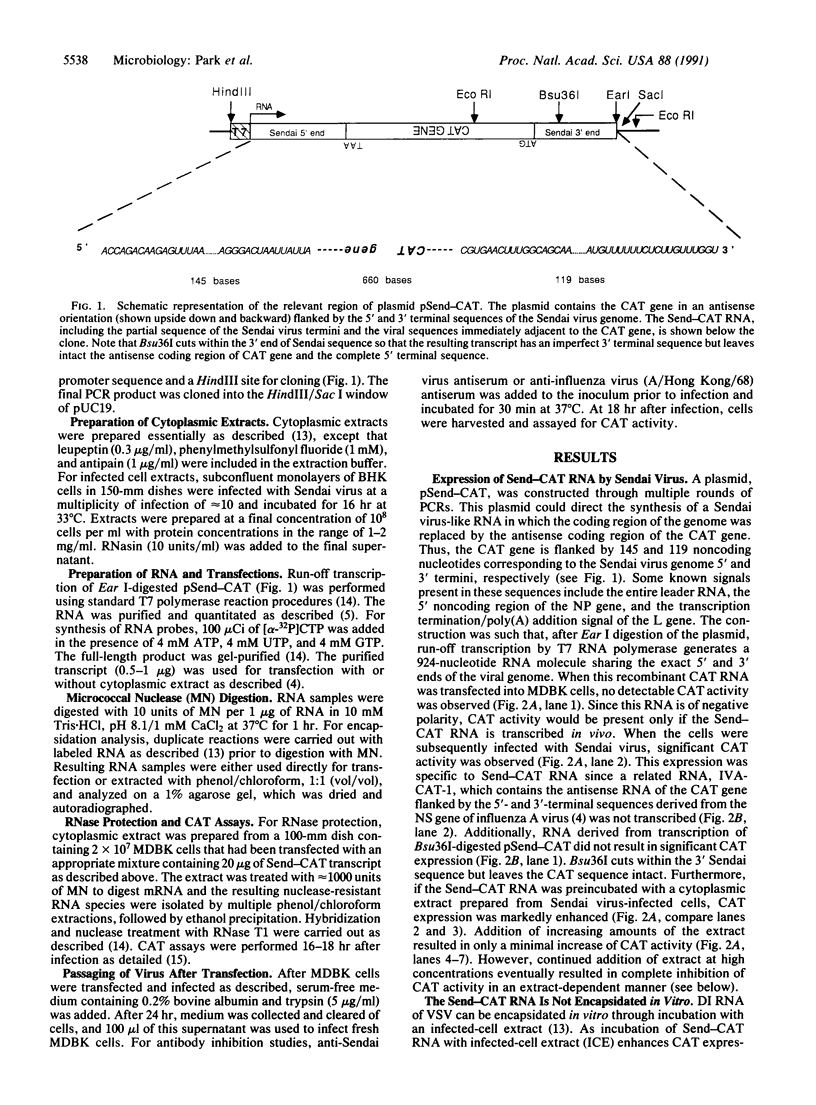
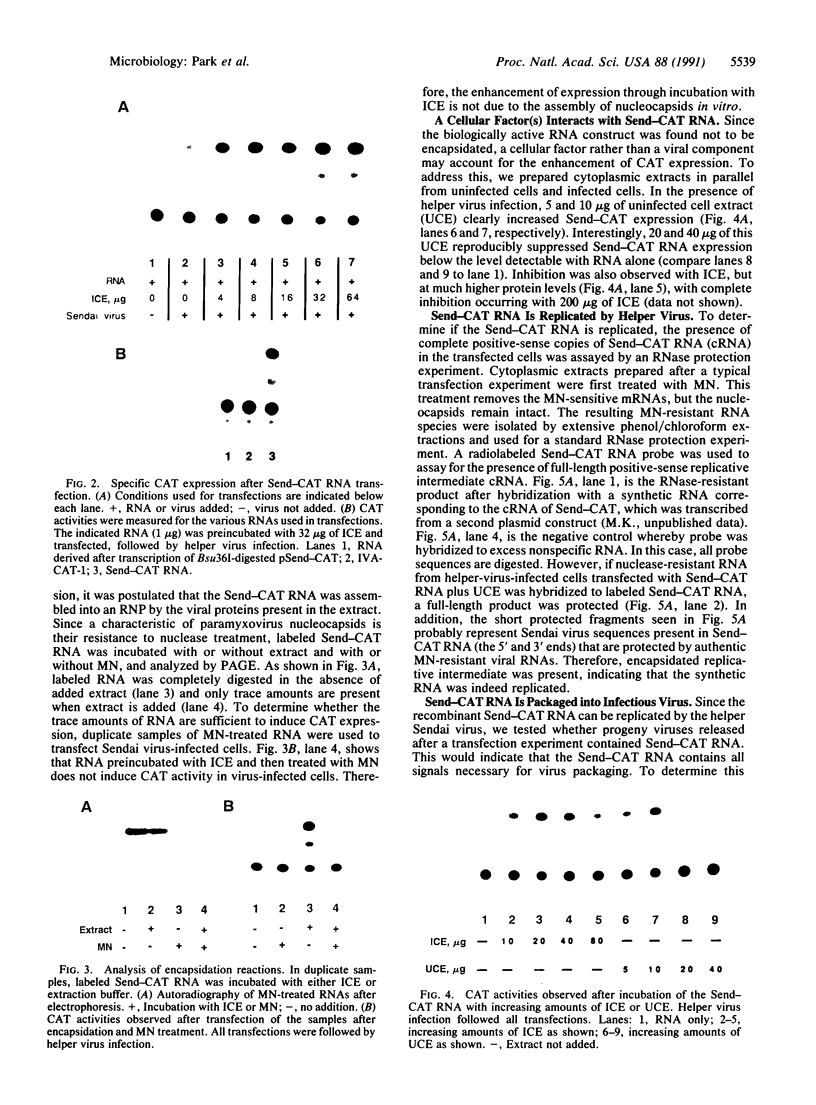
A simple protocol for the rescue of a synthetic genome into a paramyxovirus has been developed. First, a synthetic Sendai virus-like RNA, containing the antisense coding region of the chloramphenicol acetyltransferase gene replacing the coding region of the Sendai virus genome, was transcribed from a cDNA. When introduced into cells that are infected with Sendai virus, this RNA construct was transcribed, replicated, and packaged into infectious virions. The addition of infected cell extract to the RNA prior to transfection markedly enhanced levels of chloramphenicol acetyltransferase expression and rescue. However, this enhancement is not due to encapsidation of the RNA into nucleocapsids as the RNA remains nuclease-sensitive. Uninfected cell extract also enhances expression and rescue efficiency, implying involvement of a cellular factor(s) with the synthetic viral-like RNA construct that allows for enhanced polymerase recognition. This system should allow for the dissection of the various cis-acting RNA signals within the paramyxovirus genome.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amesse L. S., Pridgen C. L., Kingsbury D. W. Sendai virus DI RNA species with conserved virus genome termini and extensive internal deletions. Virology. 1982 Apr 15;118(1):17–27. doi: 10.1016/0042-6822(82)90315-4. [DOI] [PubMed] [Google Scholar]
- Ballart I., Eschle D., Cattaneo R., Schmid A., Metzler M., Chan J., Pifko-Hirst S., Udem S. A., Billeter M. A. Infectious measles virus from cloned cDNA. EMBO J. 1990 Feb;9(2):379–384. doi: 10.1002/j.1460-2075.1990.tb08121.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- De B. P., Galinski M. S., Banerjee A. K. Characterization of an in vitro system for the synthesis of mRNA from human parainfluenza virus type 3. J Virol. 1990 Mar;64(3):1135–1142. doi: 10.1128/jvi.64.3.1135-1142.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enami M., Luytjes W., Krystal M., Palese P. Introduction of site-specific mutations into the genome of influenza virus. Proc Natl Acad Sci U S A. 1990 May;87(10):3802–3805. doi: 10.1073/pnas.87.10.