Abstract
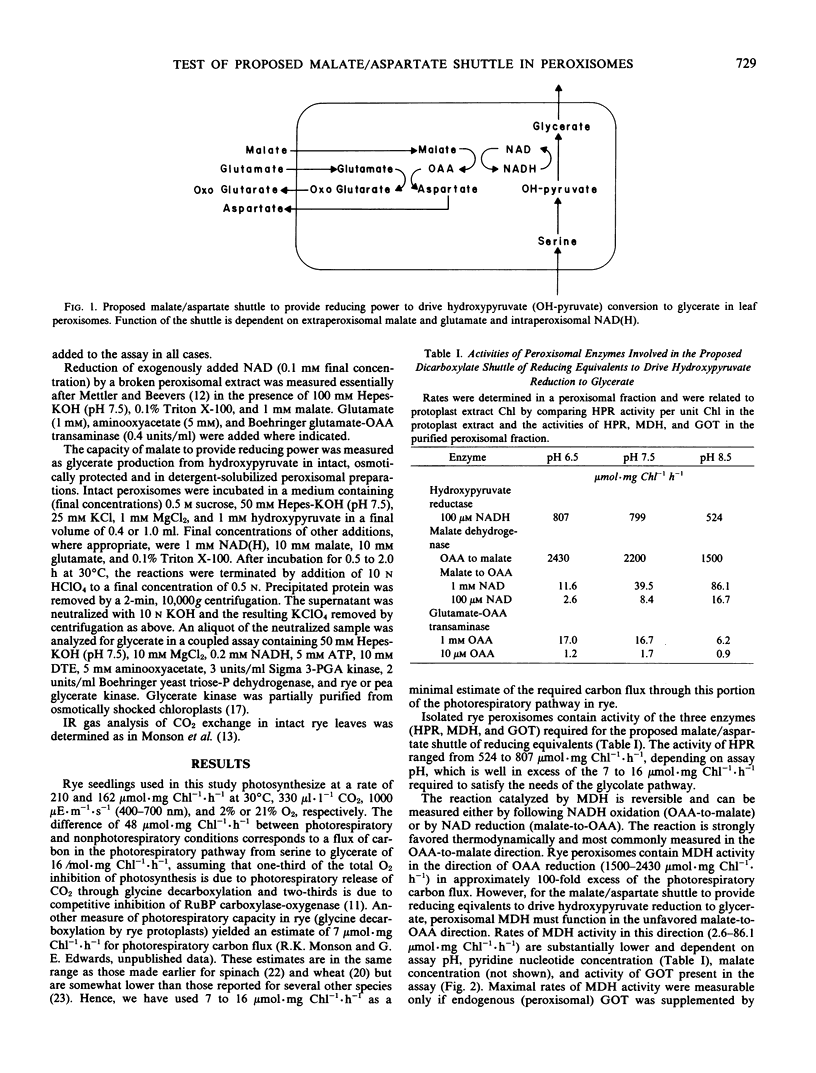
A series of experiments, with Secale cereale and Triticum aestivum var Argee, to evaluate critically the ability of a malate/aspartate shuttle to provide reducing equivalents to drive hydroxypyruvate reduction to glycerate led to the conclusion that the shuttle, as previously envisioned, does not supply NADH to the peroxisomal matrix. First, analysis of coupled malate dehydrogenase and glutamate-oxaloacetate transaminase activities in the directions required for intraperoxisomal NADH generation indicated that the peroxisomal enzyme activities were insufficient to account for necessary rates of photorespiratory carbon flux. Second, although the peroxisomal isozyme of malate dehydrogenase comprised a substantial portion (40%) of total cellular activity, less than 7% of the cellular glutamate-oxaloacetate transmaminase activity was associated with the peroxisomes. Third, a peroxisomal extract was able to reduce added NAD only slowly upon addition of malate and glutamate. The rate of NAD reduction was greatly enhanced in the presence of exogenously added glutamateoxaloacetate transaminase. Finally, intact peroxisomes were unable to reduce hydroxypyruvate to glycerate when supplied with malate and glutamate in the absence of exogenously added pyridine nucleotides, although they readily reduced hydroxypyruvate when exogenous pyridine nucleotides were supplied. Three alternative mechanisms, which are in agreement with observed data and which could serve to supply the reducing power to the peroxisomal matrix, are discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Day D. A., Wiskich J. T. Pyridine nucleotide interactions with isolated plant mitochondria. Biochim Biophys Acta. 1978 Mar 13;501(3):396–404. doi: 10.1016/0005-2728(78)90107-x. [DOI] [PubMed] [Google Scholar]
- Donaldson R. P. Nicotinamide cofactors (NAD and NADP) in glyoxysomes, mitochondria, and plastids isolated from castor bean endosperm. Arch Biochem Biophys. 1982 Apr 15;215(1):274–279. doi: 10.1016/0003-9861(82)90305-8. [DOI] [PubMed] [Google Scholar]
- Donaldson R. P. Organelle Membranes from Germinating Castor Bean Endosperm: II. ENZYMES, CYTOCHROMES, AND PERMEABILITY OF THE GLYOXYSOME MEMBRANE. Plant Physiol. 1981 Jan;67(1):21–25. doi: 10.1104/pp.67.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson R. P., Tolbert N. E., Schnarrenberger C. A comparison of microbody membranes with microsomes and mitochondria from plant and animal tissue. Arch Biochem Biophys. 1972 Sep;152(1):199–215. doi: 10.1016/0003-9861(72)90208-1. [DOI] [PubMed] [Google Scholar]
