Abstract
Metastasis associated antigen 1 (MTA1) is a recently identified candidate metastasis‐associated gene that plays an important role in tumorigenesis and tumor aggressiveness, especially tumor invasiveness and metastasis. We analyzed the relationship between MTA1 expression and variable clinicopathological features and characterized its role in tumor angiogenesis in human breast cancers. Two hundred and sixty‐three breast cancer cases that successfully underwent surgery at Hanyang University Hospital (Seoul, Korea) between January 1989 and December 1997 were enrolled. MTA1 expression was observed by immunohistochemical staining and correlated with intratumoral microvessel density (MVD) and other clinicopathological parameters. MTA1 overexpression correlated significantly with higher tumor grade (grades 1 and 2 vs grade 3, P = 0.009). However, MTA1 expression did not correlate with tumor stage, status of estrogen and progesterone receptors, or axillary lymph node metastasis. Interestingly, MTA1 expression was found to correlate significantly with tumor MVD (P = 0.002). Survival analysis did not show a significant difference between MTA1 overexpression and poorer survival. In conclusion, MTA1 overexpression was found to be closely associated with higher tumor grade and increased tumor angiogenesis. These findings suggest MTA1 as a predictor of aggressive phenotype and a possible target molecule for anti‐angiogenic drugs in breast cancer treatment. (Cancer Sci 2006)
Female breast cancer is a major public health problem, with more than 1 000 000 cases occurring worldwide annually.( 1 ) Despite major advances that have been made in understanding the biological and clinical nature of the disease, the therapeutic problem persists. Therefore, identification of novel breast cancer biomarkers could be utilized as possible therapeutic targets or prognostic predictors that would contribute to the advancement of breast cancer treatment.
Metastasis associated antigen 1 (MTA1) was originally identified by differential screening of a cDNA library from highly metastatic and non‐metastatic rat mammary adenocarcinoma cell lines.( 2 , 3 , 4 ) The expression level is estimated to be four‐fold higher in the highly metastatic cell line MTLn3 than in the non‐metastatic cell line MTC4.( 2 ) The mRNA expression level of the human homolog of this gene is approximately four‐times higher in a metastatic cell line (MDA‐MB‐231) than in a non‐invasive and non‐metastatic cell line (MDA‐MB‐468) in nude mice.( 2 ) MTA1 is expressed physiologically at low levels in all normal tissue, except the testis.( 2 ) Several studies have identified various roles for MTA1 in normal mammary gland development and human breast cancer progression, including cell proliferation and invasiveness. A study with MTA1 transgenic mice reveals that MTA1 dysregulation in mammary epithelium causes increased cell proliferation, hyper‐branched ductal structure formation and precocious development, and results in the development of hyperplastic nodules and mammary gland tumors in virgin mice.( 5 ) The growth of human MDA‐MB‐231 breast cancer cells is inhibited after treatment with MTA1 antisense phosphorothioate oligonucleotides.( 6 ) MTA1 overexpression in non‐invasive breast cancer MCF‐7 cells yields larger colony formation in soft agar, augmented colony formation, as well as increased invasiveness in a Boyden chamber assay.( 7 ) Thus, MTA1 may play an important role in tumorigenesis and tumor aggressiveness. However, the role of MTA1 in surgically resected breast cancer tissue with its clinicopathological parameters has not been investigated to date. Therefore, we analyzed the relationship between MTA1 expression and variable clinicopathological features.
Tumor microenvironment is known to play an active role in tumor progression through adhesion molecules, angiogenesis and stromal host cells.( 8 , 9 , 10 ) Previous experimental studies highlight the role of angiogenesis in tumor progression, such as tumor growth and blood‐borne metastasis.( 11 , 12 ) In breast cancer, the overall results of reported studies suggest that human breast cancer is an angiogenic‐dependent tumor, and anti‐angiogenic therapy may be beneficial for breast cancer patients.( 13 , 14 ) Therefore, we analyzed the possible role of MTA1 in tumor angiogenesis of breast cancer by counting intratumoral microvessels, which is widely used to estimate tumor angiogenesis.( 15 )
In the present study, we report a statistically significant correlation between MTA1 gene expression and increased tumor grade or tumor angiogenesis in human breast cancers. We suggest MTA1 as a predictor of aggressive phenotype and a possible target molecule for anti‐angiogenic drug design in human breast cancers.