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K. C., Kingsbury D. W. Complete sequences of the intergenic and mRNA start signals in the Sendai virus genome: homologies with the genome of vesicular stomatitis virus. Nucleic Acids Res. 1984 May 11;12(9):3829–3841. doi: 10.1093/nar/12.9.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi M., Nishikawa K., Toyoda T., Yoshida T., Hanaichi T., Nagai Y. Transcriptive complex of Newcastle disease virus. II. Structural and functional assembly associated with the cytoskeletal framework. Virology. 1985 Dec;147(2):295–308. doi: 10.1016/0042-6822(85)90132-1. [DOI] [PubMed] [Google Scholar]
- Hill V. M., Summers D. F. A minor microtubule-associated protein is responsible for the stimulation of vesicular stomatitis virus transcription in vitro. J Gen Virol. 1990 Feb;71(Pt 2):289–298. doi: 10.1099/0022-1317-71-2-289. [DOI] [PubMed] [Google Scholar]
- Huang T. S., Palese P., Krystal M. Determination of influenza virus proteins required for genome replication. J Virol. 1990 Nov;64(11):5669–5673. doi: 10.1128/jvi.64.11.5669-5673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenk R., Penman S. The cytoskeletal framework and poliovirus metabolism. Cell. 1979 Feb;16(2):289–301. doi: 10.1016/0092-8674(79)90006-0. [DOI] [PubMed] [Google Scholar]
- Luytjes W., Krystal M., Enami M., Parvin J. D., Palese P. Amplification, expression, and packaging of foreign gene by influenza virus. Cell. 1989 Dec 22;59(6):1107–1113. doi: 10.1016/0092-8674(89)90766-6. [DOI] [PubMed] [Google Scholar]
- Mirakhur B., Peluso R. W. In vitro assembly of a functional nucleocapsid from the negative-stranded genome RNA of a defective interfering particle of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7511–7515. doi: 10.1073/pnas.85.20.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer S. A., Baker S. C., Lessard J. L. Tubulin: a factor necessary for the synthesis of both Sendai virus and vesicular stomatitis virus RNAs. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5405–5409. doi: 10.1073/pnas.83.15.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olkkonen V. M., Gottlieb P., Strassman J., Qiao X. Y., Bamford D. H., Mindich L. In vitro assembly of infectious nucleocapsids of bacteriophage phi 6: formation of a recombinant double-stranded RNA virus. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9173–9177. doi: 10.1073/pnas.87.23.9173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvin J. D., Palese P., Honda A., Ishihama A., Krystal M. Promoter analysis of influenza virus RNA polymerase. J Virol. 1989 Dec;63(12):5142–5152. doi: 10.1128/jvi.63.12.5142-5152.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattnaik A. K., Wertz G. W. Cells that express all five proteins of vesicular stomatitis virus from cloned cDNAs support replication, assembly, and budding of defective interfering particles. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1379–1383. doi: 10.1073/pnas.88.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattnaik A. K., Wertz G. W. Replication and amplification of defective interfering particle RNAs of vesicular stomatitis virus in cells expressing viral proteins from vectors containing cloned cDNAs. J Virol. 1990 Jun;64(6):2948–2957. doi: 10.1128/jvi.64.6.2948-2957.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roner M. R., Sutphin L. A., Joklik W. K. Reovirus RNA is infectious. Virology. 1990 Dec;179(2):845–852. doi: 10.1016/0042-6822(90)90153-i. [DOI] [PubMed] [Google Scholar]
- Vidal S., Curran J., Kolakofsky D. Editing of the Sendai virus P/C mRNA by G insertion occurs during mRNA synthesis via a virus-encoded activity. J Virol. 1990 Jan;64(1):239–246. doi: 10.1128/jvi.64.1.239-246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal S., Kolakofsky D. Modified model for the switch from Sendai virus transcription to replication. J Virol. 1989 May;63(5):1951–1958. doi: 10.1128/jvi.63.5.1951-1958.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]