Abstract
We show that the photoreceptor rhodopsin (Rh) can exist in the membrane as a dimer or multimer using luminescence resonance energy transfer and FRET methods. Our approach looked for interactions between Rh molecules reconstituted into asolectin liposomes. The low receptor density used in the measurements ensured minimal receptor crowding and artifactual association. The fluorescently labeled Rh molecules were fully functional, as measured by their ability to activate the G protein transducin. The luminescence resonance energy transfer measurements revealed a distance of 47–50 Å between Rh molecules. The measured efficiency of FRET between receptors was close to the theoretical maximum possible, indicating nearly quantitative Rh–Rh association. Together, these results provide compelling evidence that Rh spontaneously self-associates in membranes.
Keywords: FRET, luminescence resonance energy transfer, G protein-coupled receptor, GPCR dimer, signal transduction
G protein-coupled receptors (GPCRs) are involved in diverse physiological processes and represent the single largest family of signaling molecules in the human genome. Until recently, GPCRs were assumed to exist in the membrane as monomeric proteins that are activated by the binding of one ligand to one receptor. However, a wealth of data indicates that these receptors frequently form dimers or higher order oligomeric species (1–5). In fact, some GPCRs may even function as hetero-oligomers (6).
The possibility that rhodopsin (Rh) self-associates was first indicated by atomic force microscopy measurements, which showed Rh molecules can form distinct, densely packed double rows in the rod outer segment (ROS) membranes of mouse retina (7). Other studies carried out on detergent-solubilized protein suggest that some Rh is present as dimers (8, 9). Furthermore, opsin forms dimers when expressed in COS1 cells (see accompanying article by Kota et al., ref. 10). However, the conclusion that Rh is dimeric challenges previous work that concluded Rh to be a monomer randomly distributed in the plane of the membrane (11–15).
Here, we asked whether Rh molecules associate when reconstituted into lipid vesicles. Our approach was to use luminescence and fluorescence resonance energy transfer (LRET and FRET) techniques to assess the apparent distance between Rh molecules reconstituted in asolectin liposomes at low receptor densities. Our studies indicate that nearly all of the Rh self-associates into dimers or oligomers,§ providing further independent evidence that Rh is present in the membrane in a multimeric state.
Results and Discussion
Overview.
We looked for evidence of Rh–Rh interactions using a quantitative FRET-based approach. Our strategy (outlined in Fig. 6A, which is published as supporting information on the PNAS web site) was to look for Rh–Rh interactions under conditions in which the Rh molecules were given ample opportunity to not interact. Thus, we reconstituted Rh into liposomes at low receptor densities, to maximize the amount of positive FRET signal from Rh molecules truly involved in dimeric or higher-order interactions. After reconstitution, we first measured the average distance between Rh receptors using LRET. Next, we measured FRET between Rh samples and correlated the amount of the energy transfer with the proportion of total receptors at the measured interaction distance. Our main postulate is that if the amount of measured FRET equals the maximum FRET possible, most of the Rh molecules must be self-associating.
Preparation and Characterization of Rh Samples.
Rh was labeled in the cytoplasmic face, as described in Supporting Text, which is published as supporting information on the PNAS web site. The labeling occurred at the uniquely reactive cysteine residues, C140 and C316 (17–21). The LRET studies used the label CS124-DTPA-EMCH·Tb3+ as the donor and CY3–maleimide as the acceptor. The FRET studies used CY3-maleimide as the donor and CY5–maleimide as the acceptor. These Cys-reactive fluorophores are well characterized (22–24), and their spectra are significantly red-shifted, resulting in minimal spectral overlap with the retinal chromophore in Rh (see Fig. 3B).
Fig. 3.
FRET studies show strong Rh–Rh energy transfer in liposomes. (A) Cartoon scheme of FRET studies. Excitation of Rh–CY3 (donor) will transfer energy to Rh–CY5 (acceptor) with an efficiency proportional to the distance between them (see Eq. 5). (B) Spectral overlap of CY3 and CY5. The amount of overlap, J(λ), is indicated in dark gray and results in a calculated R0 for this FRET pair of 52 Å in the dark state and 56 Å after light activation of rhodopsin. (C) The arrows indicate the strong FRET observed between Rh–CY3 and Rh–CY5 when reconstituted together into asolectin liposomes (green curve). The control (red curve) shows no FRET signal for a summation of individually labeled and reconstituted Rh–CY3 and Rh–CY5 measured at identical concentrations and conditions. In this example, reconstitution used 10,000 moles of asolectin lipids per mole of Rh. Except for buffer subtraction, the data have not been manipulated or normalized in any way.
Rh was labeled with ≈1.0 label per protein (data not shown). The C140 and C316 Cys residues labeled with roughly similar efficiencies, as assessed by V8 proteolysis and SDS/PAGE analysis (25), which produced two fragments (F1 and F2) with similar fluorescence intensity (Fig. 1A). Scanning electron microscopy indicated that the reconstituted Rh proteoliposomes ranged in size from 100 to 200 nm in diameter, with an approximate average diameter of 150 nm (Fig. 1B), consistent with previous measurements of asolectin liposomes (26).
Fig. 1.
