Abstract
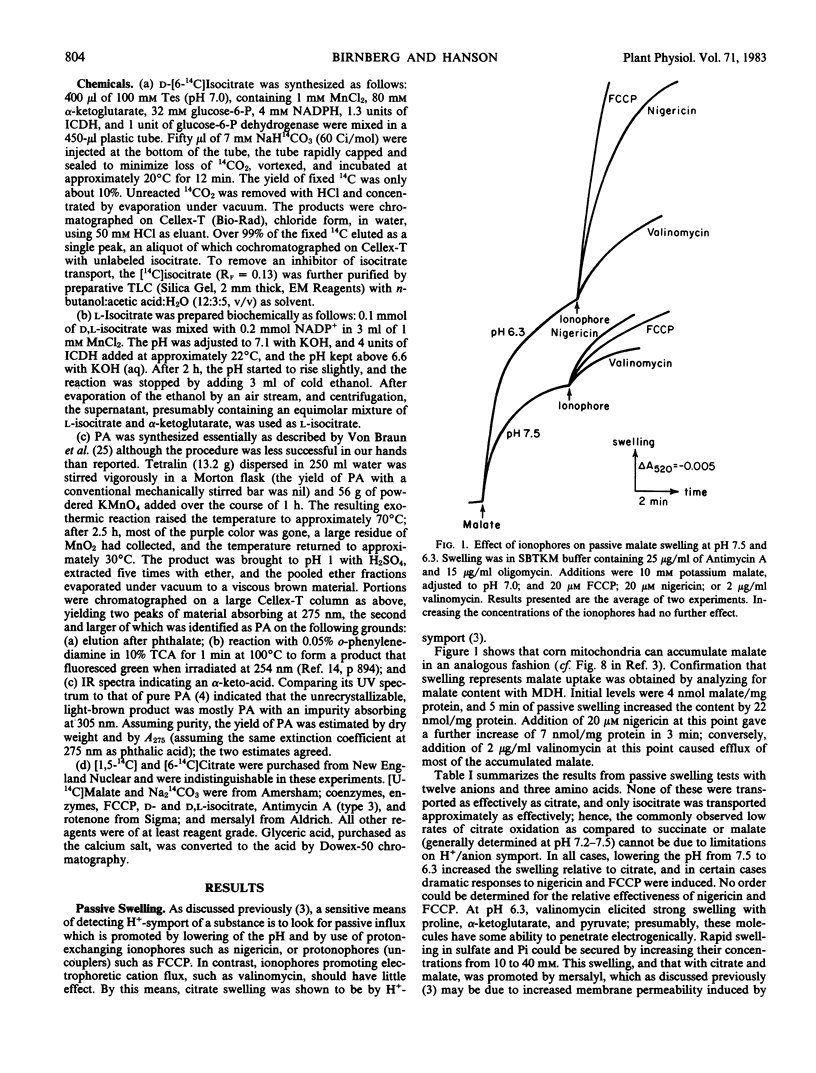
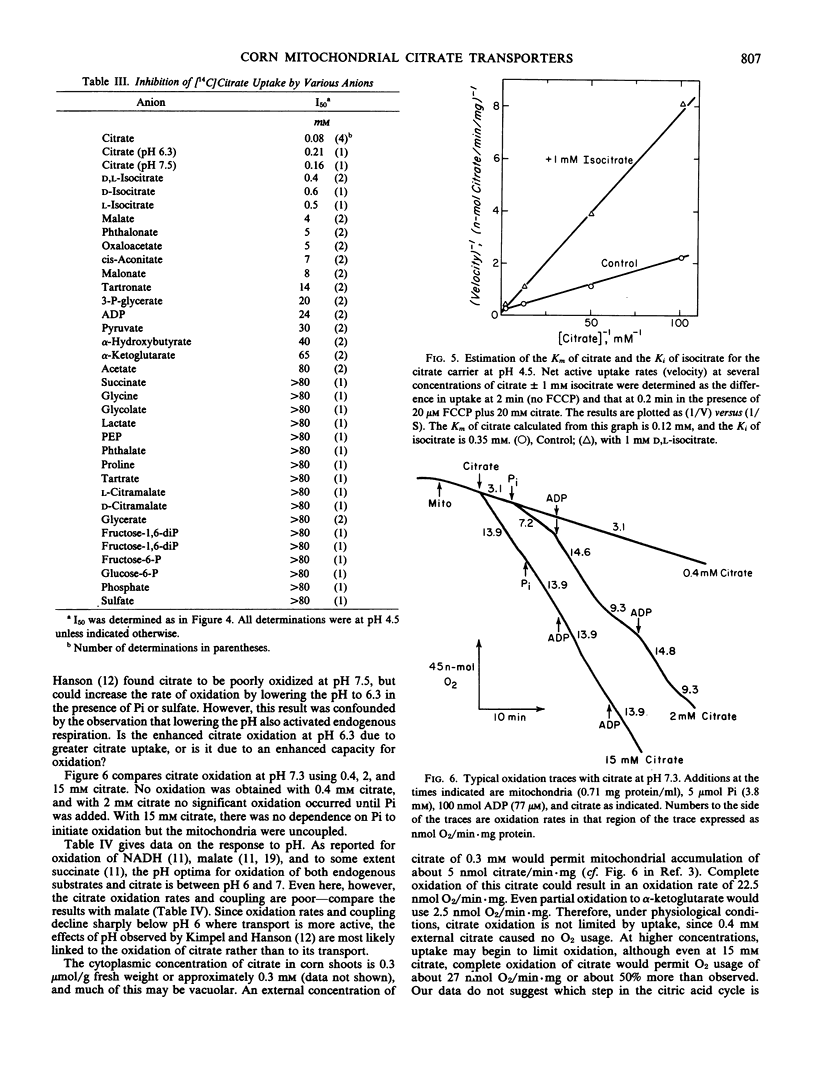
Previous work (Birnberg, Jayroe, Hanson 1982 Plant Physiol 70: 511-516) demonstrated that corn mitochondria (Zea mays L.) can accumulate citrate by a malate- and phosphate-independent proton symporter. This uptake and symport of other ions were investigated. Passive swelling experiments indicated that corn mitochondria can accumulate several other anions by proton symport, but only isocitrate is taken up nearly as effectively as citrate. At the optimal pH (4.5), active uptake of carrier-free [14C]citrate in 50 micromolar mersalyl is inhibited by fourteen anions, but only the I50 (the concentration of inhibitor required to reduce uptake of carrier-free [14C]citrate by 50%) values of citrate (0.08 millimolar) and d-and l-isocitrate (0.5 millimolar) are less than 4 millimolar. Isocitrate is a competitive inhibitor of citrate uptake and [14C]isocitrate is accumulated with a Km similar similar to its I50. Valinomycin reduces net active citrate accumulation at pH 7.5, consistent with the relatively low Vmax for citrate uptake. At pH 4.5, mersalyl reduces the rate of citrate uptake without changing the affinity of the carrier for citrate. Thus, the corn mitochondria have a high-affinity, mersalyl-insensitive carrier selective for citrate that also transports isocitrate.
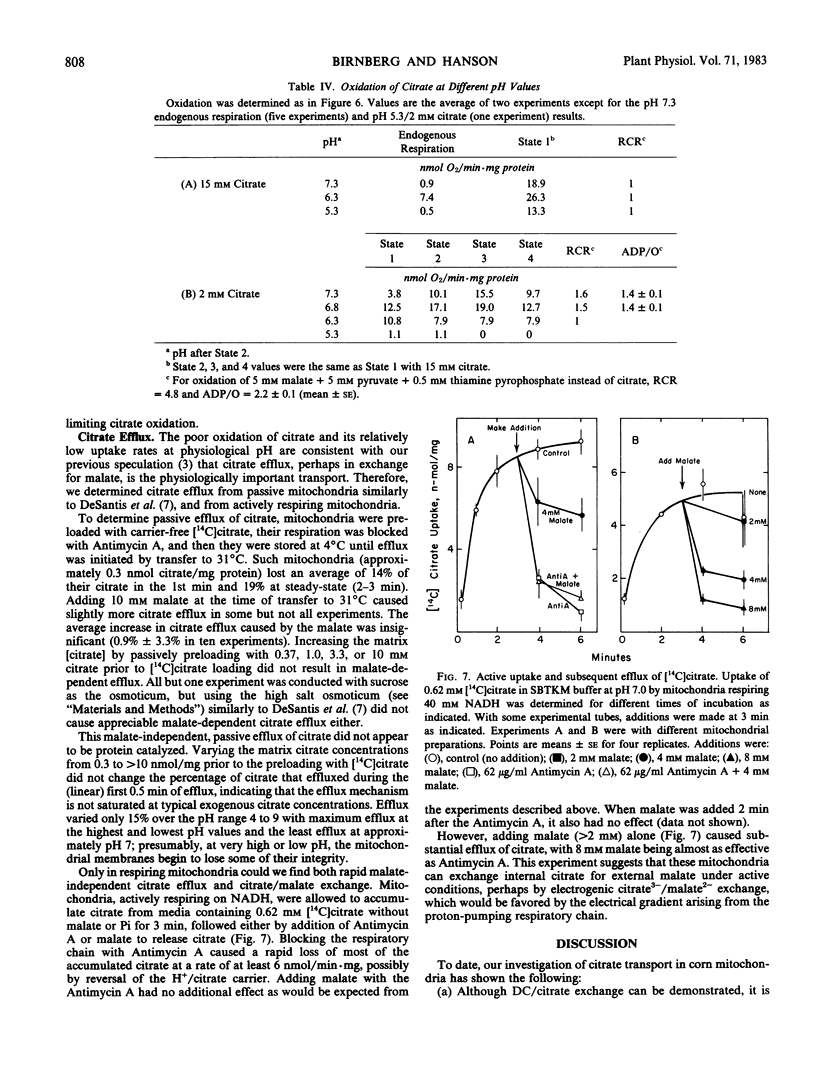
The pH optimum for oxidation of both endogenous substrates and citrate is approximately pH 6.8, but citrate oxidation is low at all pH values and is poorly coupled to ATP synthesis. Under active conditions only, at pH 7.0, malate/citrate exchange occurs with 4 millimolar malate being sufficient to remove about half the matrix citrate. Therefore, in vivo both citrate uptake by proton symport and efflux by malate/citrate exchange should occur, with the net direction of citrate movement determined by the cytoplasmic pH, and citrate and malate concentrations; in most cases, net efflux is likely to be favored.