Abstract
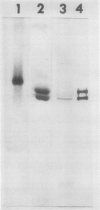
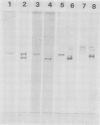
To initiate structural studies of the ADPglucose pyrophosphorylase from spinach an improved purification procedure was devised. The modified purification scheme allowed the isolation of 20 to 30 milligrams pure enzyme from 10 kilogram of spinach leaves. Electrophoresis of the purified enzyme confirmed an earlier study which showed that the enzyme was putatively composed of two subunits (Copeland L, J Preiss 1981 Plant Physiol 68: 996-1001). The two subunits migrate as 51 and 54 kilodalton proteins upon sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Both proteins can be detected on Western blots of leaf homogenates prepared under denaturing conditions suggesting that both subunits exist in vivo. Anion-exchange chromatography in the presence of urea allowed resolution of the 51 and 54 kilodalton proteins. They possess different N-terminal amino acid sequences and tryptic peptide maps. Western blot analysis reveals that the 51 and 54 kilodalton proteins are antigenically dissimilar. The 51 but not the 54 kilodalton protein is immunologically related to the ADPglucose pyrophosphorylase from maize endosperm and potato tuber.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Copeland L., Preiss J. Purification of Spinach Leaf ADPglucose Pyrophosphorylase. Plant Physiol. 1981 Nov;68(5):996–1001. doi: 10.1104/pp.68.5.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh H. P., Preiss J. Adenosine diphosphate glucose pyrophosphorylase. A regulatory enzyme in the biosynthesis of starch in spinach leaf chloroplasts. J Biol Chem. 1966 Oct 10;241(19):4491–4504. [PubMed] [Google Scholar]
- Gracy R. W. Two-dimensional thin-layer methods. Methods Enzymol. 1977;47:195–204. doi: 10.1016/0076-6879(77)47024-1. [DOI] [PubMed] [Google Scholar]
- Haugen T. H., Ishaque A., Preiss J. Biosynthesis of bacterial glycogen. Characterization of the subunit structure of Escherichia coli B glucose-1-phosphate adenylyltransferase (EC 2.7.7.27). J Biol Chem. 1976 Dec 25;251(24):7880–7885. [PubMed] [Google Scholar]
- Heldt H. W., Chon C. J., Maronde D. Role of orthophosphate and other factors in the regulation of starch formation in leaves and isolated chloroplasts. Plant Physiol. 1977 Jun;59(6):1146–1155. doi: 10.1104/pp.59.6.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan H. B., Reeves C. D., Okita T. W. ADPglucose Pyrophosphorylase Is Encoded by Different mRNA Transcripts in Leaf and Endosperm of Cereals. Plant Physiol. 1986 Jun;81(2):642–645. doi: 10.1104/pp.81.2.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Okita T. W., Greenberg E., Kuhn D. N., Preiss J. Subcellular localization of the starch degradative and biosynthetic enzymes of spinach leaves. Plant Physiol. 1979 Aug;64(2):187–192. doi: 10.1104/pp.64.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaxton W. C., Preiss J. Purification and Properties of Nonproteolytic Degraded ADPglucose Pyrophosphorylase from Maize Endosperm. Plant Physiol. 1987 Jan;83(1):105–112. doi: 10.1104/pp.83.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss J. Bacterial glycogen synthesis and its regulation. Annu Rev Microbiol. 1984;38:419–458. doi: 10.1146/annurev.mi.38.100184.002223. [DOI] [PubMed] [Google Scholar]
- SHEN L., PREISS J. BIOSYNTHESIS OF BACTERIAL GLYCOGEN. I. PURIFICATION AND PROPERTIES OF THE ADENOSINE DIPHOSPHOGLUCOSE PYROPHOSPHORYLASE OF ARTHROBACTER SPECIES NRRL B1973. J Biol Chem. 1965 Jun;240:2334–2340. [PubMed] [Google Scholar]
- Santarius K. A., Heber U. Changes in the intracellular levels of ATP, ADP, AMP and P1 and regulatory function of the adenylate system in leaf cells during photosynthesis. Biochim Biophys Acta. 1965 May 25;102(1):39–54. doi: 10.1016/0926-6585(65)90201-3. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sowokinos J. R., Preiss J. Pyrophosphorylases in Solanum tuberosum: III. PURIFICATION, PHYSICAL, AND CATALYTIC PROPERTIES OF ADPGLUCOSE PYROPHOSPHORYLASE IN POTATOES. Plant Physiol. 1982 Jun;69(6):1459–1466. doi: 10.1104/pp.69.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Perrot B., Bottomley W., Whitfeld P. R. The structure of the gene for the large subunit of ribulose 1,5-bisphosphate carboxylase from spinach chloroplast DNA. Nucleic Acids Res. 1981 Jul 24;9(14):3251–3270. doi: 10.1093/nar/9.14.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]