Preparation and functional characterization of labeled Rh samples. (A) SDS/PAGE analysis of V8 digested CY3-labeled Rh samples shows roughly equal distribution of labels on C140 and C316. (B) Electron microscopy reveals that asolectin liposomes reconstituted with Rh have an average radius of ≈75 nm. (C) Asp-N proteolysis indicates Rh–CY3 and Rh–CY5 are preferentially oriented inside-out in the liposomes. Because Asp-N cleaves Rh at the C terminus, a shift in protein mobility indicates the C terminus is located outside the liposome, and is accessible to the protease. (Upper) Imaging of CY3 fluorescence. (Lower) Coomassie stain. The individually labeled, reconstituted samples are indicated. Det. refers to a detergent solubilized control. The imaging instrument was not sensitive to CY5 fluorescence. (D) The fluorescent labels do not alter the ability of Rh, reconstituted into liposomes, to activate transducin (GαT). The initial activation rates were ≈ 1.3 pmol/min per pmol Rh for both the unlabeled (open circles) and labeled (open triangles), reconstituted Rh samples. The activation rates were determined by linear regression through the data points converging 3 min after addition of guanosine 5′-[γ-thio]triphosphate. The filled circles show the dark state control.
The Labeled and Reconstituted Rh Is Preferentially Oriented Inside-Out and Is Fully Functional.
Treatment with Asp-N protease increased the electrophoretic mobility of all liposome-bound Rh, as efficiently as for a detergent solubilized Rh control (Fig. 1C). This result indicates that the Rh is oriented inside-out in the liposomes (27), with the C-terminal tail of rhodopsin exposed to the Asp-N protease (28). Both labeled and unlabeled liposome-bound Rh samples showed essentially identical abilities to activate GαT (initial activation rates ≈ 1.3 pmol/min per pmol rhodopsin), demonstrating that the attached fluorophores do not affect rhodopsin function (Fig. 1D).
The Quantum Yield of Rh–CY3 Increases Upon Light Activation of Rhodopsin, Resulting in an Increase in the R0 Value Between Rh–CY3 and Rh–CY5.
The quantum yield of reconstituted Rh–CY3 rose from 0.13 ± 0.01 in the dark state to 0.20 ± 0.01 after light activation of Rh, presumably because energy transfer from the CY3 label to the retinal chromophore was abolished. It is important to note this increase in Rh–CY3 quantum yield changes the R0 value for the Rh–CY3/Rh–CY5 FRET pair from 52 Å in the dark state to 56 Å when light activated.
LRET Measurements Show a Rh–Rh Distance of 47–50 Å in Liposomes.
The LRET approach is illustrated in Fig. 2A. We first used the LRET method because it can accurately determine distances between two proteins, even in the presence of some labeled but noninteracting proteins (the latter are spectrally silent in LRET). Furthermore, LRET is not complicated by the false positives that often complicate FRET studies (23, 29–33). Most importantly, we could use the distances we obtained independently by LRET to quantitate the percentage of the total Rh proteins participating in the subsequent FRET studies.
Fig. 2.
LRET measurements show labeled Rh samples are 47–50 Å apart. (A) Cartoon scheme of LRET experiments. Excited Rh–Tb (donor) will transfer energy to Rh–CY3 (acceptor), with an efficiency proportional to the distance between the two proteins (see Eqs. 4 and 5). (B) Spectral overlap of Rh–Tb and Rh–CY3. The LRET experiments involve exciting Rh–Tb at 337 nm and collecting the sensitized emission from Rh–CY3 at 570 nm. (C) LRET decay data obtained from a dark state mixture of Rh–Tb and Rh–CY3. Exciting the Rh–Tb results in a strong sensitized emission signal from Rh–CY3 (green decay curve), which decays with an average lifetime 〈τAD〉 ≈ 200 μs. The average lifetime of Rh–Tb alone yields a 〈τD〉 ≈ 870 μs (blue decay curve). (D) Predicted sensitized LRET lifetimes (τAD) as a function of distance between Rh–Tb and Rh–CY3. The plot indicates the ≈200-μs τAD measured above corresponds to a Rh–Tb to Rh–CY3 distance of ≈50 Å (red line). After light activation, the Rh–Rh distance decreased slightly to 47 Å (see Table 1).
Details on the LRET studies are given in the Supporting Text. Briefly, they involved exciting the Rh–Tb (donor) at 337 nm with a laser pulse and then measuring energy transfer to Rh–CY3 (the acceptor), as indicated by the “sensitized emission” given off from Rh–CY3 at 570 nm (Fig. 2B). The rate of transfer, k, is reflected in the lifetime of the sensitized emission (τAD), because k = 1/τAD. We used the τAD value thus obtained, and Eq. 4, to determine the efficiency of luminescence resonance energy transfer. From this efficiency, we calculated the donor–acceptor distance using Eq. 5.
A representative result is shown in Fig. 2C. The data, measured from dark-state Rh reconstituted at the lowest receptor density (10,000 lipids per Rh), shows the decay of Rh–Tb donor alone (τD; blue curve) and the “sensitized emission” decay of Rh–CY3 (τAD; green curve). Two lifetime components were required to fit both the τD and τAD data, most likely because energy transfer from Rh–Tb to the retinal contributes to a short decay component. The quality of the data did not warrant a complex analysis; thus, we combined these values to calculate an “average” or amplitude-weighted lifetime, 〈τ〉 = α1τ1 + α2τ2, where α1 and α2 are the preexponential factors (α1 + α2 = 1.0) for τ1 and τ2, respectively. The complete set of amplitude-weighted LRET lifetimes, 〈τD〉 and 〈τAD〉, thus measured, are reported in Table 1.
Table 1.