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Khalil S., Hanson J. B. Energy-linked Adenosine Diphosphate Accumulation by Corn Mitochondria: II. Phosphate and Divalent Cation Requirement. Plant Physiol. 1979 Aug;64(2):281–284. doi: 10.1104/pp.64.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Khalil S., Hanson J. B. Energy-linked Sulfate Uptake by Corn Mitochondria via the Phosphate Transporter. Plant Physiol. 1979 Apr;63(4):635–638. doi: 10.1104/pp.63.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnberg P. R., Jayroe D. L., Hanson J. B. Citrate transport in corn mitochondria. Plant Physiol. 1982 Aug;70(2):511–516. doi: 10.1104/pp.70.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheema-Dhadli S., Halperin M. L., Duperrouzel P. R., Leznoff C. C. Hydrocarbon specificity for transport on the tricarboxylate transporter of rat liver mitochondria. Can J Biochem. 1980 Oct;58(10):804–808. doi: 10.1139/o80-112. [DOI] [PubMed] [Google Scholar]
- Day D. A., Wiskich J. T. Effect of phthalonic acid on respiration and metabolite transport in higher plant mitochondria. Arch Biochem Biophys. 1981 Oct 1;211(1):100–107. doi: 10.1016/0003-9861(81)90434-3. [DOI] [PubMed] [Google Scholar]
- Fluegel M. J., Hanson J. B. Mechanisms of passive potassium influx in corn mitochondria. Plant Physiol. 1981 Aug;68(2):267–271. doi: 10.1104/pp.68.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley J. R., Hanson J. B. The action of valinomycin in uncoupling corn mitochondria. Plant Physiol. 1975 Jul;56(1):13–18. doi: 10.1104/pp.56.