Abstract
The mycorrhiza helper bacterium Streptomyces strain AcH 505 improves mycelial growth of ectomycorrhizal fungi and formation of ectomycorrhizas between Amanita muscaria and spruce but suppresses the growth of plant-pathogenic fungi, suggesting that it produces both fungal growth-stimulating and -suppressing compounds. The dominant fungal-growth-promoting substance produced by strain AcH 505, auxofuran, was isolated, and its effect on the levels of gene expression of A. muscaria was investigated. Auxofuran and its synthetic analogue 7-dehydroxy-auxofuran were most effective at a concentration of 15 μM, and application of these compounds led to increased lipid metabolism-related gene expression. Cocultivation of strain AcH 505 and A. muscaria stimulated auxofuran production by the streptomycete. The antifungal substances produced by strain AcH 505 were identified as the antibiotics WS-5995 B and C. WS-5995 B completely blocked mycelial growth at a concentration of 60 μM and caused a cell stress-related gene expression response in A. muscaria. Characterization of these compounds provides the foundation for molecular analysis of the fungus-bacterium interaction in the ectomycorrhizal symbiosis between fly agaric and spruce.
Actinomycetes are known for their capacity to control plant diseases. A number of investigations have reported antagonism of root-pathogenic fungi by soil actinomycetes (6, 40) and that especially streptomycetes are a rich source of antifungal compounds (20).
Ectomycorrhizas (ECM) are widespread symbiotic organs that are formed during interactions between most tree species or perennials and soil fungi (44). The establishment of the symbiosis is affected by other soil microbes, such as bacteria and other fungi (18, 29). Understanding the interactions between the soil-inhabiting microorganisms in the mycorrhizosphere is very important, as they are key players in nutrient cycling in forest soils (29).
Under natural conditions, the plant partner is able to select for bacterial strains that are beneficial for the ECM symbiosis and for plant growth (16). Specifically, the growth of ectomycorrhizal fungi and mycorrhiza formation are promoted by some of the mycorrhizosphere bacteria (mycorrhiza helper bacteria [MHB] [18]), which belong to the genera Bacillus, Burkholderia, Pseudomonas, Rhodococcus, and Streptomyces (15, 19, 30, 37, 42).
When 12 actinomycete isolates were tested to determine their effects on mycelial growth of ectomycorrhizal fungi (39), the bacterial isolates inhibited, promoted, or had no significant effect on hyphal extension in dual cultures. Thus, there is an opportunity to select for actinomycetes that stimulate certain symbiotic fungi and plant growth but are antagonistic to pathogenic organisms. To do this, we isolated a collection of actinomycetes from the hyphosphere of a spruce (Picea abies) stand and examined their effects on symbiotic and plant-pathogenic fungi (30, 31). One member of this collection, streptomycete strain AcH 505, exhibited the desired qualities, as it significantly promoted mycelial growth and the mycorrhization rate of Amanita muscaria (fly agaric) and Suillus bovinus but suppressed mycelial extension of the plant pathogens Armillariella obscura and Heterobasidion annosum (31, 42). A dual culture with strain AcH 505 modulated A. muscaria gene expression, and the responsive fungal genes included genes encoding molecules involved in signaling pathways, metabolism, cell structure, and the cell growth response (42).
Although advances have been made in characterization of MHB belonging to different genera, the challenge of isolating the stimulating bacterial substances involved in the mycorrhization helper effect remains. In order to demonstrate the presence of such compounds produced by strain AcH 505, three dominant secondary metabolites were isolated from the culture supernatant, their structures were elucidated (27), and the compounds were determined to be auxofuran, a novel 5,6,7-trihydro-7-hydroxy-3-prolylbenzofuran-4-one, and the naphthoquinone antibiotics WS-5995 B and C (25). The effects of these compounds on the growth and gene expression of A. muscaria were analyzed. Here we provide evidence that strain AcH 505 produces not only auxofuran, which promotes the growth of A. muscaria, but also the growth-suppressing antibiotics WS-5995 B and C. The results indicate that specific antifungal substance insensitivity plays an important role in the establishment and protection of ECM.
MATERIALS AND METHODS
Organisms.
