Abstract
Rhodopsin in the disk membranes of rod outer segments serves as the dim-light photoreceptor and is a prototypic member of a G protein-coupled receptor family. Electron and atomic-force microscopy indicate that rhodopsin is present as dimers in the native membranes. Here, we have expressed the protein, opsin, in COS1 cells and have studied its molecular state by using FRET and by intermolecular cross-linking after site-directed cysteine mutagenesis. To observe FRET, the ends of the genes corresponding to the N termini of the cyan or yellow fluorescent proteins were fused to the ends of the genes corresponding to the C terminus of the opsin and the resulting fused genes were expressed in COS1 cells. The emission spectra in situ of the expressed proteins were recorded, and FRET was then calculated. The result indicated intermolecular interaction between opsin molecules in COS1 cells. To identify the amino acids involved in the interaction, those predicted by molecular modeling to be at the dimer interface were mutated one at a time to cysteine, and dimer formation was measured by the rate of disulfide bond formation in the presence of cupric orthophenanthroline. The mutants W175C and Y206C formed the dimers most rapidly, showing that the two amino acids were at the dimer interface.
Keywords: cross-linking, cysteine mutagenesis, dimerization, FRET, G protein-coupled receptors
G protein-coupled receptors (GPCRs) constitute the largest group of transmembrane receptors and mediate virtually all of the physiological processes (1). Rhodopsin is a prototypic member of this group that has been studied extensively and its crystal structure has been determined (2). Therefore, rhodopsin has served as a model for structure–function studies of the GPCR family. Rhodopsin is activated by light to a form that binds to the heterotrimeric G protein transducin, and the resulting complex undergoes a series of enzyme-mediated changes that lead to vision. It has been suggested that several of the GPCRs form and function as oligomers (refs. 3 and 4; also reviewed in refs. 5–8). Although early biochemical and biophysical studies suggested rhodopsin to be present as monomers in both membranes and detergents (9–12), atomic-force microscopy recently indicated that rhodopsin exists as dimers in the native disk membranes (13, 14). Furthermore, chemical cross-linking studies also suggest that rhodopsin is present as dimers and higher oligomers in different detergents (15–17).
Here, we have studied the molecular state of opsin as expressed in COS1 cells by using FRET and by cysteine cross-linking in the presence of copper phenanthroline (CuP). We find that the expressed opsin is present as dimers and that the amino acids at the dimer interface include tryptophan 175 and tyrosine 206.
Results
FRET Between Opsin Molecules in COS1 Cells.
To study the oligomeric nature of opsin in the heterologous COS1 expression system by FRET, the cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP) coding sequences were fused to the C terminus of opsin (Fig. 1A) (18). This fusion was followed by the addition of the sequence of the eight amino acids corresponding to the 1D4 epitope (see Materials and Methods). The genes for the fusion proteins, Ops.CFP and Ops.YFP, were transiently expressed in COS1 cells, and the proteins were purified; their UV/visible absorption spectra contained the absorption maxima characteristic of rhodopsin and that of the fluorescent proteins (data not shown), indicating the presence of the chromophores from rhodopsin and those of the fluorescence probes. The expressed fusion proteins were visualized by fluorescence imaging (data not shown) and immunoblotting (see Fig. 7 A and B, which is published as supporting information on the PNAS web site). The cells expressing the fluorescent proteins or the fusion proteins were lysed in 20 mM N-dodecyl-β-d-maltoside (DM), and the proteins in the lysate were resolved by 12% SDS/PAGE. The separated proteins were transferred to nitrocellulose membranes, where they were visualized by immunoblotting. Opsin was detected by using rho-1D4 antibody (Fig. 7A), whereas CFP and YFP proteins were visualized by using the anti-GFP antibody (Fig. 7B). The fusion proteins showed two bands (Fig. 7 A and B), as seen earlier for rho.EGFP (18, 19).