Materials and Methods
Patients and surgical specimens
Two hundred and sixty‐three breast cancer cases, consisting of 248 cases of invasive carcinoma and 15 cases of in situ carcinoma, which successfully underwent surgery at Hanyang University Hospital (Seoul, Korea) between January 1989 and December 1997, were enrolled in this retrospective study. Most patients received modified radical mastectomy or lumpectomy with axillary lymph node dissection followed by adjuvant chemotherapy and/or hormonal therapy. The patients with stage I or II received adriamycin, cyclophosphamide (AC) or cyclophophamide, methotrexate, 5‐fluorouracil chemotherapy. The patients with stage III received AC with paclitaxel chemotherapy. Adjuvant hormonal therapy with tamoxifen was administrated after chemotherapy for patients with estrogen receptor‐positive status. Histologically, the most common type was invasive ductal carcinoma (202 cases), followed by ductal carcinoma in situ (14 cases), invasive lobular carcinoma (12 cases), mucinous carcinoma (11 cases), invasive tubular medullary carcinoma (nine cases), carcinoma (six cases), apocrine carcinoma (two cases), clear cell carcinoma (two cases), invasive cribriform carcinoma (two cases), invasive tubulolobular carcinoma (one case), lobular carcinoma in situ (one case) and papillary carcinoma (one case). The mean follow‐up interval was 87 months (range: 1–168 months). Fifty‐seven (21.7%) patients died and 206 (78.3%) patients remained alive. All specimens were examined by two pathologists experienced in breast carcinoma diagnosis and the most representative paraffin‐embedded blocks were chosen for further analysis. Clinicopathological characteristics, which included age, American Joint Committee on Cancer (AJCC) stage, histological grade, histological type, the status of estrogen receptor (ER) and progesterone receptor (PR), and lymph node metastasis, were evaluated according to the general rule. Histological grade was evaluated using a modified Bloom–Richardson grading system. Steroid receptor status was evaluated by immunohistochemistry using anti‐ER and anti‐PR antibodies (Immunotech, Marseille, France).
Immunohistochemical staining of MTA1 and CD34
Immunohistochemical staining for MTA1 and CD34 was carried out using the avidin–biotin peroxidase complex method with 3,3′‐diaminobenzidine as a chromogen using an LSAB kit (DAKO, Carpentaria, CA, USA). Paraffin‐embedded tissue blocks, which included tumor and normal breast tissues, were sectioned at 5 µm. Slides were deparaffinized with xylene and rehydrated in a graded alcohol series. The slides were then incubated in 3% hydrogen peroxide for 10 min to block endogenous peroxidase activity. To enhance the immunoreactivity, microwave antigen retrieval was carried out in citrate buffer (pH 6.0) for 10 min. Primary antibodies against MTA1 (Santa Cruz Biochemistry, Santa Cruz, CA, USA) and CD34 (Immunotech) were used at dilutions of 1 : 20 and 1 : 50, respectively. Subsequently, secondary biotinylated antibody and avidin–biotin complex reagent were applied and the sections were counterstained with hematoxylin and mounted. Sections were incubated with Tris‐buffered saline containing 2% rabbit serum and 1% bovine serum albumin instead of primary antibody for negative controls.
Interpretation of MTA1 expression and microvessel density
Immunohistochemistry results were analyzed by two independent pathologists and were graded based on the nuclear staining intensity. They were scored as negative (score 0), weak (score 1), moderate (score 2) and strong (score 3) using a system that has been validated previously.( 16 ) Samples with staining of ≥10% of tumor cells were defined as positive. The slides were reinvestigated by both investigators under a multihead microscope in cases of disagreement. Microvessel density (MVD) was recorded by counting CD34‐positive vessels in the most vascularized areas of fields at ×200 magnification.( 17 ) Blood vessels with a lumen diameter exceeding approximately eight red blood cells were excluded. The mean value of the vessel count in the four fields was retained as the final value. We have used this system previously to estimate tumor angiogenesis.( 18 )
Statistical analysis
All statistical analyses were carried out using SPSS version 12.0 software (SPSS, Chicago, IL, USA). The χ2‐test was used to examine the association between MTA1 expression and the various clinicopathological characteristics, including patient age, AJCC stage, tumor grade, histological subtype, status of ER and PR, and axillary lymph node metastasis. The relationship between MTA1 expression and microvessel density was also evaluated using the Mann–Whitney test. The Kaplan–Meier method was used to calculate overall survival curves, and the log‐rank test was used to compare the difference between the survival rates of the patient subgroups, which were divided by various clinicopathological parameters and MTA1 expression. Multivariate survival analysis with Cox's proportional hazard regression model was also used to evaluate the independent prognostic factors. A difference of P < 0.05 between groups was considered significant.