Strain AcH 505 was isolated from soil of the hyphosphere of Norway spruce collected near Haigerloch, Germany (31). This isolate was cultivated on ISP-2 agar (43) in the dark at 20°C. A. muscaria strain 13 and Hebeloma cylindrosporum Romagnesi strain H1-H7 (9) were cultivated in the dark at 20°C on MMN agar (33) supplemented with 10 g liter−1 glucose. Cultures for growth experiments were started with 4-mm-diameter inocula that were placed on the center of a sterile cellophane sheet (exclusion limit, 10 kDa; Folia, Wendelstein, Germany) on MMN agar. Seeds of Norway spruce (P. abies (L.) Karst) were obtained from Staatsklenge Nagold, Germany.
Taxonomy of the producing strain.
Strain AcH 505 was inoculated onto ISP-2 agar, incubated at 27°C for 1 week, and examined visually to determine substrate and aerial mycelium pigmentation and spore color. The isomeric form of diaminopimelic acid was determined by thin-layer chromatography of a whole-organism hydrolysate by using a standard procedure (46), and menaquinones were analyzed by reversed-phase high-performance liquid chromatography (HPLC) (48).
Partial 16S rRNA gene amplification and determination of the almost complete sequence were performed as described by Rainey et al. (38). Neighbor-joining phylogenetic analysis was performed by using full-length 16S rRNA gene sequences with Streptomyces setonii as the outgroup. The sequences were aligned by using CLUSTALX (26). The resultant multiple-alignment file was edited by hand. The overhanging ends were removed from both ends of the sequence to ensure that all sequences were the same length. The phylogenetic relationships were analyzed by the neighbor-joining method using the PHYLIP software (13).
Fermentation and isolation.
Batch fermentations of strain AcH 505 were carried out in a 10-liter stirred tank fermentor (Biostat S; B. Braun International, Melsungen, Germany) containing modified MMN medium consisting of (per liter of deionized water) Bacto Casamino Acids (1 g), glucose (10 g), malt extract (5 g), CaCl2 (0.05 g), NaCl (0.025 g), KH2PO4 (0.05 g), (NH4)2HPO4 (0.025 g), MgSO4 · 7H2O (0.15 g), FeCl3 · 6H2O (1 mg), thiamine (0.1 mg), and a trace element solution (10 ml); the pH was adjusted to 5.0 after sterilization. The fermentor was inoculated with shake cultures (5%, vol/vol) that were grown in 500-ml Erlenmeyer flasks with one baffle for 48 h on a rotary shaker at 120 rpm and 27°C using the same medium. Fermentation was carried out for 13 days with an aeration rate of 0.5 airstream volumes per fermentation volume per minute (vv−1 min−1) and agitation at 200 rpm.
Cofermentation of strain AcH 505 and A. muscaria was performed in 500-ml magnetically stirred glass fermentors (home made) containing modified MMN medium. To inoculate a fermentor, the pellet from a 200-ml shake flask preculture of strain AcH 505 grown for 120 h and the pellet from a 500-ml shake flask preculture of A. muscaria grown for 1 month were resuspended in 500 ml modified MMN medium. Fermentation was carried out for 21 days with an aeration rate of 0.25 vv−1 min−1 and agitation at 100 rpm.
The secondary metabolites were analyzed and quantified by reversed-phase HPLC and diode array monitoring by using a standard protocol (14).
Hyflo Super-cel (2%) was added to the fermentation broth just prior to separation by multiple-sheet filtration into culture filtrate and mycelium. The mycelium was discarded. The culture filtrate was applied to an Amberlite XAD-16 column. Impurities were washed out with water, and auxofuran was eluted with methanol (MeOH)-H2O (80:20) and concentrated in vacuo to obtain an aqueous residue, which was extracted three times with ethyl acetate. The organic extracts were combined and concentrated in vacuo to dryness. The crude product was dissolved in CH2Cl2 and subjected to silica gel column chromatography (Silica Gel 60; Merck). Separation was performed with a linear gradient using CH2Cl2-MeOH, starting with CH2Cl2 and ending with 5% MeOH. Pure auxofuran was obtained by subsequent chromatography on Sephadex LH-20 with MeOH as the eluent and preparative reversed-phase HPLC using Nucleosil-100 C18 (Macherey & Nagel) and an H2O-MeOH linear gradient (20% to 60% MeOH). After concentration in vacuo to dryness, auxofuran was obtained as an oil.
Antibiotics WS-5995 B and C were isolated from the culture filtrate by Amberlite XAD-16 chromatography. WS-5995 B and C were separated by stepwise elution with H2O-MeOH (60:40 and 20:80, respectively). Further purification was performed by diol-modified silica gel chromatography (LiChroprep Diol; Merck), stepwise gradient elution using CH2Cl2-MeOH, and subsequent chromatography on Sephadex LH-20 with MeOH as the eluent. Pure WS-5995 B and C were obtained by preparative reversed-phase HPLC using a Nucleosil-100 C18 column and elution with 0.1% formic acid-MeOH (45:55 and 47:53, respectively).