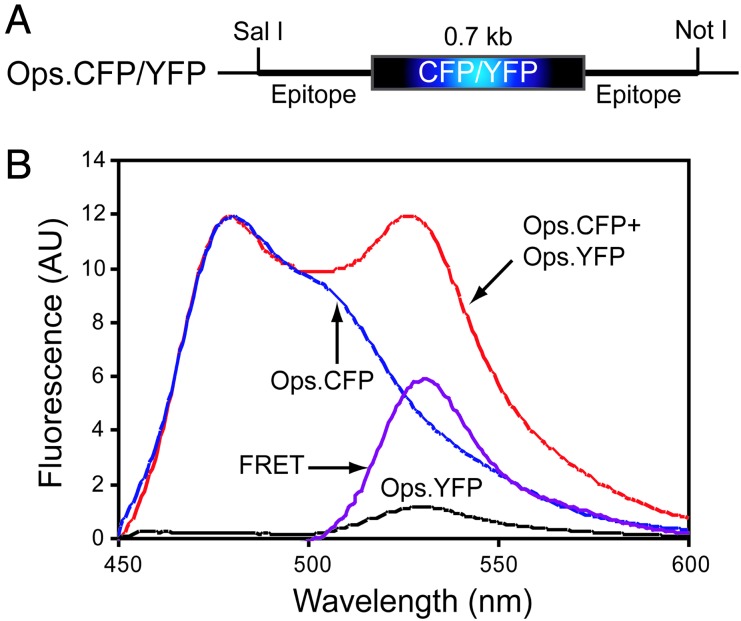
Fig. 1.
Schematic representation of plasmid constructs (A) and oligomerization of opsin in vivo detected by FRET (B). Opsin fusion proteins Ops.CFP and Ops.YFP were expressed separately and together in COS1 cells for FRET experiments. Whole cells expressing these fusion proteins were excited at 425 nm, and their emission spectra were recorded. FRET data were analyzed as described in Materials and Methods. Emission spectra were recorded from cells expressing Ops.CFP and Ops.YFP separately and together. Emission due to FRET was determined by subtracting the emission spectra of cells expressing Ops.CFP alone and cells expressing Ops.YFP alone from the cells coexpressing Ops.CFP + Ops.YFP as described in Materials and Methods.
To observe FRET between Ops.CFP and Ops.YFP, cells coexpressing the two proteins were excited at 425 nm, the excitation maximum of CFP, and fluorescence emission was recorded from 450–600 nm. Two emission peaks, at 480 nm and at 530 nm, were seen (Fig. 1B). The peak at 530 nm represented the emission from YFP due to FRET. No such peak was observed in the emission spectrum when an equal number of cells expressing only Ops.CFP or only Ops.YFP were mixed in a cuvette and irradiated at 425 nm. Furthermore, when cells coexpressing Ops.CFP and YFP, but without opsin, were excited at 425 nm, a peak at 510 nm was seen that was clearly different from that obtained from cells coexpressing Ops.CFP and Ops.YFP. In contrast, when the histone methylation reporter, consisting of histone sequences fused to YFP at its N terminus and the CFP at its C terminus, was used as a positive control (20) to detect FRET between CFP and YFP, emission peaks at 480 nm and 530 nm identical to those obtained from cells coexpressing Ops.CFP and Ops.YFP were observed (see Fig. 8, which is published as supporting information on the PNAS web site).
After allowing for differences in protein expression levels, the emission spectra of the control cells expressing either Ops.CFP or Ops.YFP were subtracted from the spectra obtained from cells coexpressing Ops.CFP and Ops.YFP. The resulting spectra (Fig. 1B) demonstrated FRET between CFP- and YFP-tagged opsins, showing that opsin exists as dimers or higher oligomers in COS1 cells.
Identification of Amino Acids at the Dimer Interface of Opsin by Using Cysteine Mutagenesis.
FRET indicates interaction between two molecules of opsin, but does not provide information about the dimer interface. For identification of the amino acids present at the interface, site-directed cysteine mutagenesis was performed. Amino acid residues (Fig. 2) predicted to be at the dimer interface by molecular modeling (21) were replaced by cysteine one at a time, and the resulting cysteine mutants were expressed in COS1 cells, separately and in combinations (see Table 1, which is published as supporting information on the PNAS web site). The protein profiles of some of the cysteine mutants and WT opsin as visualized by immunoblotting are shown in Fig. 3. The replacement of Trp 175 and Tyr 206 by cysteine residues one at a time clearly resulted in the formation of opsin dimers in the absence of DTT, but not in its presence, suggesting that dimerization was due to the spontaneous formation of a disulfide bond between two molecules of the mutant W175C or of the mutant Y206C (Fig. 3A). Therefore, the cysteine mutants W175C and Y206C were used to study the oligomeric nature of opsin in COS1 cells by FRET and by disulfide cross-linking by using CuP (see below).
