Abstract
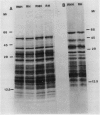
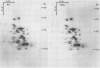
We have studied the influence of growth at low temperature on size class distribution, stability and composition of leaf cytoplasmic polysomes from rye seedlings (Secale cereale, cv Puma) grown at 5°C and at 20°C. Leaves of seedlings grown at 5°C contain 2.7 times more cytoplasmic polysomes (expressed on a DNA basis) and the polysome size class distribution is skewed toward larger polysomes. These changes were more pronounced in the free polysome fraction than in the membrane-bound fraction. The melting point of the total ribosome fraction from cold-grown leaves was decreased by 3.7°C. Electrophoresis did not reveal any difference in the rRNA or in core-ribosomal proteins (KCl nondissociable) following growth at low temperature. Some differences were noted in peripheral ribosomal proteins. This study is the first to examine the effect of growth at low and high temperatures on polysome metabolism using plants of similar developmental stage. Polysome quantity, polymerization, melting point and peripheral ribosomal proteins in rye seedlings are modified during growth at low temperature.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bixby J. A., Brown G. N. Ribosomal Changes during Induction of Cold Hardiness in Black Locust Seedlings. Plant Physiol. 1975 Nov;56(5):617–621. doi: 10.1104/pp.56.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G. Release, identification, and isolation of messenger RNA from mammalian ribosomes. Proc Natl Acad Sci U S A. 1971 Apr;68(4):832–835. doi: 10.1073/pnas.68.4.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning K. S., Yan T. F., Lauer S. J., Aquino L. A., Tao M., Ravel J. M. Phosphorylation of wheat germ initiation factors and ribosomal proteins. Plant Physiol. 1985 Feb;77(2):370–373. doi: 10.1104/pp.77.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarano P., Mazzei F., Londei P., Teichner A., de Rosa M., Gambacorta A. Secondary structure features of ribosomal RNA species within intact ribosomal subunits and efficiency of RNA-protein interactions in thermoacidophilic (Caldariella acidophila, Bacillus acidocaldarius) and mesophilic (Escherichia coli) bacteria. Biochim Biophys Acta. 1983 Aug 2;740(3):300–312. doi: 10.1016/0167-4781(83)90139-2. [DOI] [PubMed] [Google Scholar]
- Chen H. H., Li P. H. Biochemical Changes in Tuber-bearing Solanum Species in Relation to Frost Hardiness during Cold Acclimation. Plant Physiol. 1980 Sep;66(3):414–421. doi: 10.1104/pp.66.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehling E., Weidner M. Temperature characteristics and adaptive potential of wheat ribosomes. Plant Physiol. 1986 Jan;80(1):181–186. doi: 10.1104/pp.80.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroche A., Hopkins W. G. Isolation and in vitro translation of polysomes from mature rye leaves. Plant Physiol. 1987 Feb;83(2):371–376. doi: 10.1104/pp.83.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver C. J., Dyer J. A. Caution in the interpretation of plant ribosome studies. Biochem J. 1974 Oct;144(1):165–167. doi: 10.1042/bj1440165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madjar J. J., Michel S., Cozzone A. J., Reboud J. P. A method to identify individual proteins in four different two-dimensional gel electrophoresis systems: application to Escherichia coli ribosomal proteins. Anal Biochem. 1979 Jan 1;92(1):174–182. doi: 10.1016/0003-2697(79)90641-9. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak T. S., Jr, Carty E. R., Lust W. D., Passonneau J. V. An in vitro amino acid incorporation method for assessing the status of in vivo protein synthesis. Anal Biochem. 1984 Feb;136(2):285–292. doi: 10.1016/0003-2697(84)90218-5. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Stiegler P., Carbon P., Ebel J. P., Ehresmann C. A general secondary-structure model for procaryotic and eucaryotic RNAs from the small ribosomal subunits. Eur J Biochem. 1981 Dec;120(3):487–495. doi: 10.1111/j.1432-1033.1981.tb05727.x. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Uhlenbeck O. C., Levine M. D. Estimation of secondary structure in ribonucleic acids. Nature. 1971 Apr 9;230(5293):362–367. doi: 10.1038/230362a0. [DOI] [PubMed] [Google Scholar]
- VOLKIN E., COHN W. E. Estimation of nucleic acids. Methods Biochem Anal. 1954;1:287–305. doi: 10.1002/9780470110171.ch11. [DOI] [PubMed] [Google Scholar]
- Venkatesan N., Steele W. J. Isolation of ribosomes from post-nuclear fraction of rat liver in nearly quantitative yield. Biochim Biophys Acta. 1972 Sep 14;277(3):646–650. doi: 10.1016/0005-2787(72)90109-8. [DOI] [PubMed] [Google Scholar]
- Yurina N. P., Byzova N. A., Odintsova M. S. Ribosomal acidic proteins of eucaryotic cells. Isolation of a protein from pea seedlings equivalent to E. coli L7/L12. Mol Cell Biochem. 1983;50(1):17–24. doi: 10.1007/BF00225277. [DOI] [PubMed] [Google Scholar]