Antimicrobial assays.
An agar plate diffusion assay was used to determine the antibacterial and antifungal spectra of the metabolites from strain AcH 505. Ten-microliter portions of samples were applied to filter disks (diameter, 6 mm). The test plates were incubated for 24 h at a temperature that permitted optimal growth of the test organisms.
To determine the MIC, a 96-well microtiter plate assay was used. The antibiotics were dissolved in dimethyl sulfoxide, and the final dimethyl sulfoxide concentration in the cultures was 5%. The test organisms were grown on a rotary shaker (120 rpm) in a medium consisting of nutrient broth (0.8%) and NaCl (0.5%) in deionized water; 106 cells/ml was used as the inoculum, and growth inhibition was evaluated after incubation for 24 and 48 h.
Influence of streptomycetes on fungal growth.
The conditions used for bacterial and fungal axenic and dual cultures and examination of the bacterial effects on fungal growth were essentially the conditions described by Maier et al. (31). The bacterial effect on fungal growth was determined in dual cultures without direct contact between the fungus and the bacterium on MMN medium (see reference 31 for details). A single thin streak (length, 4 cm) of bacteria, obtained from a 1-week-old culture, was applied to the edge of a petri dish (outside a cellophane sheet) with a sterile inoculation loop. Ten replicates were prepared.
Synthesis of ectomycorrhizas and analysis of seedlings.
Norway spruce seedlings were cultivated until they were 4 weeks old as described by Schaeffer et al. (41). Ectomycorrhizas were synthesized using a sterile peat moss-perlite petri dish method modified from the method of Poole et al. (37), as described by Schrey et al. (42). Four-month-old seedlings were harvested for analysis. For all inoculations, 10 replicates were prepared.
Biological cultures for evaluation of the effect of AcH 505-derived metabolites on hyphal growth and gene expression.
Stock solutions of auxofuran and WS-5995 B and C were prepared by dissolving the substances in MeOH at concentrations of 50 mM for auxofuran and 30 mM for WS-5995 B and C. 7-Dehydroxy-auxofuran, which is liquid at room temperature, was diluted to obtain a concentration of 50 mM in MeOH. The stock solutions were stored in 500-μl aliquots at −20°C. After a short incubation at room temperature, the stock solutions were vortexed vigorously to ensure that the compounds were completely dissolved before use. For all substances, concentrations ranging from 150 nM to 150 μM were used to assess the effect on fungal growth. For the analysis of mycelial growth on solid media, petri dishes were filled with 35 ml MMN medium. The compounds were added in 100 μl of MeOH in the middle of the petri dishes, spread with a glass triangle, and allowed to dissolve into the agar overnight. Introduction of specific substances directly into 40°C culture medium gave results comparable to the results obtained when substances were applied to the top of the agar (data not shown). A cellophane sheet was placed on the MMN medium, and four fungal inocula were positioned equally spaced from the petri dish margin. The radii of fungal colonies were determined after 3 and 6 weeks of growth. Fungal suspension cultures were started by using actively growing hyphae that were finely cut, transferred to MMN medium, homogenized, and incubated at 20°C on a rotary shaker at 80 rpm. The MMN medium was changed once a week. Prior to substance application, the fungal suspension cultures were washed twice with MMN medium without glucose, and the substances were added in 100 μl of MeOH to the culture medium. An MeOH-treated fungal suspension culture served as a control. At harvest, suspension cultures were filtered through a 100-μm nylon mesh (Seidengazefabrik, Thal, Switzerland). The material was immediately frozen in liquid nitrogen and stored at −80°C.
Nucleic acid extraction and analysis of gene expression.
Total RNA of A. muscaria was extracted as described by Nehls et al. (35). A Uga4 cDNA insert was amplified from the subtractive cDNA library of A. muscaria, and the 3′-untranslated regions of acetoacyl coenzyme A (acetoacyl-CoA) and cyclophilin 40 cDNAs were amplified from the corresponding cDNAs as described by Schrey et al. (42). PCR-based labeling of the cDNA fragments with digoxigenin and Northern analyses were performed according to the instructions provided in a PCR DIG probe synthesis kit (Roche, Mannheim, Germany). The Northern analyses were replicated twice using RNA from two independent cultures.