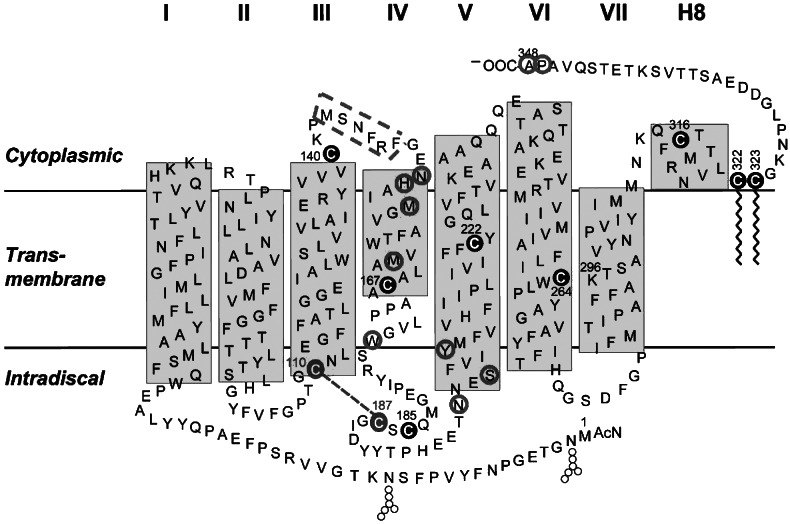
Fig. 2.
A secondary structure model of rhodopsin, showing cysteines occurring naturally (shaded circles) and cysteines that replaced amino acid residues (open circles).
Fig. 3.
Immunoblot analysis of WT opsin and cysteine mutants expressed in COS1 cells. Cells were harvested 48 h after transfection, and the extracts were prepared after lysis in 20 mM DM. Proteins were resolved by 12% SDS/PAGE in the presence or absence of DTT, and opsins were visualized by immunoblotting. The dimers are indicated by arrows. Single expression (A) or coexpression (B) of cysteine mutants in COS1 cells.
Dimer formation was also observed in lysates prepared from cells coexpressing the mutants W175C and S202C in the absence of DTT (Fig. 3B). The dimers could result from disulfide bond formation either between two molecules of the W175C mutant or between the mutants W175C and S202C.
The CFP and YFP proteins were fused to the C termini of the W175C and Y206C mutants, as described for WT opsin in Fig. 1, and the resulting fusion proteins were expressed separately or together in COS1 cells. After excitation of the cells at 425 nm (Fig. 4A and B), the emission spectra were recorded from 450–600 nm. Only a minor difference was observed in the FRET between fusion proteins of WT opsin (Fig. 4, purple trace) and those of the W175C and Y206C mutants. This difference was more apparent in the presence of the detergent DM. Fig. 4C shows the effect of changes in the DM concentration on FRET between CFP and YFP in the various fusion proteins: WT opsin and the W175C and Y206C mutants. A decrease in FRET of >80% was seen for WT opsin fusion proteins at a DM concentration of 0.2 mM, whereas the fusion proteins containing the cysteine mutants W175C or Y206C required ≥1 mM DM concentration for a similar decrease (Fig. 4C). In addition, whereas FRET from the WT opsin fusion protein was completely abolished in 0.5 mM DM, higher concentrations of DM were required to reduce the FRET in the cysteine mutants. Even at 20 mM DM, FRET was not completely abolished, at least in the case of the W175C mutant. These results suggest that, at high concentration of DM, mutants W175C and Y206C are present as both monomers and dimers, whereas WT opsin is present as monomers.
Fig. 4.
Oligomerization of WT opsin and of opsin cysteine mutants W175C and Y206C in vivo as studied by FRET. Whole cells expressing various rhodopsin fusion proteins were excited at 425 nm, and their emission spectra were recorded. FRET data were analyzed as described in Materials and Methods. (A) Emission spectra of cells expressing W175C.CFP and W175C.YFP separately and together. (B) Emission spectra of cells expressing Y206C.CFP and Y206C.YFP separately and together. The dotted line (WT FRET) is the FRET spectrum of Ops.CFP + Ops.YFP. (C) Effect of DM on FRET. Cells expressing opsin fusion proteins were suspended in PBS and 0.1 mM PMSF in the presence of varying concentrations of DM and excited at 425 nm, and their emission spectra were recorded.
Chemical Cross-Linking by Using CuP.
To identify the amino acids at the dimer interface, disulfide cross-linking was carried out by using CuP. Membranes from cells expressing WT opsin or the mutants W175C or Y206C were isolated and treated with 400 μM CuSO4 containing 1,600 μM 1,10-phenanthroline for different periods of time at room temperature. The resulting proteins were fractionated on a 12% SDS-polyacrylamide gel in the absence of DTT, and the opsins were visualized by immunoblotting. In the absence of CuP, WT opsin migrated predominantly as a 38-kDa species, corresponding to the monomer. In the presence of CuP, there was a small time-dependent decrease in amount of the 38-kDa species, accompanied by a small increase in a species approximately twice the size of the monomer (Fig. 5A). In the case of the W175C and Y206C mutants, the major species, even in the absence of CuP, corresponded to the dimer. Addition of CuP resulted in the complete disappearance of the opsin monomer within 5 min. However, the decrease in the monomer species did not show good correlation with the increase in the dimer species (Fig. 5 B and C), possibly because of the concomitant formation of higher oligomers.
