Abstract
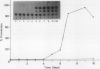
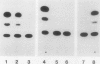
Proteinase inhibitor genes are expressed in solanaceous and leguminous plants following wounding of the foliage by mechanical methods. Previous studies have shown that a cloned proteinase inhibitor II-chloramphenicol acetyl transferase (pin2-CAT) chimeric gene is regulated in a wound-inducible manner in transgenic plants. In this study, we analyzed transgenic plant tissues for expression of the pin2-CAT gene in response to various plant hormones. We found that CAT activity was induced in tobacco (Nicotiana tabacum) callus incubated in the absence of any plant growth regulators. Addition of growth regulators to the medium thus permitted us to measure the effects of these substances on the activity of the pin2-CAT gene construction. Cytokinin (BAP) and ethylene (ethophon) even at low concentrations stimulated the expression of CAT activity by 25 to 50%. Abscisic acid at concentrations up to 4.4 × 10−5 molar had no effect upon CAT activity, but increasing auxin (naphthalene acetic acid) levels completely inhibited the synthesis of CAT protein. Gibberellic acid had little effect except at very high concentration (2.9 × 105 molar). The kinetics of activation of the pin2-CAT gene were quite long (5 to 7 days) when unwounded calli were plated on media lacking auxin. This effect was documented for calli derived from several transformed plants, containing the full, chimeric pin2-CAT (pRT45) gene. In addition, calli from tissues transformed with wild-type vectors or from several plants transformed with pRT50 (a noninducible derivative of pRT45) were not induced by plating on media lacking auxin. Other naturally occurring and synthetic auxins had similar effects to naphthalene acetic acid in inhibiting the induction of the chimeric gene fusion. Finally, leaf discs from transformed plants were induced by incubation in MS liquid medium in the presence and absence of naphthalene acetic acid. NAA was also effective in down regulating the chimeric gene in whole plant tissues.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop P. D., Makus D. J., Pearce G., Ryan C. A. Proteinase inhibitor-inducing factor activity in tomato leaves resides in oligosaccharides enzymically released from cell walls. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3536–3540. doi: 10.1073/pnas.78.6.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Corbin D. R., Sauer N., Lamb C. J. Differential regulation of a hydroxyproline-rich glycoprotein gene family in wounded and infected plants. Mol Cell Biol. 1987 Dec;7(12):4337–4344. doi: 10.1128/mcb.7.12.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDELMAN J., HALL M. A. EFFECT OF GROWTH HORMONES ON THE DEVELOPMENT OF INVERTASE ASSOCIATED WITH CELL WALLS. Nature. 1964 Jan 18;201:296–297. doi: 10.1038/201296b0. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J. S., Pearce G., Merryweather J., Titani K., Ericsson L. H., Ryan C. A. Wound-induced proteinase inhibitors from tomato leaves. II. The cDNA-deduced primary structure of pre-inhibitor II. J Biol Chem. 1985 Jun 10;260(11):6561–6564. [PubMed] [Google Scholar]
- Green T. R., Ryan C. A. Wound-Induced Proteinase Inhibitor in Plant Leaves: A Possible Defense Mechanism against Insects. Science. 1972 Feb 18;175(4023):776–777. doi: 10.1126/science.175.4023.776. [DOI] [PubMed] [Google Scholar]
- Gustafson G., Ryan C. A. Specificity of protein turnover in tomato leaves. Accumulation of proteinase inhibitors, induced with the wound hormone, PIIF. J Biol Chem. 1976 Nov 25;251(22):7004–7010. [PubMed] [Google Scholar]
- Keil M., Sanchez-Serrano J., Schell J., Willmitzer L. Primary structure of a proteinase inhibitor II gene from potato (Solanum tuberosum). Nucleic Acids Res. 1986 Jul 25;14(14):5641–5650. doi: 10.1093/nar/14.14.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton M. A., Lamb C. J. Transcriptional activation of plant defense genes by fungal elicitor, wounding, and infection. Mol Cell Biol. 1987 Jan;7(1):335–341. doi: 10.1128/mcb.7.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Brown W. E., Graham J. S., Pearce G., Fox E. A., Dreher T. W., Ahern K. G., Pearson G. D., Ryan C. A. Molecular characterization and phylogenetic studies of a wound-inducible proteinase inhibitor I gene in Lycopersicon species. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7277–7281. doi: 10.1073/pnas.83.19.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockerse R., Siegel B. Z., Galston A. W. Hormone-induced repression of a peroxidase isozyme in plant tissue. Science. 1966 Jan 28;151(3709):452–453. doi: 10.1126/science.151.3709.452. [DOI] [PubMed] [Google Scholar]
- Ryan C. A., Bishop P., Pearce G. A sycamore cell wall polysaccharide and a chemically related tomato leaf polysaccharide possess similar proteinase inhibitor-inducing activities. Plant Physiol. 1981 Sep;68(3):616–618. doi: 10.1104/pp.68.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C. A. Synthesis of chymotrypsin inhibitor I protein in potato leaflets induced by detachment. Plant Physiol. 1968 Nov;43(11):1859–1865. doi: 10.1104/pp.43.11.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J. A., Tseng J., Williams R., Cabello A. Wound-Induced RNase Activity in Sweet Potato : EVIDENCE FOR REGULATION AT TRANSCRIPTION. Plant Physiol. 1982 May;69(5):1060–1065. doi: 10.1104/pp.69.5.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs T. Plant tumors resulting from unregulated hormone synthesis. J Theor Biol. 1975 Dec;55(2):445–453. doi: 10.1016/s0022-5193(75)80092-0. [DOI] [PubMed] [Google Scholar]
- Simons T. J., Israel H. W., Ross A. F. Effect of 2,4-dichlorophenoxyacetic acid on tobacco mosaic virus lesions in tobacco and on the fine structure of adjacent cells. Virology. 1972 May;48(2):502–515. doi: 10.1016/0042-6822(72)90061-x. [DOI] [PubMed] [Google Scholar]
- Thornburg R. W., An G., Cleveland T. E., Johnson R., Ryan C. A. Wound-inducible expression of a potato inhibitor II-chloramphenicol acetyltransferase gene fusion in transgenic tobacco plants. Proc Natl Acad Sci U S A. 1987 Feb;84(3):744–748. doi: 10.1073/pnas.84.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]