Amino acid extraction and analysis of γ-aminobutyric acid (GABA) concentrations.
Amino acids were extracted from liquid nitrogen-ground material first in 80% MeOH and then in 20% MeOH. The two extracts were pooled. Amino acids were separated by HPLC and analyzed as described by Pilot et al. (36). The amino acid analyses were replicated three times using fungal material from three independent cultures.
RESULTS
Taxonomy of the producing strain.
Strain AcH 505 produced a white aerial spore mass and a yellow-brown-pigmented substrate mycelium on ISP-2 agar. Whole-organism hydrolysates were rich in ll-diaminopimelic acid, and the main menaquinone was MK-9(H6). The 120-bp α-region of the 16S rRNA gene from AcH 505 exhibited the highest level of identity to the corresponding 16S rRNA gene region of Streptomyces laceyi (31).
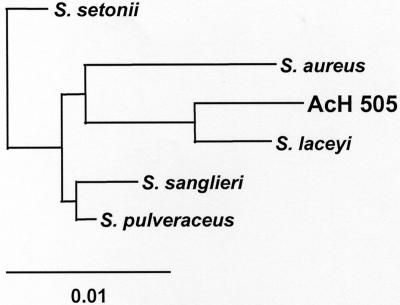
To analyze the relatedness of AcH 505 to other strains belonging to the family Streptomycetaceae in more detail, the complete 16S rRNA gene sequence of AcH 505 was isolated. As expected, 16S rRNA gene signature nucleotides of the Streptomycetaceae family (45) were conserved in the AcH 505 16S rRNA gene. The level of nucleotide identity between AcH 505 and the its closest homologue, S. laceyi, was 98.7%, indicating that AcH 505 is a close relative of S. laceyi but may constitute a new species in the genus Streptomyces. To verify this hypothesis, a phylogenetic analysis was performed using the 16S rRNA gene sequence of AcH 505 and the most closely related sequences based on sequence identity. The neighbor-joining tree analysis (Fig. 1) confirmed that AcH 505 forms a distinct phylogenetic line and groups most closely with S. laceyi.
FIG. 1.
AcH 505-related streptomycetes based on a neighbor-joining analysis of 16S rRNA gene sequences. The sequence data for five AcH 505-related streptomycete-like cultures were obtained from the GenBank database and included in the tree. Scale bar = 0.01 estimated mutation per sequence position. The accession numbers for the sequences are as follows: Streptomyces sp. strain AcH505, DQ231567; Streptomyces aureus, AY094368; S. laceyi, AY094367; Streptomyces pulveraceus, AJ781377; S. sanglieri, AY094363; and S. setonii, D63872.
Fermentation and isolation of secondary metabolites produced by strain AcH 505.
Production of auxofuran started in the optimized 10-liter fermentor after 4 days of fermentation, and the concentration reached the maximal value, 80 mg/liter, after 13 days. Production of the antibiotics WS-5995 B and C began at the same time, and the maximal concentrations (7 and 3 mg/liter, respectively) were observed after 6 and 13 days of fermentation, respectively. The production of auxofuran and the antibiotics WS-5995 B and C was dependent on the pH of the culture. While auxofuran was produced preferably in fermentations at a low pH (e.g., in pH 5-controlled fermentations), no production of WS-5995 B and C was observed under these conditions. These metabolites were preferably produced at a neutral pH, which resulted in a decreased amount of auxofuran.
The metabolites were isolated from the culture filtrate and were purified by chromatography with Amberlite XAD-16, silica gel, Sephadex, and LH-20 and by preparative reversed-phase HPLC. The structures of auxofuran and WS-5995 B and C (Fig. 2) were determined by high-resolution electron impact mass spectrometry and nuclear magnetic resonance (NMR). The NMR signals were assigned to two-dimensional NMR experiments (27). The data for WS-5995 B and C were compared with the data in reference 25 (R. D. Süßmuth, personal communication). The molecular weights were determined to be 194.0127 for auxofuran and 338.3136 and 354.3126 for WS-5995 B and WS-5995 C, respectively.
FIG. 2.
Structural formulas for auxofuran and the antibiotics WS-5995 B and C, produced by Streptomyces strain AcH 505.
Cofermentation of strain AcH 505 with A. muscaria.