Fig. 5.
Cross-linking of opsin by CuP. Cells were harvested 48 h after transfection, and membranes were prepared as described in Materials and Methods. The membranes were treated with 400 μM CuSO4 and 1600 μM 1,10-phenanthroline for 1, 2, 5, 10, and 15 min and, then, with 20 mM NEM containing 20 mM EDTA. The proteins were resolved by SDS/PAGE and visualized by immunoblotting. (A) WT. (B) W175C. (C) Y206C.
The reactivity of the cysteines in WT opsin and in the W175C and Y206C mutants was assessed by using different concentrations of CuP. The WT opsin showed a small concentration-dependent increase in the dimer species concomitant with a decrease in the monomer species (Fig. 6A). The W175C mutant was mostly dimeric, even in the absence of CuP, the residual monomer being converted to the dimer at the lowest concentration of CuP used (10/40 μM) (Fig. 6B). These results are similar to those of Falke et al. (22) on site-specific cysteine mutants of Salmonella typhimurium aspartate receptor. The Y206C mutant contained a relatively low amount of the dimeric species in the absence of CuP compared with the W175C mutant, and it required a higher concentration of CuP for complete conversion to the dimeric species (Fig. 6C). In the case of the W175C and the Y206C mutants, a few faint bands, presumably corresponding to tri- and tetramers, were also seen in the presence of CuP (Fig. 6 B and C).
Fig. 6.
Cross-linking of opsin by CuP. Cells were harvested 48 h after transfection, and membranes were prepared as described in Materials and Methods. The membranes were treated with CuP (0, 10/40, 25/100, 50/200, and 100/400 μM) for 10 min at room temperature and, then, with 20 mM NEM containing 20 mM EDTA. The proteins were resolved by SDS/PAGE and visualized by immunoblotting. (A) WT. (B) W175C. (C) Y206C.
Discussion
By using FRET, site-directed cysteine mutagenesis, and disulfide cross-linking, we have demonstrated intermolecular interaction between opsin molecules in COS1 cells and have identified the amino acids present at the dimer interface. Guided by intermolecular interactions between opsin molecules, as indicated by FRET, the amino acids, predicted by molecular modeling to be at the dimer interface (21), were mutated to cysteine one at a time. Of these, the cysteine mutants W175C and Y206C formed DTT-sensitive homodimers, and, furthermore, these mutants formed CuP-induced disulfide cross-linked dimers most rapidly. These results provide experimental evidence that the amino acids W175 and Y206 are present at the interface.
Several lines of evidence have indicated that rhodopsin exists as dimers in ROS (13–17), and molecular modeling studies have predicted the amino acids involved in the interaction between the monomers (21). However, the contact points in the interaction between the monomers remain unknown. Because identification of the amino acids involved in the interaction between rhodopsin monomers in the native disk membranes is not feasible, a heterologous expression system has been used in this study. The use of a heterologous expression system has enabled a study of the molecular arrangement of opsin by using techniques, such as FRET and disulfide cross-linking after site-directed cysteine mutagenesis.
FRET is a powerful tool for the study of molecular interaction between two functional receptors in living cells (23). Here, we have used FRET to study possible interaction between opsin molecules in COS1 cells. Furthermore, resonance energy transfer has been used in studies of rhodopsin (24), β2-adrenergic (25), CCR5 (26), oxytocin and vasopressin (27), and somatostatin receptors (28). In this study, the GFP variants CFP or YFP, considered to be the best FRET pair (29), were attached to the C terminus of opsin. The resulting fusion proteins were expressed separately and together in COS1 cells. FRET was observed only in the cells coexpressing Ops.CFP and Ops.YFP. The negative controls in our FRET studies further confirm that FRET is not just due to expression of CFP and YFP in the same cells. We can also rule out the possibility of the interaction between CFP and YFP as a mediator of rhodopsin dimer formation, because it has been shown that GFP molecules can dimerize only at very high concentrations (30). Independently, Mansoor et al. (31) have used luminescence and FRET to show dimer formation between rhodopsin molecules in liposomes.