Results
Expression of MTA1 in human breast cancers and its relationships with other clinicopathological parameters Upon staining for MTA1, tumor cells showed diffuse nuclear staining from negative to strong intensity, whereas normal breast ducts and lobules showed generally negative or weak focal expression (Fig. 1). The breast cancer cases were classified by intensity scores: negative (score 0), 113 cases (43.0%); weak (score 1), 83 cases (31.6%); moderate (score 2), 48 cases (18.3%); and strong expression (score 3), 19 cases (7.2%). Tumors with scores of more than 2 (moderate and strong expression) were considered to show MTA1 overexpression( 19 ) and 67 of the 263 cases (25.5%) showed overexpression of MTA1 (Table 1). MTA1 overexpression was found to be significantly higher in the younger age group than in the older age group (<50 years old vs >50 years old, P = 0.029), and higher in invasive ductal carcinoma than in other histological subtypes of invasive breast cancers, such as medullary carcinoma, mucinous carcinoma, invasive cribriform carcinoma or papillary carcinoma (P = 0.002). No difference was found between MTA1 expression in invasive ductal carcinoma and ductal carcinoma in situ (P = 0.613). There was a significant correlation between MTA1 overexpression and higher tumor grade (grade 1 and 2 vs grade 3, P = 0.009). However, MTA1 expression did not correlate with tumor stage, status of ER and PR, or axillary lymph node metastasis. In the analyses with invasive breast cancers, MTA1 expression did not correlate with patient's age, tumor stage, ER, PR or lymph node metastasis.
Figure 1.
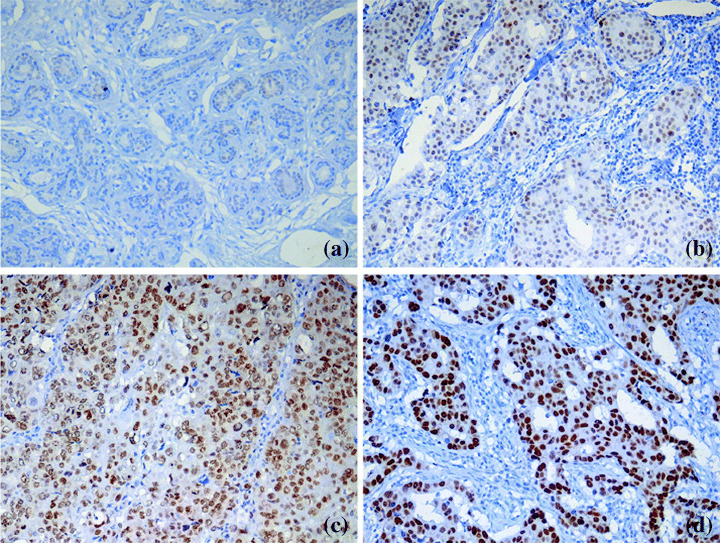
(a) Immunohistochemical staining for metastasis associated antigen 1 (MTA1), normal duct and lobule of breast tissue shows generally negative staining. Representative sections of (b) weak, (c) moderate and (d) strong positive staining for MTA1 expression. MTA1 expression is localized in the nucleus of tumor cells.
Table 1.
Relationship between metastasis associated antigen 1 (MTA1) expression and clinicopathological data in breast cancers (n = 263)
| Variable | n | Expression of MTA1 | P‐value (χ2‐test) | |
|---|---|---|---|---|
| Low (n = 196) | High (n = 67) | |||
| Age (years) | 0.029 | |||
| <50 | 157 | 110 | 47 | |
| >50 | 106 | 86 | 20 | |
| Tumor stage | 0.467 | |||
| 0–I | 62 | 47 | 15 | |
| II–III | 201 | 149 | 52 | |
| Tumor grade (IDC only) | 0.009 | |||
| 1–2 | 131 | 101 | 30 | |
| 3 | 70 | 42 | 28 | |
| Histological type | ||||
| IDC | 202 | 143 | 59 | |
| DCIS | 14 | 10 | 4 | 0.613 |
| Other invasive cancer | 46 | 42 | 4 | 0.002 |
| Estrogen receptor | 0.269 | |||
| Positive | 140 | 107 | 33 | |
| Negative | 123 | 89 | 34 | |
| Progesterone receptor | 0.109 | |||
| Positive | 158 | 113 | 45 | |
| Negative | 105 | 83 | 22 | |
| Lymph node metastasis | 0.426 | |||
| Positive | 125 | 92 | 33 | |
| Negative | 138 | 104 | 34 | |
DCIS, ductal carcinoma in situ; IDC, invasive ductal carcinoma.