LRET lifetime data indicate distances of ≈47–50 Å between Rh molecules reconstituted into asolectin liposomes
| Rh–Tb + Rh–CY3 sample | 〈τD〉 Lifetime, μs | 〈τAD〉 Lifetime, μs | Rh–Rh distance, Å |
|---|---|---|---|
| Dark state | 870 ± 14 | 196 ± 12 | 50 ± 1 |
| +hυ, 0 min | 970 ± 20 | 185 ± 1 | 48 ± 1 |
| +hυ, 30 min | 982 ± 29 | 171 ± 14 | 47 ± 1 |
Measurements were carried out as described in Materials and Methods. Note that due to the long luminescent lifetimes of Rh–Tb, we cannot completely rule out some contributions to the measured LRET distance caused by simple diffusion bringing two Rh proteins together. See Supporting Text for more details.
These 〈τD〉 and 〈τAD〉 values yield a distance of 50 Å between Rh–Tb and Rh–CY3 in the dark state (Fig. 2D). Similar measurements were made for the samples immediately and 30 min after light activation (see Table 1). In each case, the distance between Rh proteins undergoing LRET was between 47 and 50 Å. Interestingly, this value is in good agreement with an expected Rh–Rh distance based on the known diameter of Rh (34).
FRET Measurements Show Substantial Energy Transfer Between Rh Samples in Liposomes.
To determine the proportion of Rh protein molecules that are close enough to interact, we used quantitative FRET measurements using CY3 as the energy transfer donor and CY5 as the acceptor (Fig. 3A and B).
In these experiments, we reconstituted Rh–CY3 and Rh–CY5, both together, and separately as a control (in the latter case with equimolar amounts of unlabeled Rh). We ensured that each reconstitution resulted in an identical amount of Rh in the liposomes. The results from these studies, shown in Fig. 3C, reveal substantial FRET, but only when the samples were combined and reconstituted together (green curves). The control samples show no FRET (red curves). The amount of FRET appears to increase immediately upon light activation of Rh and continues to increase over time (Fig. 3C Left), although we suspect this increase is mainly due to the inherent increase in the quantum yield for Rh–CY3 upon bleaching.
We quantified the amount of FRET observed in these excitation spectra by using standard approaches (35, 36). Our results showed a FRET efficiency of 32% in the dark, which increased to 38% efficiency immediately after light activation and up to 46% 30 min after light activation. Importantly, these donor- and acceptor-labeled ROS samples were prepared separately, their concentrations were determined and matched, and only then were the samples mixed for reconstitution. These stringent conditions ensured that a positive FRET signal unequivocally reflects intermolecular FRET occurring between at least two different receptors. We stress that the data in Fig. 3C are raw data that, aside from buffer subtraction, have not been normalized or manipulated in any other way.
Substantial FRET is also apparent in the emission spectra and fluorescence lifetimes. For example, the sensitized acceptor emission spectra (Fig. 3C Right) show both a decrease in fluorescence intensity of Rh–CY3 emission at 570 nm and a concomitant increase in the sensitized Rh–CY5 emission at 670 nm (again, the FRET signal in the dark state appears to increase after light activation). Similarly, a high FRET efficiency is observed by measuring the fluorescence lifetime of the donor, Rh–CY3, in the absence (τD) and presence (τDA) of the acceptor, Rh–CY5. These measurements indicate FRET efficiencies ranging from 25% in the dark state to 32% at 30 min after light activation (Table 2). These values are in qualitative agreement with the steady-state FRET efficiencies. Imperfect correlation with the steady-state data are most likely due to the short lifetimes of the donor fluorophore and to the limitations of our instrumental setup.
Table 2.
FRET efficiencies calculated using steady-state excitation spectra and fluorescence lifetime decays of Rh reconstituted into liposomes
| Rh-CY3 + Rh-CY5 sample | FRET efficiency steady-state excitation data, % | FRET efficiency lifetime data, % |
|---|---|---|
| Dark state | 32 ± 1 | 25 ± 2 |
| +hυ, 0 min | 38 ± 1 | 21 ± 1 |
| +hυ, 30 min | 46 ± 1 | 32 ± 2 |
Measurements were carried out as described in Materials and Methods. See Supporting Text for more details.
To assess possible error in the LRET and FRET results, we measured the steady-state anisotropy of the samples to determine the degree of fluorophore mobility (Table 3, which is published as supporting information on the PNAS web site). These results indicate the maximum possible error associated with the LRET distances (Table 1), due to changes in κ2, are <8% in both the dark state and light-activated states, whereas the maximum error in the FRET efficiency is between 20% and 30%. Note that the actual errors are likely much less significant than these absolute possible extremes (37).
FRET Efficiency at Different Receptor Densities.
