Abstract
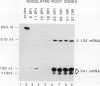
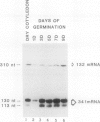
In Pisum sativum, two classes of genes encode distinct isoforms of cytosolic glutamine synthetase (GS). The first class comprises two nearly identical or “twin” GS genes (GS341 and GS132), while the second comprises a single GS gene (GS299) distinct in both coding and noncoding regions from the “twin” GS genes. Gene-specific analyses were used to monitor the individual contribution of each gene for cytosolic GS during root nodule development and in cotyledons during germination, two contexts where large amounts of ammonia must be assimilated by GS for nitrogen transport. mRNAs corresponding to all three genes for cytosolic GS were shown to accumulate coordinately during a time course of nodule development. All the GS mRNAs also accumulate to wild-type levels in mutant nodules formed by a nifD− strain of Rhizobium leguminosarum indicating that induced GS expression in pea root nodules does not depend on the production of ammonia. Distinct patterns of expression for the two classes of GS genes were observed in certain mutant root nodules and most dramatically in cotyledons of germinating seedlings. The different patterns of expression between the two classes of genes for cytosolic GS suggests that their distinct gene products may serve nonoverlapping functions during pea development.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coruzzi G., Broglie R., Edwards C., Chua N. H. Tissue-specific and light-regulated expression of a pea nuclear gene encoding the small subunit of ribulose-1,5-bisphosphate carboxylase. EMBO J. 1984 Aug;3(8):1671–1679. doi: 10.1002/j.1460-2075.1984.tb02031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullimore J. V., Gebhardt C., Saarelainen R., Miflin B. J., Idler K. B., Barker R. F. Glutamine synthetase of Phaseolus vulgaris L.: organ-specific expression of a multigene family. J Mol Appl Genet. 1984;2(6):589–599. [PubMed] [Google Scholar]
- Dilworth M. F., Dure L. Developmental biochemistry of cotton seed embryogenesis and germination: x. Nitrogen flow from arginine to asparagine in germination. Plant Physiol. 1978 Apr;61(4):698–702. doi: 10.1104/pp.61.4.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke S. H., Schrader L. E., Miller M. G. Low Temperature Effects on Soybean (Glycine max [L.] Merr. cv. Wells) Free Amino Acid Pools during Germination. Plant Physiol. 1978 Oct;62(4):642–647. doi: 10.1104/pp.62.4.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke S. H., Schrader L. E., Miller M. G. Low Temperature Effects on Soybean (Glycine max [L.] Merr. cv. Wells) Free Amino Acid Pools during Germination. Plant Physiol. 1978 Oct;62(4):642–647. doi: 10.1104/pp.62.4.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn K., Dickstein R., Feinbaum R., Burnett B. K., Peterman T. K., Thoidis G., Goodman H. M., Ausubel F. M. Developmental regulation of nodule-specific genes in alfalfa root nodules. Mol Plant Microbe Interact. 1988 Feb;1(2):66–74. doi: 10.1094/mpmi-1-066. [DOI] [PubMed] [Google Scholar]
- Edwards J. W., Coruzzi G. M. Photorespiration and light act in concert to regulate the expression of the nuclear gene for chloroplast glutamine synthetase. Plant Cell. 1989 Feb;1(2):241–248. doi: 10.1105/tpc.1.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt C., Oliver J. E., Forde B. G., Saarelainen R., Miflin B. J. Primary structure and differential expression of glutamine synthetase genes in nodules, roots and leaves of Phaseolus vulgaris. EMBO J. 1986 Jul;5(7):1429–1435. doi: 10.1002/j.1460-2075.1986.tb04379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirel B., Bouet C., King B., Layzell D., Jacobs F., Verma D. P. Glutamine synthetase genes are regulated by ammonia provided externally or by symbiotic nitrogen fixation. EMBO J. 1987 May;6(5):1167–1171. doi: 10.1002/j.1460-2075.1987.tb02350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson R. A., Kavanagh T. A., Bevan M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987 Dec 20;6(13):3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schetgens T. M., Bakkeren G., van Dun C., Hontelez J. G., van den Bos R. C., van Kammen A. Molecular cloning and functional characterization of Rhizobium leguminosarum structural nif-genes by site-directed transposon mutagenesis and expression in Escherichia coli minicells. J Mol Appl Genet. 1984;2(4):406–421. [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Suissa M. Spectrophotometric quantitation of silver grains eluted from autoradiograms. Anal Biochem. 1983 Sep;133(2):511–514. doi: 10.1016/0003-2697(83)90117-3. [DOI] [PubMed] [Google Scholar]
- Tingey S. V., Tsai F. Y., Edwards J. W., Walker E. L., Coruzzi G. M. Chloroplast and cytosolic glutamine synthetase are encoded by homologous nuclear genes which are differentially expressed in vivo. J Biol Chem. 1988 Jul 15;263(20):9651–9657. [PubMed] [Google Scholar]
- Tingey S. V., Walker E. L., Coruzzi G. M. Glutamine synthetase genes of pea encode distinct polypeptides which are differentially expressed in leaves, roots and nodules. EMBO J. 1987 Jan;6(1):1–9. doi: 10.1002/j.1460-2075.1987.tb04710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquhart A. A., Joy K. W. Use of Phloem exudate technique in the study of amino Acid transport in pea plants. Plant Physiol. 1981 Sep;68(3):750–754. doi: 10.1104/pp.68.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallsgrove R. M., Turner J. C., Hall N. P., Kendall A. C., Bright S. W. Barley mutants lacking chloroplast glutamine synthetase-biochemical and genetic analysis. Plant Physiol. 1987 Jan;83(1):155–158. doi: 10.1104/pp.83.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter H. C., Powell G. K., Dekker E. E. Glutamine Synthetase of Germinating Peanuts : PROPERTIES OF TWO CHROMATOGRAPHICALLY DISTINCT FORMS AND THEIR ACTIVITY TOWARD 4-METHYLENEGLUTAMIC ACID. Plant Physiol. 1982 Jan;69(1):41–47. doi: 10.1104/pp.69.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]