MTA1 overexpression is closely associated with tumor angiogenesis in invasive breast cancers
To investigate the association between MTA1 overexpression and tumor angiogenesis, we examined the intratumoral MVD in 238 invasive breast cancers using CD34 immunohistochemical staining. As shown in 2, 3, MTA1 and CD34 staining in serial sections showed that weak expression of MTA1 was associated with fewer microvessels, and strong MTA1 expression was associated with abundant microvessels (P = 0.002, Mann–Whitney test). In further analysis with subgroups according to ER status and lymph node metastasis, we found that MTA1 overexpression was significantly associated with tumor angiogenesis regardless of ER status. In the lymph node‐negative subgroup, MTA1 overexpression was also significantly associated with tumor angiogenesis. In the lymph node‐positive subgroup, MTA1 expression and tumor angiogenesis showed no significant association, but the correlation tended to increase in samples with higher MTA1 expression (Table 2).
Figure 2.
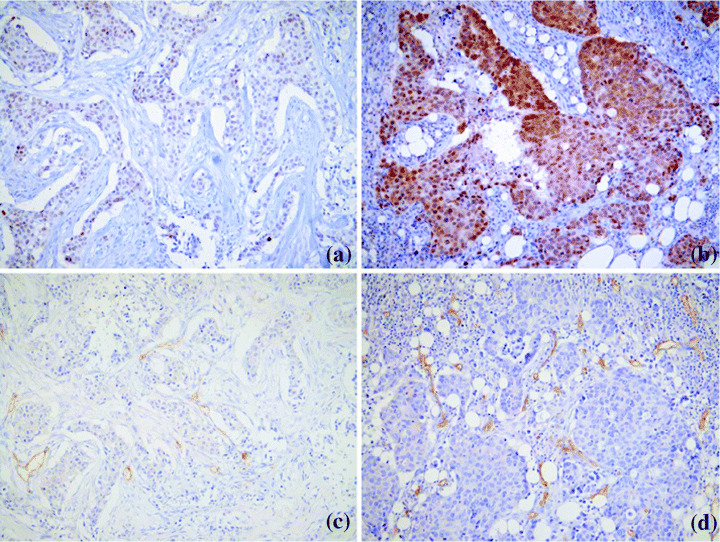
(a) Metastasis associated antigen 1 (MTA1) and (c) CD34 staining in serial sections showed weak expression of MTA1 in association with low microvessel density. (b) MTA1 and (d) CD34 staining in a tumor with strong MTA1 expression and abundant microvessels.
Figure 3.
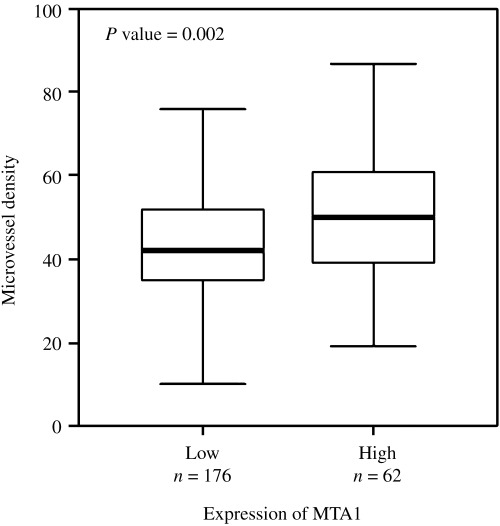
Analysis of subgroups with negative or weak (low) and moderate or strong (high) metastasis associated antigen 1 (MTA1) expression is presented by bars. Tumors with higher MTA1 expression showed significantly higher microvessel density than tumors with lower MTA‐1 expression (Mann–Whitney test).
Table 2.