We found that the opsins containing the W175C and Y206C mutations as isolated from the membranes existed mostly in the dimeric forms (Figs. 5 and 6). Thus, the cysteines in these mutants had spontaneously oxidized during membrane preparation to form intermolecular disulfide bonds. This result means that the α-carbon atoms of the cysteines are in close proximity (≈7 Å) (32). Further evidence for this came from the finding that the mutant opsins W175C and Y206C readily formed dimers in membrane preparations in the presence of the oxidizing agent CuP. Spontaneous intramolecular disulfide bond formation has been described in cysteine mutants of rhodopsin (33) and of the aspartate receptor from S. typhimurium (22). Furthermore, CuP has been used in intermolecular disulfide bond formation between rhodopsin and transducin (34) and in homodimerization of aspartate, C5a, and D2 dopamine receptors (22, 35–37). Interestingly, in the presence of CuP, the W175C opsin mutant formed dimers more readily than did the Y206C mutant (Fig. 6), possibly because of differences in proximity of the cysteines, the inherent reactivity of the cysteines, or accessibility of the cysteines to CuP.
Whole-cell extracts prepared from the mutants W175C and Y206C, at pH 7.2 in 20 mM DM, showed the presence of mostly monomers and some dimers on SDS polyacrylamide gels (Fig. 3), whereas dimers were the major species in the cell membranes (Figs. 5 and 6). In agreement with these results, higher concentrations of DM significantly reduced the efficiency of FRET of the cysteine mutants, indicating that in 20 mM DM at pH 7.2 (Fig. 4C), opsin is present as both monomers and dimers, with the monomers predominating.
Tryptophan 175 is present on the extracellular loop connecting TM4 and TM5, and tyrosine 206 is present on the extracellular side of the transmembrane domain (Fig. 2). In the model proposed for mouse rhodopsin dimers (21), TM4 and TM5 are believed to be at the dimer interface. The dimer interface for squid rhodopsin has been shown to involve TM4 (38). Also, using disulfide cross-linking with CuP, Guo et al. (35, 36) showed that dopamine D2 receptors can form dimers and that TM4 dimer interface is part of the receptor activation mechanism. Our finding that the W175C and Y206C mutants readily form dimers is in agreement with the proposed model and is most easily explained by disulfide bond formation between 175C to 175C and 206C to 206C in these mutants. The possibility that cysteines at position 175 or 206 are forming disulfide bonds with other cysteines in opsin is extremely unlikely, because 175C or 206C mutants of opsin lacking 6 of the 10 cysteine residues of opsin still form dimers (data not shown). The 4 remaining cysteines in opsin are either already in disulfide form (C110 and C187) or are palmitoylated (C322 and C323). Our results thus provide experimental support to the hypothesis that, in WT opsin, hydrophobic interactions between two tryptophans at 175 and two tyrosines at 206 contribute toward dimerization of opsin. Finally, although our studies have demonstrated close interaction between opsin molecules in the membrane, they have not addressed the question of the role of dimerization in opsin function.
Materials and Methods
The detergent DM was purchased from Anatrace (Maumee, OH). The monoclonal anti-rhodopsin antibody rho-1D4 (39) was obtained from the National Cell Culture Center (Minneapolis), where it was prepared from a cell line provided by R. S. Molday (University of British Columbia, Vancouver). CuSO4, 1,10-phenanthroline, and N-ethylmaleimide were purchased from Sigma.
Construction of Opsin Cysteine Mutants and the Fusion Plasmids Ops.CFP and Ops.YFP.
Cysteine mutants were constructed by the QuickChange mutagenesis procedure, developed by Stratagene. DNA sequences coding for CFP or YFP were cloned between the SalI and NotI sites of the mammalian cell expression vector pMT4, bearing the WT opsin gene or its cysteine mutants W175C or Y206C. The resulting DNAs code for fusion proteins that contain, at the N terminus, the WT opsin gene or its mutants containing W175C or Y206C replacements, followed by the fluorescent protein CFP or YFP and, at the C terminus, the eight amino acids corresponding to the epitope for the rho-1D4 antibody (Fig. 1A). Two copies of the 1D4 epitope are present in these constructs to ensure that the fusion protein is recognized by the rho-1D4 antibody.
Expression of the Opsin Fusion Proteins and Opsin Cysteine Mutants.
For expression of opsin in COS-1 cells, a plate (150 × 25 mm) of confluent COS1 cells was transfected with either 15 μg of plasmid DNA for single transfections or 7.5 μg of each plasmid DNA for cotransfections. Forty-eight hours after transfection, the plates were washed two times with cold PBS, and the cells were harvested by using 4 ml of PBS containing 0.1 mM PMSF.
FRET.