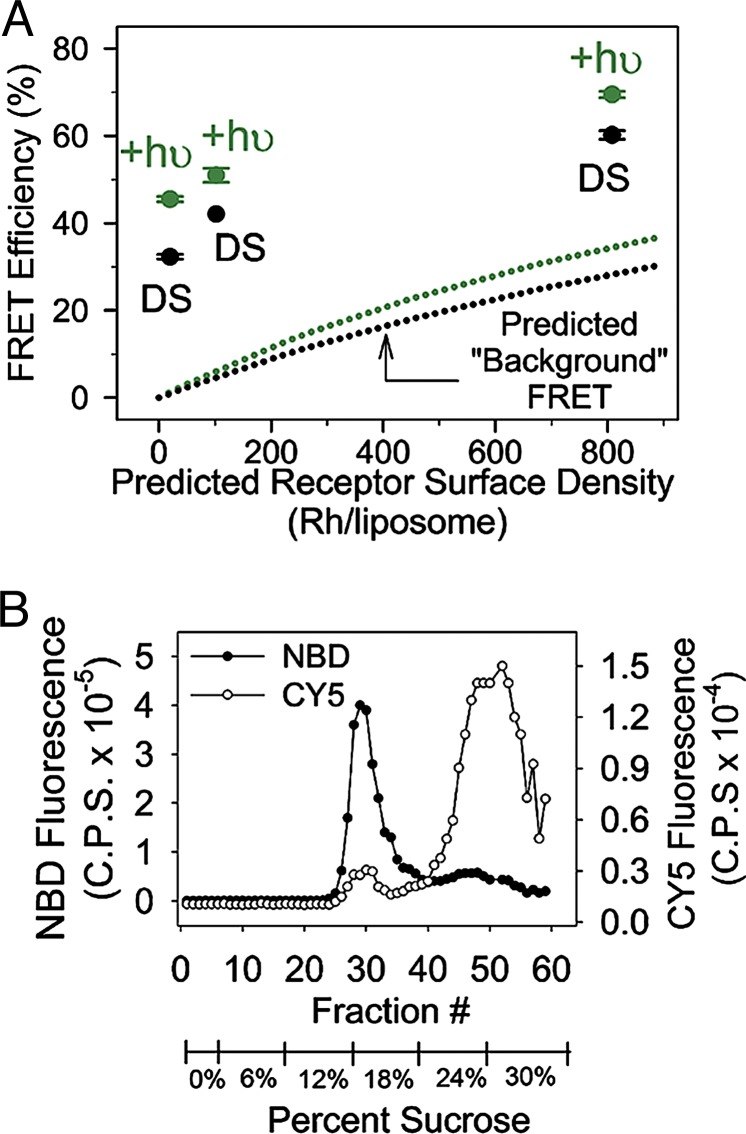
We determined the amount of nonspecific “background FRET” (due to receptor crowding) by measuring FRET for samples reconstituted at varying molar lipid/Rh ratios (10,000:1, 2,000:1, and 250:1). These conditions should theoretically produce receptor densities of ≈20, ≈100, and ≈800 proteins per vesicle, respectively. We used these receptor densities, along with the concept of a reduced acceptor surface density (CA), to calculate the amount of background FRET expected at each lipid/Rh concentration. The FRET results, and the “background FRET” expected for the different concentrations, are shown in Fig. 4A. Notice that the measured FRET signal is strongly dependent on the receptor density, and appears to be superimposed on the predicted background FRET at each concentration.
Fig. 4.
FRET signal as a function of receptor density. (A) Experimentally determined FRET efficiencies for Rh in the dark-state (DS), and after illumination (+hυ), at different predicted receptor densities (Rh/liposome). The FRET signals are well above the nonspecific or “background FRET” predicted to occur due to molecular crowding with increasing receptor density (dotted lines) (46). (B) Isopycnic centrifugation at the lowest receptor concentration (10,000× lipid/Rh). Open circles indicate the Rh–CY5 fluorescence, and filled circles indicate the NBD-labeled lipid fluorescence. The data indicate ≈90% of Rh incorporates into ≈11% of the total liposomes. Thus, Rh-containing vesicles have ≈200 Rh per liposome, yielding a predicted background FRET signal of ≈8% in the dark state and ≈11% after light activation (note that these values are still well below the measured FRET signals in A).
Experimentally Determined Receptor Density.
The above calculation assumes a random distribution of Rh among the liposomes. We tested this assumption at the lowest Rh/lipid ratio (one Rh per 10,000 lipid molecules) by carrying out isopycnic density centrifugation analysis (38). These studies showed that Rh, in these preparations, is not uniformly distributed (Fig. 4B). Instead, ≈90% of the Rh appears to be present in ≈11% of the available vesicles. This interesting result (which may suggest that Rh is forming higher-order oligomers) indicates that in most vesicles containing Rh, the “true” receptor density is actually ≈10× higher than predicted (≈200 rhodopsin molecules per liposome). The correct CA values are thus 0.039 in the dark state and 0.045 after light activation, yielding a more accurate assessment of the “background FRET” at this lipid/Rh ratio of ≈8% in the dark and ≈11% after light activation (see dotted curve in Fig. 4A).
The Measured FRET Efficiencies Are near the Theoretical Maximum Possible, Suggesting That the Majority of Rh Molecules Interact.
Our main postulate in this work is that quantitative Rh–Rh self-association is indicated if the measured FRET efficiencies equal the theoretically maximum FRET possible at the Rh–Rh distances measured from the LRET studies (Fig. 2D and Table 1).
We estimated the total amount of interacting Rh proteins as follows. We analyzed the excitation FRET spectra (35, 36) to determine the percentage of FRET in each sample (see Table 2). We then compared this value to the maximal FRET possible under these conditions. To calculate the theoretical maximum FRET, we had to take into account two factors. First, only half of the labeled Rh can form donor–acceptor pairs (see Fig. 5A). Second, the R0 values for a specific donor–acceptor pair must be used. When these factors are accounted for, the following theoretically maximum FRET efficiencies are predicted: 28% for the Rh–CY3–Rh–CY5 pair in the dark state (distance = 50 Å), and 37% for the Rh–CY3–Rh–CY5 pair in the light-activated state (distance = 47 Å) (Fig. 5B).
Fig. 5.