Relationship between metastasis associated antigen 1 (MTA1) expression and microvessel density according to the estrogen receptor status and lymph node involvement in invasive breast cancers (n = 238)
| MTA1 expression | n = 238 | Microvessel density | P‐value (Mann–Whitney test) |
|---|---|---|---|
| Total | 0.002 | ||
| Low | 176 | 44.54 ± 18.10 | |
| High | 62 | 52.34 ± 19.50 | |
| Estrogen receptor‐positive | 0.031 | ||
| Low | 96 | 45.75 ± 17.94 | |
| High | 31 | 52.94 ± 16.38 | |
| Estrogen receptor‐negative | 0.023 | ||
| Low | 80 | 43.09 ± 18.30 | |
| High | 31 | 51.74 ± 22.45 | |
| Lymph node‐positive | 0.121 | ||
| Low | 88 | 45.63 ± 15.94 | |
| High | 30 | 50.97 ± 17.12 | |
| Lymph node‐negative | 0.004 | ||
| Low | 88 | 43.45 ± 20.07 | |
| High | 32 | 53.63 ± 21.69 |
All data are presented as mean ± standard deviation.
MTA1 overexpression is not significantly related with a poorer survival rate
Because we found a correlation between MTA1 expression and tumor grade and angiogenesis, we further examined the effect of MTA1 expression on clinicopathological prognostic factors in breast cancer patients. As shown in Table 3, a significant prognostic influence of tumor stage and node metastasis on overall survival was found by univariate analysis. However, no impact of MTA1 expression on overall survival was observed in univariate and multivariate survival analyses. Kaplan–Meier survival curves and log‐rank tests showed no significant correlation between patient survival and MTA1 overexpression (Fig. 4). Although we found that MTA1 expression correlated with tumor grade and angiogenesis, which are generally considered to be poor prognostic factors,( 20 , 21 ) MTA1 overexpression was not associated with patient survival. In the analyses with invasive breast cancers, no correlation was found between MTA1 expression and patient survival.
Table 3.
Univariate and multivariate survival analysis of 263 breast cancer patients
| Overall survival | Significance | Exp (B) | 95% CI for Exp (B) | |
|---|---|---|---|---|
| Univariate | Multivariate | |||
| MTA1 expression (low vs high) | 0.444 | 0.513 | 0.791 | 0.393–1.596 |
| Patient age (<50 years vs >50 years) | 0.665 | 0.936 | 1.025 | 0.557–1.888 |
| Histological grade (1, 2 vs 3) | 0.719 | 0.720 | 1.122 | 0.599–2.102 |
| AJCC stage (I, II vs III) | <0.001 | 0.002 | 4.684 | 1.770–12.395 |
| Lymph node metastasis (yes vs no) | <0.001 | 0.632 | 1.304 | 0.439–3.876 |
| Estrogen receptor (positive vs negative) | 0.943 | 0.603 | 0.844 | 0.445–1.599 |
| Progesterone receptor (positive vs negative) | 0.798 | 0.902 | 1.041 | 0.546–1.984 |
AJCC, American Joint Committee on Cancer; CI, confidence interval; Exp (B), hazard ratio; MTA1, metastasis associated antigen 1.
Figure 4.
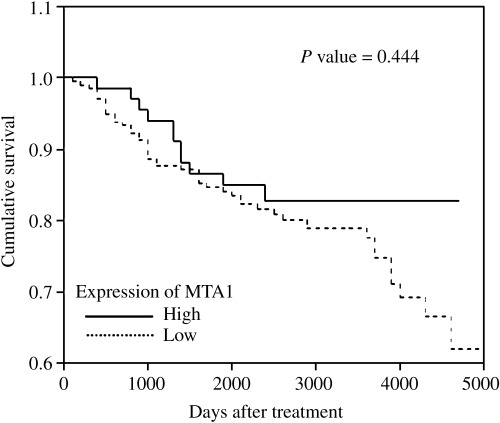
Cumulative survival according to metastasis associated antigen 1 (MTA1) expression in breast cancer patients (Kaplan–Meier method and log‐rank test). Higher MTA1 expression did not correlate with poorer prognosis.
Discussion
In the present study, we found that MTA1 expression was generally absent or focally weak in the normal duct epithelium of human breast tissue by immunohistochemistry. In contrast, breast cancer cells expressed MTA1 diffusely and its staining intensity was negative to strong. Therefore, MTA1 expression with more than moderate intensity, defined as MTA1 overexpression, occupied 25.5% of breast cancers in our series. Here we showed that MTA1 overexpression correlated with higher tumor grade and abundant intratumoral microvessels in surgically resected samples.
