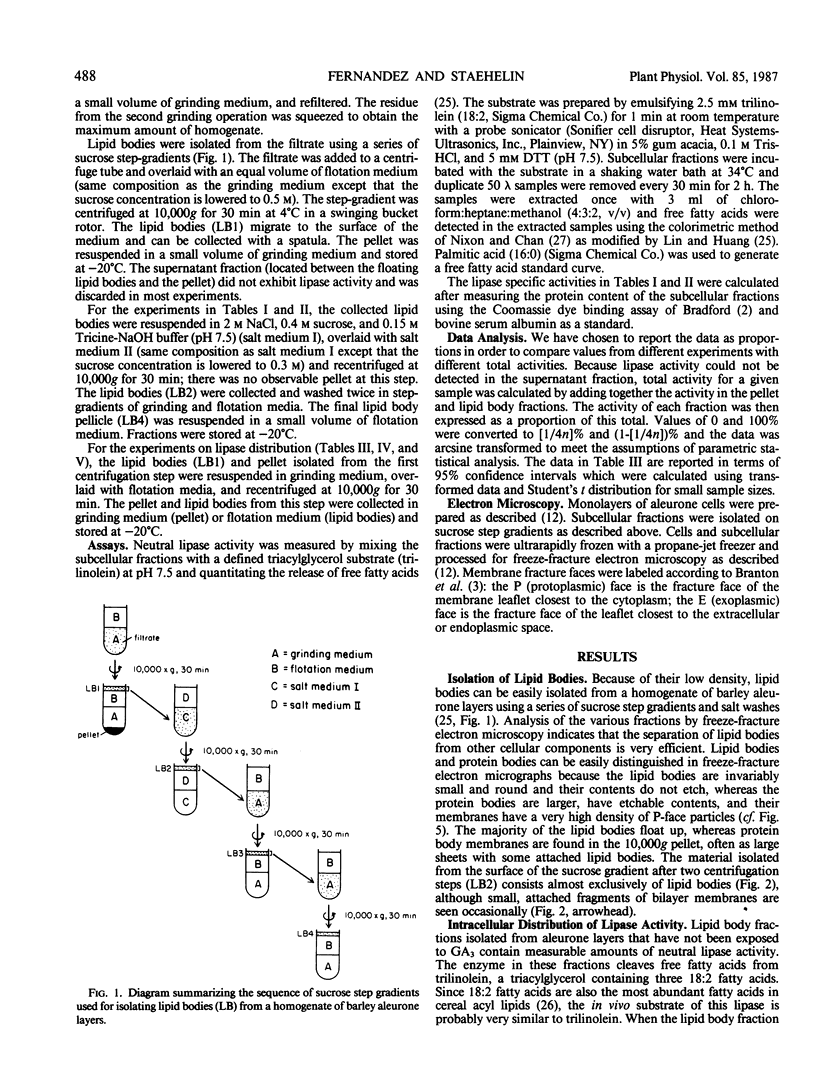
Abstract
We have examined the effect of gibberellic acid (GA3) on the distribution of the enzyme responsible for mobilizing storage triacylglycerol in aleurone cells of Hordeum vulgare L. cv Himalaya. Using cellular fractionation techniques, we find that, in cells that have not been exposed to hormone, neutral lipase activity is principally associated with a pellet containing the membranes of protein bodies. If the cells are exposed to GA3 for at least 1 hour, the majority of the lipase activity becomes associated with the lipid body fraction. The nature of the in vivo association between lipid bodies and protein bodies was examined using ultrarapid freezing followed by freeze-fracture electron microscopy. Our analysis indicates that the phospholipid monolayer surrounding the lipid body is directly continuous with the outer leaflet of the bilayer surrounding the protein body. Based on our data, we propose that lipase can be transferred from protein bodies (storage form) to lipid bodies (active form) by lateral diffusion within the plane of the fused phospholipid monolayer, and that the transfer can be controlled by gibberellic acid by an unknown mechanism.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Tal Y. An early response to gibberellic Acid not requiring protein synthesis. Plant Physiol. 1974 Dec;54(6):813–816. doi: 10.1104/pp.54.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Branton D., Bullivant S., Gilula N. B., Karnovsky M. J., Moor H., Mühlethaler K., Northcote D. H., Packer L., Satir B., Satir P. Freeze-etching nomenclature. Science. 1975 Oct 3;190(4209):54–56. doi: 10.1126/science.1166299. [DOI] [PubMed] [Google Scholar]
- Chrispeels M. J., Varner J. E. Gibberellic Acid-enhanced synthesis and release of alpha-amylase and ribonuclease by isolated barley and aleurone layers. Plant Physiol. 1967 Mar;42(3):398–406. doi: 10.1104/pp.42.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evins W. H., Varner J. E. Hormonal control of polyribosome formation in barley aleurone layers. Plant Physiol. 1972 Mar;49(3):348–352. doi: 10.1104/pp.49.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evins W. H., Varner J. E. Hormone-controlled synthesis of endoplasmic reticulum in barley aleurone cells. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1631–1633. doi: 10.1073/pnas.68.7.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firn R. D., Kende H. Some effects of applied gibberellic Acid on the synthesis and degradation of lipids in isolated barley aleurone layers. Plant Physiol. 1974 Dec;54(6):911–915. doi: 10.1104/pp.54.6.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. D., Kende H. Hormonal Control of Lecithin Synthesis in Barley Aleurone Cells: Regulation of the CDP-Choline Pathway by Gibberellin. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2674–2677. doi: 10.1073/pnas.68.11.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler D. E., Varner J. E. Hormonal control of orthophosphate incorporation into phospholipids of barley aleurone layers. Plant Physiol. 1973 Sep;52(3):208–214. doi: 10.1104/pp.52.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. H., Huang A. H. Purification and initial characterization of lipase from the scutella of corn seedlings. Plant Physiol. 1984 Nov;76(3):719–722. doi: 10.1104/pp.76.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon M., Chan S. H. A simple and sensitive colorimetric method for the determination of long-chain free fatty acids in subcellular organelles. Anal Biochem. 1979 Sep 1;97(2):403–409. doi: 10.1016/0003-2697(79)90093-9. [DOI] [PubMed] [Google Scholar]
- Qu R., Wang S. M., Lin Y. H., Vance V. B., Huang A. H. Characteristics and biosynthesis of membrane proteins of lipid bodies in the scutella of maize (Zea mays L.). Biochem J. 1986 Apr 1;235(1):57–65. doi: 10.1042/bj2350057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Wilden W., Herman E. M., Chrispeels M. J. Protein bodies of mung bean cotyledons as autophagic organelles. Proc Natl Acad Sci U S A. 1980 Jan;77(1):428–432. doi: 10.1073/pnas.77.1.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. M., Huang A. H. Biosynthesis of lipase in the scutellum of maize kernel. J Biol Chem. 1987 Feb 15;262(5):2270–2274. [PubMed] [Google Scholar]
- Yatsu L. Y., Jacks T. J. Association of lysosomal activity with aleurone grains in plant seeds. Arch Biochem Biophys. 1968 Mar 20;124(1):466–471. doi: 10.1016/0003-9861(68)90354-8. [DOI] [PubMed] [Google Scholar]
- Yatsu L. Y., Jacks T. J. Spherosome membranes: half unit-membranes. Plant Physiol. 1972 Jun;49(6):937–943. doi: 10.1104/pp.49.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]