To detect FRET, fluorescence spectra of intact COS1 cells transiently coexpressing CFP- and YFP-tagged opsin were recorded by using a spectrofluorometer and felix 1.42b software (Photon Technology International). FRET data were analyzed, and calculations were performed essentially as described by Overton and Blumer (40). In every FRET experiment, four samples containing the same number of cells are used as follows: sample 1, cells that express opsin alone without tags; sample 2, cells that express only Ops.CFP; sample 3, cells that express only Ops.YFP; and sample 4, cells that coexpress Ops.CFP and Ops.YFP. FRET was observed by using the following three-step procedure. Step 1, the autofluorescence emission spectrum of cells that contained opsin alone without a tag was subtracted from that of an equivalent number of cells coexpressing Ops.CFP and Ops.YFP, resulting in the emission curve labeled Ops.CFP + Ops.YFP in Fig. 1B. This Ops.CFP + Ops.YFP emission spectrum is due to Ops.CFP emission, Ops.YFP emission due to direct excitation, and Ops.YFP emission due to FRET. The autofluorescence of cells expressing opsin alone was also subtracted from the cells expressing Ops.CFP or Ops.YFP. Step 2, cells expressing Ops.CFP alone were excited at 425 nm, the λexcitation of CFP, and the emission spectrum was recorded. This spectrum, designated Ops.CFP in Fig. 1B, was normalized to that of Ops.CFP + Ops.YFP spectrum from the preceding step by matching its peak height at 480 nm to that of Ops.CFP + Ops.YFP. The normalized Ops.CFP spectrum was subtracted from that of Ops.CFP + Ops.YFP spectrum obtained in the above step. The resulting spectrum gives YFP emission due to FRET and due to direct excitation of YFP. Step 3, to obtain the YFP emission spectrum due to FRET, we subtracted YFP emission that is due to direct excitation of YFP from the spectrum obtained from step 2. The YFP emission spectrum of cells coexpressing Ops.CFP and Ops.YFP receptors and the spectrum of cells expressing only Ops.YFP were corrected for differences in YFP expression level by exciting these cells at λexcitation maximum, 475 nm, for YFP and recording their YFP emission spectra. These emission spectra specifically quantify YFP, because CFP is not excited at this wavelength. The ratio of YFP emissions was used to normalize the background emission spectrum obtained when cells expressing only Ops.YFP were excited at 425 nm. The normalized emission spectrum, Ops.YFP in Fig. 1B, was subtracted from the emission spectrum obtained in step 2 from cells coexpressing Ops.CFP and Ops.YFP. The resulting YFP emission spectrum is due to apparent FRET (designated FRET in Fig. 1B).
Preparation of COS1 Cell Membranes.
Forty-eight hours after transfection of a culture of cells, the culture medium was removed, and the cells were washed twice with 25 ml each of PBS and once with 5 ml of lysis buffer (10 mM Tris·HCl, pH 7.4, 1 mM EDTA, and 0.4 mM PMSF). The cells were harvested and treated with 15 ml of the lysis buffer containing 5 μg/ml leupeptin, 0.7 μg/ml pepstatin A, 10 μg/ml benzamidin, and 0.4 mM PMSF. The suspension was homogenized in a 15-ml hand-held glass homogenizer (30 strokes), and the homogenate, after passage through a 26 3/8-gauge needle, was centrifuged (300 × g for 10 min at 4°C) to remove unbroken cells and nuclei. The supernatant solution was carefully transferred to a new tube, which was centrifuged at 100,000 × g for 20 min in a Ti60 rotor. The resulting pellet was resuspended in buffer A (20 mM Tris·HCl, pH 7.4, 150 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, and 1 mM EDTA) by using a hand-held glass homogenizer and the suspension centrifuged again at 100,000 × g for 20 min. The resulting pellet containing the membranes was resuspended in 1 ml of buffer A. A portion of the suspension was used for cross-linking, and the rest was frozen in 100-μl aliquots at −80°C. Protein concentration was determined by using the Bradford reagent with BSA as standard.
Chemical Cross-Linking of Opsin by Using CuP.
Chemical cross-linking of opsin in COS1 cell membranes with CuP was performed in a reaction mixture (100 μl) containing 20 mM Tris·HCl, pH 8, 1 mM CaCl2, 5 mM MgCl2, and 100 mM NaCl. The membrane suspension was treated with either 400 μM CuSO4 containing 1,600 μM 1,10-phenanthroline for different times at room temperature or with different concentrations of CuP (10, 25, 50, and 100 μM CuSO4 per 40, 100, 200, and 400 μM 1,10 phenanthroline) for 10 min at room temperature. The reaction was terminated by addition of SDS loading buffer, which contained 20 mM NEM, 20 mM EDTA, and 0.5 mM PMSF. The proteins were separated on a 12% SDS-polyacrylamide gel in the absence of any reducing agents. In the control, the membranes were first incubated for 10 min at room temperature with 20 mM NEM, 20 mM EDTA, and 0.5 mM PMSF and then were treated with 400 μM CuSO4 and 1600 μM 1,10-phenanthroline for 10 min at room temperature. The SDS loading buffer was then added, and the proteins were resolved by SDS/PAGE and transferred to nitrocellulose membrane, and opsins were visualized by immunoblotting with rho-1D4 antibody.
