Abstract
A chilling-sensitive mutant of Arabidopsis thaliana was isolated and subjected to genetic, physiological, and biochemical analysis. The chilling-sensitive nature of the mutant line is due to a single recessive nuclear mutation at a locus designated chs1. In contrast to wild-type plants, which are not adversely affected by low temperatures, the chs1 mutant is killed by several days of exposure to temperatures below 18°C. Following exposure to chilling temperatures, the mutant displays two common symptoms of chilling injury—leaf chlorosis and electrolyte leakage. In these respects, the physiological response of the mutant to low temperatures mimics the response observed in some naturally occurring chilling sensitive species. The biochemical basis of chilling sensitivity was explored by examining the pattern of incorporation of 14CO2 into soluble metabolites and lipids in wild-type and mutant plants. The only difference observed between the mutant and wild type was that following low temperature treatment, the mutant accumulated 10-fold more radioactivity in a specific class of neutral lipids which were identified by a variety of criteria to be steryl-esters. The accumulation of radioactivity in the steryl-ester fraction occurs 24 hours before there is any visible evidence of chilling injury. These results suggest one of two possible explanations: either the mutation directly affects sterol metabolism, which in turn leads to chilling sensitivity, or the mutation affects another unidentified function and the accumulation of radioactivity in steryl-esters is a secondary consequence of chilling injury.
Full text
PDF








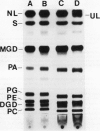
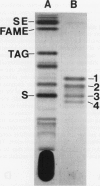
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brady D. R., Gaylor J. L. Enzymic formation of esters of methyl sterol precursors of cholesterol. J Lipid Res. 1971 May;12(3):270–276. [PubMed] [Google Scholar]
- Browse J., McCourt P., Somerville C. R. A mutant of Arabidopsis lacking a chloroplast-specific lipid. Science. 1985 Feb 15;227(4688):763–765. doi: 10.1126/science.227.4688.763. [DOI] [PubMed] [Google Scholar]
- Browse J., McCourt P., Somerville C. A mutant of Arabidopsis deficient in c(18:3) and c(16:3) leaf lipids. Plant Physiol. 1986 Jul;81(3):859–864. doi: 10.1104/pp.81.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Bowman J. L., DeJohn A. W., Lander E. S., Meyerowitz E. M. Restriction fragment length polymorphism linkage map for Arabidopsis thaliana. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6856–6860. doi: 10.1073/pnas.85.18.6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald C. Effects of free sterols, steryl ester, and steryl glycoside on membrane permeability. Plant Physiol. 1971 Nov;48(5):653–655. doi: 10.1104/pp.48.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald C. Sterol distribution in intracellular organelles isolated from tobacco leaves. Plant Physiol. 1970 Jun;45(6):663–666. doi: 10.1104/pp.45.6.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg L., Hull R., Hull S., Falkow S., Freter R., Svanborg Edén C. Contribution of adhesion to bacterial persistence in the mouse urinary tract. Infect Immun. 1983 Apr;40(1):265–272. doi: 10.1128/iai.40.1.265-272.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp R. J., Mercer E. I. Studies on the sterols and sterol esters of the intracellular organelles of maize shoots. Biochem J. 1968 Nov;110(1):119–125. doi: 10.1042/bj1100119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons J. M., Raison J. K. Oxidative activity of mitochondria isolated from plant tissues sensitive and resistant to chilling injury. Plant Physiol. 1970 Apr;45(4):386–389. doi: 10.1104/pp.45.4.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCourt P., Kunst L., Browse J., Somerville C. R. The effects of reduced amounts of lipid unsaturation on chloroplast ultrastructure and photosynthesis in a mutant of Arabidopsis. Plant Physiol. 1987 Jun;84(2):353–360. doi: 10.1104/pp.84.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suiter K. A., Wendel J. F., Case J. S. LINKAGE-1: a PASCAL computer program for the detection and analysis of genetic linkage. J Hered. 1983 May-Jun;74(3):203–204. doi: 10.1093/oxfordjournals.jhered.a109766. [DOI] [PubMed] [Google Scholar]
- Yao J. K., Rastetter G. M. Microanalysis of complex tissue lipids by high-performance thin-layer chromatography. Anal Biochem. 1985 Oct;150(1):111–116. doi: 10.1016/0003-2697(85)90447-6. [DOI] [PubMed] [Google Scholar]