To examine the influence of streptomycete strain AcH 505 on the fungus A. muscaria and vice versa during submerged cocultivation with regard to the production of auxofuran, the two organisms were cultivated at the 500-ml scale separately and with each other. As expected, no production of auxofuran was observed in the culture of A. muscaria alone. In the case of fermentation of strain AcH 505, a nearly constant level of about 1.5 mg/liter auxofuran was observed over a 21-day fermentation period. On the other hand, cocultivation of the two organisms stimulated auxofuran production by the streptomycete and resulted in a continuously increasing amount of auxofuran; the maximal value was 6 mg/liter after incubation for 21 days.
Effect of AcH 505 and its dominant secondary substances on mycelial growth of fly agaric.
Fly agaric (A. muscaria) isolates generally grow slowly in standard culture media. In contrast, in a dual culture with strain AcH 505 the fungus is capable of significantly faster saprophytic growth (Table 1). As a first step toward formation of ectomycorrhizas between spruce and A. muscaria, fungal hyphae reach and cover the surfaces of fine roots. A tripartite culture with strain AcH 505 aided this process, and the increase in the amount of mycorrhizal fine roots of 4-month-old spruce seedlings correlated with the level of mycelial growth promotion (Table 1), indicating that increased rates of mycelial extension are at least partially responsible for the mycorrhization helper effect.
TABLE 1.
Effect of strain AcH 505 on the mycelial growth of A. muscaria and mycorrhiza formationa
| Bacterial inoculum | Mycelial growth (cm2) | Mycorrhiza (% of secondary roots) |
|---|---|---|
| AcH 505 | 50.2 ± 7.8b | 66 ± 8.6b |
| None | 30.3 ± 5.9 | 44.4 ± 5.6 |
| Ratio (AcH 505/none) | 1.67 ± 0.26 | 1.47 ± 0.19 |
Mycelial growth is expressed as the colony area, and mycorrhiza formation is expressed as the percentage of mycorrhizal secondary roots of Norway spruce with A. muscaria.
The values for the treatments with bacterial inoculation are significantly higher than values for the control treatments as determined by Student's t test (P< 0.01).
Auxofuran, the dominant fungal-growth-promoting substance excreted by AcH 505, stimulated A. muscaria hyphal growth most effectively at a concentration of 15 μM, and the fungus responded significantly to concentrations in the nanomolar range (Fig. 3A). It is possible that auxofuran and its synthetic dehydroxylated derivative, 7-dehydroxy-auxofuran, could exhibit specificity in their effects as growth-stimulating substances because of differences in their structures and solubilities. In order to investigate this possibility, fungal mycelia were grown on solid media supplemented with one of the compounds. As observed for auxofuran, the strongest positive effect on the growth of A. muscaria on solid media was observed with 15 μM 7-dehydroxy-auxofuran (data not shown). When the effects of the two substances were compared, we found that A. muscaria responded until 4 weeks to both substances, although it responded to a significantly greater extent to 7-dehydroxy-auxofuran (Fig. 3B). At 6 weeks, further stimulation of growth was detected for 7-dehydroxy-auxofuran, whereas the growth-promoting effect of auxofuran disappeared, indicating that 7-dehydroxy-auxofuran has not only a stronger but also a more persistent stimulatory effect on fly agaric than auxofuran has.
FIG. 3.
Growth-promoting activity of auxofuran. (A) Radial growth of A. muscaria mycelia after 2 weeks of incubation on solid media. The P values (as determined by Student's t test) for pairwise comparisons with the control treatments (C) were <0.01 for 150 nM to 50 μM auxofuran. (B) Comparison of the effects of auxofuran (A) and its synthetic analog 7-dehydroxy-auxofuran (dOH-A) on A. muscaria mycelia on solid media. Both substances were added to the culture medium at a concentration of 15 μM. The P values (as determined by Student's t test) for pairwise comparisons were <0.001 for all times. The P value for the comparison of control and auxofuran treatments after 6 weeks of culture was 0.02.
In addition, we analyzed whether the hydroxy group of WS-5995 C, which is absent from WS-5995 B, could have an effect on the response of A. muscaria. We found that at a concentration of 5 μM in solid medium, only WS-5995 B significantly suppressed fungal growth and that A. muscaria responded similarly to 30 μM WS-5995 B and 60 μM WS-5995 C (Fig. 4).
FIG. 4.
Growth-suppressing effects of WS-5995 B and C on A. muscaria hyphae on solid media. Substances were added to MMN culture medium in 100 μl methanol. Control treatments without and with methanol are indicated by Me− and Me+, respectively. Bars with different letters are significantly different according to a one-way analysis of variance and the Tukey test (P < 0.01).
Antimicrobial activities of the antibiotics WS-5995 B and C.