The expression of MTA1 has been investigated in several types of human cancers and was found to correlate closely with some clinicopathological parameters that increase tumor aggressiveness.( 16 , 22 , 23 , 24 , 25 ) In a previous report with gastrointestinal carcinomas, 13 of 36 colon cancer patients that expressed higher MTA1 mRNA exhibited significantly deeper wall invasion and frequent lymph node metastasis, and tended to be at a more advanced Dukes’ stage.( 22 ) In gastric carcinomas, higher expression of MTA1 mRNA is significantly associated with frequent serosal invasion and lymph node metastasis, and tends to have a higher rate of vascular invasion.( 22 ) Similar observations have been reported in esophageal cancers. In a previous study, 12 out of 16 esophageal carcinomas overexpressing MTA1 mRNA invaded into the adventitia or neighboring structures, whereas 13 out of 31 remaining tumors were restricted in the proper muscle layer.( 23 ) In an analysis of 74 non‐small cell lung cancers, MTA1 mRNA expression levels were higher in tumors with higher stage and lymph node metastasis.( 24 ) Hofer et al. analyzed 1940 prostate tissue microarray samples from more than 300 patients using immunohistochemistry.( 16 ) They found that MTA1 protein expression was elevated in hormone‐refractory metastatic prostate cancer compared with clinically localized prostate cancer or benign prostatic tissue. Additionally, increased expression of MTA1 protein was also detected in prostate cancer precursor lesions.( 16 ) Another study revealed that pancreatic cancer tissues overexpressing MTA1 mRNA tended to have a higher frequency of lymph node metastasis.( 26 ) In thymoma, stage IV patients had higher expression of MTA1 mRNA than patients with stage I.( 27 ) In the present study, higher expression of MTA1 significantly correlated with increased tumor grade. This finding, together with previous studies, suggests the possible role of MTA1 in tumor progression and the development of aggressive phenotypes. However, there was no difference between in situ lesions and invasive carcinoma in the breast. No association was found between MTA1 expression and axillary lymph node metastasis in our breast cancer series, whereas a correlation with regional lymph node metastasis has been reported in gastrointestinal carcinoma, lung cancer and pancreatic cancer.( 22 , 24 , 26 ) Such discrepancies suggest that the role of MTA1 may be organ‐specific, and further consecutive studies are required.
Angiogenesis, the branching and sprouting of capillaries from the endothelium of the pre‐existing vasculature,( 28 ) plays a central role in tumor progression of invasive breast cancers and is related to the development of blood‐borne metastasis.( 12 , 14 ) Thus, MTA1 may be related to tumor angiogenesis and its angiogenic activity represents a potentially novel anticancer therapeutic target.( 13 ) MTA1 is a subunit of the nuclear remodeling and deacetylation (NuRD) complex, which is associated with chromatin remodeling and histone deacetylase activity, leading to transcriptional regulation of specific genes.( 29 , 30 ) Qian et al. have reported recently that the use of chromatin remodeling agents, such as histone deacetylase inhibitor NVP‐LAQ824, inhibits angiogenesis and has a greater antitumor effect in combination with the vascular endothelial growth factor receptor tyrosine kinase inhibitor PTK787/ZK222584.( 31 ) In view of MTA1 as a subunit of the NuRD complex, this finding suggests a possible angiogenic property for MTA1. Therefore, we analyzed the association between MTA1 expression and intratumoral MVD, the most commonly used technique to quantify tumor angiogenesis. Interestingly, we found that higher expression of MTA1 was associated significantly with increased intratumoral MVD. This result suggests a novel role for MTA1 in tumor angiogenesis and MTA1 as a possible target for anti‐angiogenic therapy. However, the exact role of MTA1 as an angiogenic factor remains to be elucidated at the molecular level.