Supplementary Material
Acknowledgments
We thank Dr. Alice Ting, Department of Chemistry, Massachusetts Institute of Technology (MIT), for the histone methylation reporter plasmid; Dr. Peter So, Department of Biological Engineering, MIT, for valuable discussions on FRET; and Ms. Judy Carlin for enthusiastic assistance in the preparation of the manuscript. This work was supported by National Institutes of Health Grant GM28289, National Eye Institute Grant EY11716, and National Science Foundation Grant EIA-0225609.
Abbreviations
- CuP
copper phenanthroline
- DM
N-dodecyl-β-d-maltoside
- CFP
cyan fluorescent protein
- YFP
yellow fluorescent protein.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Bouvier M. Nat. Rev. Neurosci. 2001;2:274–286. doi: 10.1038/35067575. [DOI] [PubMed] [Google Scholar]
- 2.Palczewski K., Kumasaka T., Hori T., Behnke C. A., Motoshima H., Fox B. A., LeTrong I., Teller D. C., Okada T., Stenkamp R. E., et al. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 3.White J. H., Wise A., Main M. J., Green A., Fraser N. J., Disney G. H., Barnes A. A., Emson P., Foord S. M., Marshall F. H. Nature. 1998;396:679–682. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]
- 4.Nelson G., Hoon M. A., Chandrasekhar J., Zhang Y., Ryba N. J. P., Zuker C. S. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 5.George S.R., O’Dowd B. F., Lee S. P. Nat. Rev. Drug Discov. 2002;1:808–820. doi: 10.1038/nrd913. [DOI] [PubMed] [Google Scholar]
- 6.Milligan G., Ramsay D., Pascal G., Carrillo J. J. Life Sci. 2003;74:181–188. doi: 10.1016/j.lfs.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Park P.S.-H., Filipek S., Wells J. W., Palczewski K. Biochemistry. 2004;43:15643–15656. doi: 10.1021/bi047907k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prinster S. C., Hague C., Hall R. A. Pharmacol. Rev. 2005;57:289–298. doi: 10.1124/pr.57.3.1. [DOI] [PubMed] [Google Scholar]
- 9.Saibil H., Chabre M., Worcester D. Nature. 1976;262:266–270. doi: 10.1038/262266a0. [DOI] [PubMed] [Google Scholar]
- 10.Kuhn H. Nature. 1980;283:587–589. doi: 10.1038/283587a0. [DOI] [PubMed] [Google Scholar]
- 11.Klingenberg M. Nature. 1981;290:449–454. doi: 10.1038/290449a0. [DOI] [PubMed] [Google Scholar]
- 12.Chabre M., Maire M. L. Biochemistry. 2005;44:9395–9403. doi: 10.1021/bi050720o. [DOI] [PubMed] [Google Scholar]
- 13.Fotiadis D., Liang Y., Filipek S., Saperstein D. A., Engel A., Palczewski K. Nature. 2003;421:127–128. doi: 10.1038/421127a. [DOI] [PubMed] [Google Scholar]
- 14.Liang Y., Fotiadis D., Filipek S., Saperstein D. A., Palczewski K., Engel A. J. Biol. Chem. 2003;278:21655–21662. doi: 10.1074/jbc.M302536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jastrzebska B., Maeda T., Zhu L., Fotiadis D., Filipek S., Engel A., Stenkamp R. E., Palczewski K. J. Biol. Chem. 2004;279:54663–54675. doi: 10.1074/jbc.M408691200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medina R., Perdomo D., Bubis J. J. Biol. Chem. 2004;279:39565–39573. doi: 10.1074/jbc.M402446200. [DOI] [PubMed] [Google Scholar]
- 17.Suda K., Filipek S., Palczewski K., Engel A., Fotiadis D. Mol. Membr. Biol. 2004;21:435–446. doi: 10.1080/09687860400020291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin S., McKee T. D., Oprian D. D. FEBS Lett. 2003;542:142–146. doi: 10.1016/s0014-5793(03)00368-5. [DOI] [PubMed] [Google Scholar]