Rh samples show near quantitative self-association in liposomes. (A) Cartoon illustrating how only half of the mixed samples can form donor, D, and acceptor, A, FRET pairs. (B) Plot of energy transfer efficiency as a function of distance between the Rh–CY3 and Rh–CY5 pairs. The predicted FRET efficiency for Rh–CY3:Rh–CY5 is ≈56% at the ≈50 Å dark-state Rh–Rh distance measured by LRET. The maximum FRET for an equimolar mixture Rh–CY3 and Rh–CY5 in the dark-state is ≈28% (half of 56%). For light-activated Rh, the maximum possible FRET signal at the LRET distance of 47 Å is ≈ 37% (half of 74%). (C) The theoretical maximum and measured FRET efficiencies are nearly identical, indicating that essentially all of the Rh samples are close enough to participate in a dimeric (or other higher order) interaction. The theoretical maximum FRET efficiencies are shown in the blue bars, and the experimentally determined FRET efficiencies (corrected for the background FRET predicted in Fig. 4) are shown by the gray bars.
Importantly, as shown in Fig. 5C, these predicted maximum possible FRET values are nearly identical to the measured FRET efficiencies (once the expected background FRET is subtracted; Fig. 4A). We conclude that, for this result to be possible, almost all of the Rh molecules must be within interacting distance.
Implications of Rh–Rh Association.
Although the idea that visual Rh may self-associate in the membrane has been debated (39), our results clearly favor Rh dimerization and are consistent with evidence found for other GPCRs (1–4). In fact, our studies found no evidence for a substantial amount of monomeric Rh. Because Rh constitutes the majority of volume of the membrane in ROS, with a concentration as high as 3 mM (40), and only ≈65 phospholipids solvating each Rh molecule (40), an interesting question may be “what could prevent Rh from interacting with itself?” The complete absence of any protein–protein interactions at such high concentrations would seem to require an extremely low affinity between Rh molecules, a possibility that is not supported by our present work or the accompanying work of Kota et al. (10).
Thinking about Rh as a dimer, instead of a monomer, is compelling when examining the architecture of various downstream signaling components in the visual pathway. For example, the interface surface area of Rh in a monomeric model of the Rh–transducin interaction is too small to cover all of the regions of transducin known to be critical for interaction with the receptor. However, subsequent modeling studies have demonstrated that the surface area of one transducin molecule is large enough to accommodate the docking of four rhodopsin molecules (40).
Similarly, the concept of a multimeric Rh may help explain Rh desensitization by visual arrestin. Arrestin acts by binding to activated, phosphorylated Rh, thus blocking further signaling (41). Interestingly, crystal structures of arrestin show a bilobed protein with two concave surfaces (42, 43). Both concave surfaces have been demonstrated by mutagenesis studies to be involved in Rh–arrestin interactions (44). Like transducin, the putative Rh-interaction surface on visual arrestin is highly striking: the two concave grooves can physically accommodate two molecules of rhodopsin (5). Although the specific reasons why Rh may function as a dimer remain to be established, it is clear a dimeric state may have a profound impact on the kinetics of Rh activation, signaling through transducin, and desensitization through arrestin.
Summary and Conclusions.
We have found that Rh molecules in reconstituted asolectin liposomes are ≈47–50 Å apart. Furthermore, the energy transfer between donor and acceptor-modified Rh is close to the theoretically possible maximum FRET efficiency, showing that most of the Rh molecules are in a dimeric state (if not higher-order oligomers). Finally, we anticipate the approach described here may prove generally useful for quantitatively studying GPCR self-association in membranes.
Materials and Methods
Materials.
The origin of the materials used can be found in Supporting Text, which is published as supporting information on the PNAS web site.
Buffers.
Buffer A (137 mM NaCl/8 mM Na2HPO4/2.7 mM KCl/1.5 mM KH2PO4, pH 7.2), buffer B [137 mM NaCl/8 mM Na2HPO4/2.7 mM KCl/1.5 mM KH2PO4/4% 1-O-n-octyl-β-glucoside (OG)/0.1% asolectin, pH 7.2], buffer C (137 mM NaCl/8 mM Na2HPO4/2.7 mM KCl/1.5 mM KH2PO4/1.46% OG/0.1% asolectin, pH 7.2), buffer D (137 mM NaCl/8 mM Na2HPO4/2.7 mM KCl/1.5 mM KH2PO4/1.46% OG/0.1% asolectin/0.3 M Methyl α-d-Mannopyranoside, pH 7.2), and Transducin Assay Buffer (10 mM Tris·HCl, pH 7.5/0.1 M NaCl/5 mM MgCl2/1 mM EDTA) were used.
Nomenclature.
Here, rhodopsin is abbreviated as Rh. Abbreviations for rhodopsin derivatives are identified by Rh, followed by the fluorophore used in the labeling. For example, Rh–CY5 stands for Rh labeled with CY5-maleimide and Rh–Tb stands for Rh labeled with the Tb3+ chelator.
Purification and Fluorescent Labeling of Rh.
Rh was purified from ROS membranes and modified with the appropriate fluorophore. V8 proteolysis was used to assess the sites of fluorescent labeling. Specific details on these procedures are provided in Supporting Text.
Reconstitution of Purified, Fluorescently Labeled Rh.
Reconstitution of fluorescently labeled Rh into asolectin liposomes was performed in the dark under dim red light, as described (27). Different receptor densities were achieved by reconstituting samples with varying molar ratios of asolectin and Rh. The lipid/Rh ratios, 250:1, 2,000:1, or 10,000:1, should theoretically produce receptor densities of ≈800, 100, and 20 Rh protein molecules per liposome, respectively. More details are provided in Supporting Text.