MTA1 expression and its prognostic effect were investigated previously in several human tumors. No significant prognostic effect was found in lung cancer( 24 ) or thymoma.( 27 ) In prostate cancer, higher MTA1 expression is associated with a longer prostate‐specific antigen (PSA)‐free survival time after radical prostatectomy and negative MTA1 expression is associated with a 2.8 relative risk for developing PSA recurrence.( 16 ) This study shows an inverse relationship between MTA1 expression and PSA recurrence‐free survival. In our study with breast cancer, MTA1 overexpression was not associated with patient survival but significantly correlated with angiogenesis, which is generally considered to be a poor prognostic factor.( 21 ) However, the recent report of Uzzan et al. argues against the generalization of angiogenesis as a poor prognostic factor.( 32 ) Such a recent view may partially explain our seemingly controversial relationship between MTA1 expression and angiogenesis or patient survival. Another possible explanation would be that additional clinical parameters, such as post‐surgical hormonal therapy or chemotherapy, can alter the biological behavior of tumors that may affect the outcome of the disease.
In conclusion, MTA1 overexpression was found to be closely associated with higher tumor grade and increased tumor angiogenesis. These findings suggest MTA1 as an indicator of an aggressive phenotype and a possible target molecule for anti‐angiogenic drugs in breast cancer treatment.
Acknowledgments
This work was supported by the research fund of Hanyang University (HY‐2003‐T) to S. S. Paik.
References
- 1. Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer 2001; 94: 153–6. [DOI] [PubMed] [Google Scholar]
- 2. Toh Y, Pencil SD, Nicolson GL. Analysis of the complete sequence of the novel metastasis‐associated candidate gene, mta1, differentially expressed in mammary adenocarcinoma and breast cancer cell lines. Gene 1995; 159: 97–104. [DOI] [PubMed] [Google Scholar]
- 3. Toh Y, Pencil SD, Nicolson GL. A novel candidate metastasis‐associated gene, mta1, differentially expressed in highly metastatic mammary adenocarcinoma cell lines. cDNA cloning, expression, and protein analyses. J Biol Chem 1994; 269: 22 958–63. [PubMed] [Google Scholar]
- 4. Pencil SD, Toh Y, Nicolson GL. Candidate metastasis‐associated genes of the rat 13762NF mammary adenocarcinoma. Breast Cancer Res Treat 1993; 25: 165–74. [DOI] [PubMed] [Google Scholar]
- 5. Bagheri‐Yarmand R, Talukder AH, Wang RA, Vadlamudi RK, Kumar R. Metastasis‐associated protein 1 deregulation causes inappropriate mammary gland development and tumorigenesis. Development 2004; 131: 3469–79. [DOI] [PubMed] [Google Scholar]
- 6. Nawa A, Nishimori K, Lin P et al. Tumor metastasis‐associated human MTA1 gene: its deduced protein sequence, localization, and association with breast cancer cell proliferation using antisense phosphorothioate oligonucleotides. J Cell Biochem 2000; 79: 202–12. [PubMed] [Google Scholar]
- 7. Mazumdar A, Wang RA, Mishra SK et al. Transcriptional repression of oestrogen receptor by metastasis‐associated protein 1 corepressor. Nat Cell Biol 2001; 3: 30–7. [DOI] [PubMed] [Google Scholar]
- 8. Weiss L, Orr FW, Honn KV. Interactions of cancer cells with the microvasculature during metastasis. FASEB J 1988; 2: 12–21. [DOI] [PubMed] [Google Scholar]
- 9. Weiss L, Orr FW, Honn KV. Interactions between cancer cells and the microvasculature: a rate‐regulator for metastasis. Clin Exp Metastasis 1989; 7: 127–67. [DOI] [PubMed] [Google Scholar]
- 10. Cunha GR. Role of mesenchymal–epithelial interactions in normal and abnormal development of the mammary gland and prostate. Cancer 1994; 74: 1030–44. [DOI] [PubMed] [Google Scholar]
- 11. Kim KJ, Li B, Winer J et al. Inhibition of vascular endothelial growth factor‐induced angiogenesis suppresses tumour growth in vivo . Nature 1993; 362: 841–4. [DOI] [PubMed] [Google Scholar]
- 12. Ellis LM, Fidler IJ. Angiogenesis and breast cancer metastasis. Lancet 1995; 346: 388–90. [DOI] [PubMed] [Google Scholar]