- 19.Moritz O. L., Tam B. M., Papermaster D. S., Nakayama T. J. Biol. Chem. 2001;276:28242–28251. doi: 10.1074/jbc.M101476200. [DOI] [PubMed] [Google Scholar]
- 20.Lin C.-W., Jao C. Y., Ting A. Y. J. Am. Chem. Soc. 2004;126:5982–5983. doi: 10.1021/ja038854h. [DOI] [PubMed] [Google Scholar]
- 21.Fotiadis D., Liang Y., Filipek S., Saperstein D. A., Engel A., Palczewski K. FEBS Lett. 2004;564:281–288. doi: 10.1016/S0014-5793(04)00194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falke J. J., Dernburg A. F., Sternberg D. A., Zalkin N., Milligan D. L., Koshland D. E. J. Biol. Chem. 1988;263:14850–14858. [PubMed] [Google Scholar]
- 23.Tsien R. Y., Miyawak A. Science. 1998;280:1954–1955. doi: 10.1126/science.280.5371.1954. [DOI] [PubMed] [Google Scholar]
- 24.Rajan R. S., Kopito R. R. J. Biol. Chem. 2005;280:1284–1291. doi: 10.1074/jbc.M406448200. [DOI] [PubMed] [Google Scholar]
- 25.Angers S., Salahpour A., Joly E., Hilairet S., Chelsky D., Dennis M., Bouvier M. Proc. Natl. Acad. Sci. USA. 2000;97:3684–3689. doi: 10.1073/pnas.060590697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Issafras H., Angers S., Bulenger S., Blanpain C., Parmentier M., Labbe-Jullie C., Bouvier M., Marullo S. J. Biol. Chem. 2002;277:34666–34673. doi: 10.1074/jbc.M202386200. [DOI] [PubMed] [Google Scholar]
- 27.Terrillon S., Durroux T., Mouillac B., Briet A., Ayoub M. A., Taulan M., Jockers R., Barberis C., Bouvier M. Mol. Endocrinol. 2003;17:677–691. doi: 10.1210/me.2002-0222. [DOI] [PubMed] [Google Scholar]
- 28.Rocheville M., Lange D. C., Kumar U., Sasi R., Patel R. C., Patel Y. C. J. Biol. Chem. 2000;275:7862–7869. doi: 10.1074/jbc.275.11.7862. [DOI] [PubMed] [Google Scholar]
- 29.Miyawak A., Tsien R. Y. Methods Enzymol. 2000;327:472–500. doi: 10.1016/s0076-6879(00)27297-2. [DOI] [PubMed] [Google Scholar]
- 30.Zacharias D. A., Violin J. D., Newton A. C., Tsien R. Y. Science. 2002;296:913–916. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- 31.Mansoor S. E., Palczewski K., Farrens D. L. Proc. Natl. Acad. Sci. USA. 2006;103:3060–3065. doi: 10.1073/pnas.0511010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Careaga C. L., Falke J. J. J. Mol. Biol. 1992;226:1219–1235. doi: 10.1016/0022-2836(92)91063-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang K., Farrens D. L., Altenbach C., Farahbakhsh Z. T., Hubbell W. L., Khorana H. G. Biochemistry. 1996;35:14040–14046. doi: 10.1021/bi962113u. [DOI] [PubMed] [Google Scholar]
- 34.Bubis J., Khorana H. G. J. Biol. Chem. 1990;265:12995–12999. [PubMed] [Google Scholar]
- 35.Guo W., Shi L., Javitch J. A. J. Biol. Chem. 2003;278:4385–4388. doi: 10.1074/jbc.C200679200. [DOI] [PubMed] [Google Scholar]
- 36.Guo W., Shi L., Filizola M., Weinstein H., Javitch J. A. Proc. Natl. Acad. Sci. USA. 2005;102:17495–17500. doi: 10.1073/pnas.0508950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klco J. M., Lassre T. B., Baranski T. J. J. Biol. Chem. 2003;278:35345–35353. doi: 10.1074/jbc.M305606200. [DOI] [PubMed] [Google Scholar]
- 38.Davies A., Gowen B. E., Krebs A. M., Schertler G. F. X., Saibil H. R. J. Mol. Biol. 2001;314:455–463. doi: 10.1006/jmbi.2001.5167. [DOI] [PubMed] [Google Scholar]
- 39.MacKenzie D., Arendt A., Hargrave P., McDowell J. H., Molday R. S. Biochemistry. 1984;23:6544–6549. doi: 10.1021/bi00321a041. [DOI] [PubMed] [Google Scholar]
- 40.Overton M. C., Blumer K. J. Methods. 2002;27:324–332. doi: 10.1016/s1046-2023(02)00090-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.