Analysis of Rh-Reconstituted Proteoliposomes Using Electron Microscopy, Asp-N Proteolysis, and Transducin Activation Assays.
After reconstitution, the size of the proteoliposomes was determined by electron microscopy. Asp-N proteolysis was used to determine the relative orientation of Rh in the liposomes (27). The effect of the fluorescent labels on Rh function was measured by performing transducin activation assays (45). For details, see Supporting Text.
Determination of the Quantum Yield of Rh–CY3 Donor and R0 Value for CY3–CY5 FRET Pair.
The quantum yield for Rh–CY3 reconstituted into liposomes was measured and used to determine the overlap integral (R0) for the Rh–CY3 and Rh–CY5 FRET pair. Details are given in Supporting Text.
Isopycnic Density Centrifugation.
Isopycnic density centrifugation analysis was carried out on Rh–CY3 and Rh–CY5 reconstituted into asolectin liposomes. The liposomes were supplemented with NBD-labeled phosphatidyl serine (0.4% of the total lipid content) to enable independent fluorescent monitoring of the lipid fractions. The proteoliposomes were subjected to discontinuous flotation gradients (38), and fractions were analyzed for Rh and lipid content. See Supporting Text for more details.
Predicted Receptor Density.
The number of Rh molecules per liposome was calculated as follows. The average radius of the asolectin liposomes was ≈ 75 nm (see our EM data, Fig. 1B, and ref. 26) thus producing a liposome surface area of ≈7,000,000 Å2. Assuming the surface area of one lipid molecule to be 70 Å2, and the vesicle membranes is a bilayer, yields ≈200,000 lipids per vesicle. If equally distributed, the number of Rh molecules per liposome is
 |
where Rh/Lipid Ratio is the inverse of the lipid/Rh ratio used during the reconstitution.
Calculation of Reduced Acceptor Surface Density (CA).
The reduced acceptor surface density (CA) is equal to the R02 of the FRET pair multiplied by the surface density of acceptor-labeled proteins (46). For the CY3–CY5 FRET pair on Rh, we measured an R0 of ≈ 52 Å in the dark state and 56 Å after light activation. These values are similar to those published in refs. 22 and 24. We then calculated the CA at each lipid/Rh ratio using the following relationship (46)
 |
Note that the change in the R0 value between dark- and light-activated Rh–CY3 and Rh–CY5 requires the CA values to be calculated for each state.
Calculation of Expected Random Energy Transfer (Erandom) Based on the Reduced Acceptor Surface Density (CA).
The CA values (described above) were used to assess the amount of random “background” energy transfer expected under the different reconstitution conditions, as follows (46–48)
 |
where Erandom is the amount of random energy transfer expected, CA is the reduced acceptor surface density, and r is the distance of closest approach of the donors and acceptors (which can be approximated by the protein diameter) (46–52). The value of r for rhodopsin was approximated to be ≈48 Å, the diameter across the face of an ellipsoid shape observed for rhodopsin from the crystal structure (34).
Measurement of the Rate of LRET Between Labeled Rh Samples in Asolectin Proteolipsomes.
LRET studies were made by using a PTI LaserStrobe phosphorescence lifetime system. The Rh–Tb samples were excited with a 337-nm laser pulse, and the emission was monitored at 545 nm to obtain the lifetime of the Rh–Tb donor alone (τD), and at 570 nm, when Rh–Tb and Rh–CY3 were reconstituted together, to obtain the sensitized emission lifetime (τAD) from Rh–CY3. For further details, see Supporting Text.
Determination of Distance from LRET Measurements.
The lifetime of Rh–Tb luminescence (τD) and the Rh–CY3 sensitized emission (τAD) were used to calculate the efficiency of energy transfer (E)
 |
This efficiency was then used to calculate the distance between the two probes (35, 53)
 |
where E is the efficiency of energy transfer, R is the distance between the probes, and R0 is the distance at which the energy transfer is 50%. Eqs. 4 and 5 were used to plot τAD as a function of R. This plot yields the appropriate distance for any experimentally measured τAD (see Fig. 2D). An R0 = 61.2 Å was used for the Rh–Tb and Rh–CY3 pair (23).
To assess the maximum possible error in the LRET measurements due to uncertainties in the orientation factor (κ2), the steady-state anisotropies of Rh–CY3 and Rh–CY5 in the liposomes were measured. Details are given in Supporting Text.
FRET Steady-State and Lifetime Measurements.
The steady-state fluorescence measurements were carried out on Rh–CY3 and Rh–CY5 samples reconstituted individually and together. FRET was measured in two ways, first by measuring the emission spectrum of the acceptor while exciting the donor and then by measuring the excitation spectrum of the donor while collecting emission from the acceptor. Fluorescence lifetimes were measured on the samples reconstituted at the lowest receptor density. Further details are given in Supporting Text.
FRET Efficiency Calculated From Steady-State Fluorescence Intensity and Fluorescence Lifetimes.
FRET efficiency was determined by measuring the sensitized emission from steady-state excitation spectra using standard analysis procedures (35, 36). The FRET efficiency was also determined from the fluorescence lifetimes of the donor (Rh–CY3) in the presence and absence of the acceptor (Rh–CY5) (35). See Supporting Text for details.