- 13. Gasparini G. Biological and clinical role of angiogenesis in breast cancer. Breast Cancer Res Treat 1995; 36: 103–7. [DOI] [PubMed] [Google Scholar]
- 14. Gasparini G. Clinical significance of the determination of angiogenesis in human breast cancer: update of the biological background and overview of the Vicenza studies. Eur J Cancer 1996; 32A: 2485–93. [DOI] [PubMed] [Google Scholar]
- 15. Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis − correlation in invasive breast carcinoma. N Engl J Med 1991; 324: 1–8. [DOI] [PubMed] [Google Scholar]
- 16. Hofer MD, Kuefer R, Varambally S et al. The role of metastasis‐associated protein 1 in prostate cancer progression. Cancer Res 2004; 64: 825–9. [DOI] [PubMed] [Google Scholar]
- 17. Hansen S, Grabau DA, Rose C, Bak M, Sorensen FB. Angiogenesis in breast cancer: a comparative study of the observer variability of methods for determining microvessel density. Lab Invest 1998; 78: 1563–73. [PubMed] [Google Scholar]
- 18. Lee KT, Lee YW, Lee JK et al. Overexpression of Id‐1 is significantly associated with tumour angiogenesis in human pancreas cancers. Br J Cancer 2004; 90: 1198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schoppmann SF, Schindl M, Bayer G et al. Overexpression of Id‐1 is associated with poor clinical outcome in node negative breast cancer. Int J Cancer 2003; 104: 677–82. [DOI] [PubMed] [Google Scholar]
- 20. Merkel DE, Osborne CK. Prognostic factors in breast cancer. Hematol Oncol Clin North Am 1989; 3: 641–52. [PubMed] [Google Scholar]
- 21. Arora R, Joshi K, Nijhawan R, Radotra BD, Sharma SC. Angiogenesis as an independent prognostic indicator in node‐negative breast cancer. Anal Quant Cytol Histol 2002; 24: 228–33. [PubMed] [Google Scholar]
- 22. Toh Y, Oki E, Oda S et al. Overexpression of the MTA1 gene in gastrointestinal carcinomas: correlation with invasion and metastasis. Int J Cancer 1997; 74: 459–63. [DOI] [PubMed] [Google Scholar]
- 23. Toh Y, Kuwano H, Mori M, Nicolson GL, Sugimachi K. Overexpression of metastasis‐associated MTA1 mRNA in invasive oesophageal carcinomas. Br J Cancer 1999; 79: 1723–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sasaki H, Moriyama S, Nakashima Y et al. Expression of the MTA1 mRNA in advanced lung cancer. Lung Cancer 2002; 35: 149–54. [DOI] [PubMed] [Google Scholar]
- 25. Moon WS, Chang K, Tarnawski AS. Overexpression of metastatic tumor antigen 1 in hepatocellular carcinoma: Relationship to vascular invasion and estrogen receptor‐alpha. Hum Pathol 2004; 35: 424–9. [DOI] [PubMed] [Google Scholar]
- 26. Iguchi H, Imura G, Toh Y, Ogata Y. Expression of MTA1, a metastasis‐associated gene with histone deacetylase activity in pancreatic cancer. Int J Oncol 2000; 16: 1211–14. [DOI] [PubMed] [Google Scholar]
- 27. Sasaki H, Yukiue H, Kobayashi Y et al. Expression of the MTA1 mRNA in thymoma patients. Cancer Lett 2001; 174: 159–63. [DOI] [PubMed] [Google Scholar]
- 28. Risau W. Mechanisms of angiogenesis. Nature 1997; 386: 671–4. [DOI] [PubMed] [Google Scholar]
- 29. Xue Y, Wong J, Moreno GT, Young MK, Cote J, Wang W. NuRD, a novel complex with both ATP‐dependent chromatin‐remodeling and histone deacetylase activities. Mol Cell 1998; 2: 851–61. [DOI] [PubMed] [Google Scholar]
- 30. Toh Y, Kuninaka S, Endo K et al. Molecular analysis of a candidate metastasis‐associated gene, MTA1: possible interaction with histone deacetylase 1. J Exp Clin Cancer Res 2000; 19: 105–11. [PubMed] [Google Scholar]
- 31. Qian DZ, Wang X, Kachhap SK et al. The histone deacetylase inhibitor NVP‐LAQ824 inhibits angiogenesis and has a greater antitumor effect in combination with the vascular endothelial growth factor receptor tyrosine kinase inhibitor PTK787/ZK222584. Cancer Res 2004; 64: 6626–34. [DOI] [PubMed] [Google Scholar]
- 32. Uzzan B, Nicolas P, Cucherat M, Perret GY. Microvessel density as a prognostic factor in women with breast cancer: a systematic review of the literature and meta‐analysis. Cancer Res 2004; 64: 2941–55. [DOI] [PubMed] [Google Scholar]