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn M. E., Mertz D. Cyanide-Resistant Respiration in Suspension Cultured Cells of Nicotiana glutinosa L. Plant Physiol. 1982 Jun;69(6):1439–1443. doi: 10.1104/pp.69.6.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimpel J. A., Hanson J. B. Activation of endogenous respiration and anion transport in corn mitochondria by acidification of the medium. Plant Physiol. 1977 Dec;60(6):933–934. doi: 10.1104/pp.60.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaNoue K. F., Schoolwerth A. C. Metabolite transport in mitochondria. Annu Rev Biochem. 1979;48:871–922. doi: 10.1146/annurev.bi.48.070179.004255. [DOI] [PubMed] [Google Scholar]
- Lips S. H., Beevers H. Compartmentation of Organic Acids in Corn Roots II. The Cytoplasmic Pool of Malic Acid. Plant Physiol. 1966 Apr;41(4):713–717. doi: 10.1104/pp.41.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclennan D. H., Beevers H., Harley J. L. 'Compartmentation' of acids in plant tissues. Biochem J. 1963 Nov;89(2):316–327. doi: 10.1042/bj0890316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa T., Asami K., Suzuki H., Yukawa O. Appearance of energy conservation system in rat liver mitochondria during development. The role of adenine nucleotide translocation. J Biochem. 1973 Feb;73(2):397–406. [PubMed] [Google Scholar]
- Osmond C. B., Laties G. G. Compartmentation of malate in relation to ion absorption in beet. Plant Physiol. 1969 Jan;44(1):7–14. doi: 10.1104/pp.44.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. K., Wemmer D., Ray P. M., Jardetzky O. Regulation of Cytoplasmic and Vacuolar pH in Maize Root Tips under Different Experimental Conditions. Plant Physiol. 1982 Jun;69(6):1344–1347. doi: 10.1104/pp.69.6.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]