Supplementary Material
Acknowledgments
We thank Mr. J. Fay for technical assistance on the transducin activation assays, Dr. E. Barklis for performing the electron microscopy analysis, and Dr. T. Huber for suggesting the isopycnic density centrifugation analysis. We thank Dr. T. Heyduk for providing lanthanide reagents for initial LRET trials, and Drs. T. Heyduk and S. Lutsenko for valuable comments on the manuscript. Finally, we thank Drs. I. Sokal and G. Jang for help in initiating this project. This work was supported by National Institute of Drug Abuse Grants DA14896 (to D.L.F.) and F30DA15584 (to S.E.M.) and in part by U.S. Public Health Service Grant EY08061 from the National Eye Institute, National Institutes of Health, Bethesda (to K.P.).
Abbreviations
- GPCR
G protein-coupled receptor
- Rh
rhodopsin
- ROS
rod outer segment
- LRET
luminescence resonance energy transfer.
Footnotes
Conflict of interest statement: No conflicts declared.
§For simplicity, we have limited our interpretation to a dimeric interaction, as suggested by others (7–10, 16), but, formally, our data cannot discriminate between Rh dimers and other higher-order oligomers.
References
- 1.Zeng F. Y., Wess J. J. Biol. Chem. 1999;274:19487–19497. doi: 10.1074/jbc.274.27.19487. [DOI] [PubMed] [Google Scholar]
- 2.Devi L. A. Trends Pharmacol. Sci. 2001;22:532–537. doi: 10.1016/s0165-6147(00)01799-5. [DOI] [PubMed] [Google Scholar]
- 3.Guo W., Shi L., Filizola M., Weinstein H., Javitch J. A. Proc. Natl. Acad. Sci. USA. 2005;102:17495–17500. doi: 10.1073/pnas.0508950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramsay D., Kellett E., McVey M., Rees S., Milligan G. Biochem. J. 2002;365:429–440. doi: 10.1042/BJ20020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park P. S., Filipek S., Wells J. W., Palczewski K. Biochemistry. 2004;43:15643–15656. doi: 10.1021/bi047907k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrillo J. J., Pediani J., Milligan G. J. Biol. Chem. 2003;278:42578–42587. doi: 10.1074/jbc.M306165200. [DOI] [PubMed] [Google Scholar]
- 7.Fotiadis D., Liang Y., Filipek S., Saperstein D. A., Engel A., Palczewski K. Nature. 2003;421:127–128. doi: 10.1038/421127a. [DOI] [PubMed] [Google Scholar]
- 8.Medina R., Perdomo D., Bubis J. J. Biol. Chem. 2004;279:39565–39573. doi: 10.1074/jbc.M402446200. [DOI] [PubMed] [Google Scholar]
- 9.Jastrzebska B., Maeda T., Zhu L., Fotiadis D., Filipek S., Engel A., Stenkamp R. E., Palczewski K. J. Biol. Chem. 2004;279:54663–54675. doi: 10.1074/jbc.M408691200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kota P., Reeves P. J., RajBhandary U. L., Khorana H. G. Proc. Natl. Acad. Sci. USA. 2006;103:3054–3059. doi: 10.1073/pnas.0510982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blasie J. K., Worthington C. R. J. Mol. Biol. 1969;39:407–416. doi: 10.1016/0022-2836(69)90135-1. [DOI] [PubMed] [Google Scholar]
- 12.Blasie J. K., Worthington C. R. J. Mol. Biol. 1969;39:417–439. doi: 10.1016/0022-2836(69)90136-3. [DOI] [PubMed] [Google Scholar]
- 13.Chabre M. Biochim. Biophys. Acta. 1975;382:322–335. doi: 10.1016/0005-2736(75)90274-6. [DOI] [PubMed] [Google Scholar]
- 14.Saibil H., Chabre M., Worcester D. Nature. 1976;262:266–270. doi: 10.1038/262266a0. [DOI] [PubMed] [Google Scholar]
- 15.Roof D. J., Heuser J. E. J. Cell Biol. 1982;95:487–500. doi: 10.1083/jcb.95.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fotiadis D., Liang Y., Filipek S., Saperstein D. A., Engel A., Palczewski K. FEBS Lett. 2004;564:281–288. doi: 10.1016/S0014-5793(04)00194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelasco A., Crouch R. K., Knapp D. R. Biochemistry. 2000;39:4907–4914. doi: 10.1021/bi992736i. [DOI] [PubMed] [Google Scholar]
- 18.Imamoto Y., Kataoka M., Tokunaga F., Palczewski K. Biochemistry. 2000;39:15225–15233. doi: 10.1021/bi0018685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang K., Farrens D. L., Altenbach C., Farahbakhsh Z. T., Hubbell W. L., Khorana H. G. Biochemistry. 1996;35:14040–14046. doi: 10.1021/bi962113u. [DOI] [PubMed] [Google Scholar]
- 20.Yu H., Kono M., Oprian D. D. Biochemistry. 1999;38:12028–12032. doi: 10.1021/bi990948+. [DOI] [PubMed] [Google Scholar]
- 21.Cai K., Klein-Seetharaman J., Hwa J., Hubbell W. L., Khorana H. G. Biochemistry. 1999;38:12893–12898. doi: 10.1021/bi9912443. [DOI] [PubMed] [Google Scholar]
- 22.Kenworthy A. K. Methods. 2001;24:289–296. doi: 10.1006/meth.2001.1189. [DOI] [PubMed] [Google Scholar]