- Hicks D. B., Donaldson R. P. Electron transport in glyoxysomal membranes. Arch Biochem Biophys. 1982 Apr 15;215(1):280–288. doi: 10.1016/0003-9861(82)90306-x. [DOI] [PubMed] [Google Scholar]
- Huang A. H., Liu K. D., Youle R. J. Organelle-specific Isozymes of Aspartate-alpha-Ketoglutarate Transaminase in Spinach Leaves. Plant Physiol. 1976 Jul;58(1):110–113. doi: 10.1104/pp.58.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku S. B., Edwards G. E. Oxygen Inhibition of Photosynthesis: II. Kinetic Characteristics as Affected by Temperature. Plant Physiol. 1977 May;59(5):991–999. doi: 10.1104/pp.59.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler P., Saz H. J. Demonstration and possible function of NADH:NAD+ transhydrogenase from ascaris muscle mitochondria. J Biol Chem. 1976 Apr 25;251(8):2217–2225. [PubMed] [Google Scholar]
- Mettler I. J., Beevers H. Oxidation of NADH in Glyoxysomes by a Malate-Aspartate Shuttle. Plant Physiol. 1980 Oct;66(4):555–560. doi: 10.1104/pp.66.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson R. K., Stidham M. A., Williams G. J., Edwards G. E., Uribe E. G. Temperature Dependence of Photosynthesis in Agropyron smithii Rydb. : I. FACTORS AFFECTING NET CO(2) UPTAKE IN INTACT LEAVES AND CONTRIBUTION FROM RIBULOSE-1,5-BISPHOSPHATE CARBOXYLASE MEASURED IN VIVO AND IN VITRO. Plant Physiol. 1982 Apr;69(4):921–928. doi: 10.1104/pp.69.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld D. W., Tolbert N. E. Aminotransferases in peroxisomes from spinach leaves. J Biol Chem. 1972 Aug 10;247(15):4803–4811. [PubMed] [Google Scholar]
- Schmitt M. R., Edwards G. E. Isolation and purification of intact peroxisomes from green leaf tissue. Plant Physiol. 1982 Oct;70(4):1213–1217. doi: 10.1104/pp.70.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E. Metabolic pathways in peroxisomes and glyoxysomes. Annu Rev Biochem. 1981;50:133–157. doi: 10.1146/annurev.bi.50.070181.001025. [DOI] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]
- Yamazaki R. K., Tolbert N. E. Enzymic characterization of leaf peroxisomes. J Biol Chem. 1970 Oct 10;245(19):5137–5144. [PubMed] [Google Scholar]
- Yamazaki R. K., Tolbert N. E. Malate dehydrogenase in leaf peroxisomes. Biochim Biophys Acta. 1969 Mar 18;178(1):11–20. doi: 10.1016/0005-2744(69)90127-2. [DOI] [PubMed] [Google Scholar]
- Zschoche W. C., Ting I. P. Malate Dehydrogenases of Pisum sativum: Tissue Distribution and Properties of the Particulate Forms. Plant Physiol. 1973 Jun;51(6):1076–1081. doi: 10.1104/pp.51.6.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]