- 23.Selvin P. R. Annu. Rev. Biophys. Biomol. Struct. 2002;31:275–302. doi: 10.1146/annurev.biophys.31.101101.140927. [DOI] [PubMed] [Google Scholar]
- 24.Li E., You M., Hristova K. Biochemistry. 2005;44:352–360. doi: 10.1021/bi048480k. [DOI] [PubMed] [Google Scholar]
- 25.Farrens D. L., Altenbach C., Yang K., Hubbell W. L., Khorana H. G. Science. 1996;274:768–770. doi: 10.1126/science.274.5288.768. [DOI] [PubMed] [Google Scholar]
- 26.Fenske D. B., MacLachlan I., Cullis P. R. Methods Enzymol. 2002;346:36–71. doi: 10.1016/s0076-6879(02)46048-x. [DOI] [PubMed] [Google Scholar]
- 27.Niu L., Kim J. M., Khorana H. G. Proc. Natl. Acad. Sci. USA. 2002;99:13409–13412. doi: 10.1073/pnas.212518899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palczewski K., Buczylko J., Kaplan M. W., Polans A. S., Crabb J. W. J. Biol. Chem. 1991;266:12949–12955. [PubMed] [Google Scholar]
- 29.Heyduk T., Heyduk E. Anal. Biochem. 2001;289:60–67. doi: 10.1006/abio.2000.4925. [DOI] [PubMed] [Google Scholar]
- 30.Heyduk T. Curr. Opin. Biotechnol. 2002;13:292–296. doi: 10.1016/s0958-1669(02)00332-4. [DOI] [PubMed] [Google Scholar]
- 31.Heyduk E., Heyduk T. Anal. Biochem. 1997;248:216–227. doi: 10.1006/abio.1997.2148. [DOI] [PubMed] [Google Scholar]
- 32.Selvin P. R., Hearst J. E. Proc. Natl. Acad. Sci. USA. 1994;91:10024–10028. doi: 10.1073/pnas.91.21.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Selvin P. R., Rana T. M., Hearst J. E. J. Am. Chem. Soc. 1994;116:6029–6030. [Google Scholar]
- 34.Palczewski K., Kumasaka T., Hori T., Behnke C. A., Motoshima H., Fox B. A., Le Trong I., Teller D. C., Okada T., Stenkamp R. E., et al. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 35.Stryer L. Annu. Rev. Biochem. 1978;47:819–846. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- 36.Fisher L. E., Engelman D. M., Sturgis J. N. J. Mol. Biol. 1999;293:639–651. doi: 10.1006/jmbi.1999.3126. [DOI] [PubMed] [Google Scholar]
- 37.Cha A., Snyder G. E., Selvin P. R., Bezanilla F. Nature. 1999;402:809–813. doi: 10.1038/45552. [DOI] [PubMed] [Google Scholar]
- 38.Rigaud J. L., Paternostre M. T., Bluzat A. Biochemistry. 1988;27:2677–2688. doi: 10.1021/bi00408a007. [DOI] [PubMed] [Google Scholar]
- 39.Chabre M., Cone R., Saibil H. Nature. 2003;426:30–31. doi: 10.1038/426030b. discussion, 31. [DOI] [PubMed] [Google Scholar]
- 40.Filipek S., Krzysko K. A., Fotiadis D., Liang Y., Saperstein D. A., Engel A., Palczewski K. Photochem. Photobiol. Sci. 2004;3:628–638. doi: 10.1039/b315661c. [DOI] [PubMed] [Google Scholar]
- 41.Hofmann K. P., Pulvermuller A., Buczylko J., Van Hooser P., Palczewski K. J. Biol. Chem. 1992;267:15701–15706. [PubMed] [Google Scholar]
- 42.Han M., Gurevich V. V., Vishnivetskiy S. A., Sigler P. B., Schubert C. Structure (Cambridge, U.K.) 2001;9:869–880. doi: 10.1016/s0969-2126(01)00644-x. [DOI] [PubMed] [Google Scholar]
- 43.Granzin J., Wilden U., Choe H. W., Labahn J., Krafft B., Buldt G. Nature. 1998;391:918–921. doi: 10.1038/36147. [DOI] [PubMed] [Google Scholar]
- 44.Gurevich V. V., Gurevich E. V. Trends Pharmacol. Sci. 2004;25:105–111. doi: 10.1016/j.tips.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 45.Oprian D. D., Molday R. S., Kaufman R. J., Khorana H. G. Proc. Natl. Acad. Sci. USA. 1987;84:8874–8878. doi: 10.1073/pnas.84.24.8874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kenworthy A. K., Edidin M. J. Cell Biol. 1998;142:69–84. doi: 10.1083/jcb.142.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dewey T. G., Hammes G. G. Biophys. J. 1980;32:1023–1035. doi: 10.1016/S0006-3495(80)85033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolber P. K., Hudson B. S. Biophys. J. 1979;28:197–210. doi: 10.1016/S0006-3495(79)85171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yguerabide J. Biophys. J. 1994;66:683–693. doi: 10.1016/s0006-3495(94)80842-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fung B. K., Stryer L. Biochemistry. 1978;17:5241–5248. doi: 10.1021/bi00617a025. [DOI] [PubMed] [Google Scholar]
- 51.Shaklai N., Yguerabide J., Ranney H. M. Biochemistry. 1977;16:5585–5592. doi: 10.1021/bi00644a031. [DOI] [PubMed] [Google Scholar]
- 52.Snyder B., Freire E. Biophys. J. 1982;40:137–148. doi: 10.1016/S0006-3495(82)84468-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stryer L., Haugland R. P. Proc. Natl. Acad. Sci. USA. 1967;58:719–726. doi: 10.1073/pnas.58.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.