A great variety of gram-positive and gram-negative bacteria and fungi were tested using the agar plate diffusion assay. Only WS-5995 B inhibited the growth of gram-positive bacteria and Haemophilus influenzae (Table 2), whereas other gram-negative bacteria, such as Escherichia coli K-12, Pseudomonas fluorescens DSM 50090, and Proteus mirabilis ATCC 35501, yeasts, such as Saccharomyces cerevisiae ATCC 9080 and Candida albicans Tü 164, and filamentous fungi, such as Botrytis cinerea Tü 157, Aspergillus viridinutans CBS 12756, Penicillium notatum Tü 136, and Paecilomyces variotii Tü 137, were not sensitive to WS-5995 B and C. Using a microtiter plate assay, the MIC of WS-5995 B was determined to be 33 μM for Arthrobacter aurescens, Bacillus subtilis, and Staphylococcus aureus.
TABLE 2.
Antibacterial spectrum of the antibiotic WS-5995 B as determined by the agar plate diffusion assay
| Organism | Inhibition zone diam (mm) with WS-5995 B at a concn of:
|
||
|---|---|---|---|
| 1 mg/ml | 0.3 mg/ml | 0.1 mg/ml | |
| Arthrobacter aurescens DSM 20166 | 15 | 12 | 7 |
| Bacillus subtilis DSM 10a | 15 | 10 | 8 |
| Bacillus subtilis DSM 10b | 24 | 17 | 9 |
| Brevibacillus brevis DSM 30 | 15 | 10 | 7 |
| Staphylococcus aureus DSM 20231 | 15 | 10 | 7 |
| Streptococcus pneumoniae ATCC 49619 | 10 | ||
| Streptomyces viridochromogenes Tü 57 | 27 | 16 | 10 |
| Haemophilus influenzae ATCC 49766 | 25 | 19 | 14 |
Inhibition zone diameters were determined in complex medium.
Inhibition zone diameters were determined in chemically defined medium.
Sensitivity of H. cylindrosporum to auxofuran and WS-5995 B.
The responses of the ectomycorrhizal fungi A. muscaria and H. cylindrosporum to strain AcH 505 vary significantly. Although growth of the A. muscaria strains tested increased in dual cultures with strain AcH 505, this bacterium suppresses the growth of H. cylindrosporum (42). The isolation of bacterial substances enabled us to investigate if the sensitivities to auxofuran or WS-5995 B or C were different in A. muscaria and H. cylindrosporum. First, we analyzed the growth of H. cylindrosporum in the presence of different auxofuran concentrations. We found that 1.5 μM auxofuran resulted in the greatest (50%) increase in the growth of H. cylindrosporum, which is similar to the results obtained with A. muscaria (Fig. 5A). To determine if the inhibition of H. cylindrosporum growth in dual cultures was due to increased sensitivity to WS-5995 B, we analyzed whether A. muscaria and H. cylindrosporum responded differently to WS-5995 B application (Fig. 5B). We observed that at all WS-5995 B concentrations tested, H. cylindrosporum appeared to be significantly more sensitive to the antibiotic than A. muscaria. At a WS-5995 B concentration of 15 μM, H. cylindrosporum growth was drastically decreased (3% growth compared to the control treatment), whereas A. muscaria mycelium was still actively growing (41%). Simultaneous application of auxofuran and WS-5995 B resulted in fungal growth similar to that observed with WS-5995 B, indicating that the effect of the antibiotic overcomes the effect of the growth-promoting factor (data not shown).
FIG. 5.
Response of H. cylindrosporum to auxofuran and WS-5995 B. (A) Promotion of H. cylindrosporum growth with 1.5 μM auxofuran (A 1,5 μM). (B) Activity of WS-5995 B against the ectomycorrhizal fungi A. muscaria and H. cylindrosporum. The radial growth of 2-week-old mycelia was measured. Note the different scales.
Modulated levels of gene expression in A. muscaria in response to 7-dehydroxy-auxofuran and WS-5995 B.
In an initial examination of the effects of 7-dehydroxy-auxofuran and WS-5995 B on the level of A. muscaria gene expression, fungal suspension cultures were supplemented with 15 μM 7-dehydroxy-auxofuran or 30 μM WS-5995 B, and fungal hyphae were harvested after 1 h of incubation. Three genes from an AcH 505-induced A. muscaria cDNA library (42) were selected for this analysis, the genes encoding acetoacyl-CoA synthetase (Aacs), cyclophilin 40 (Cyp40), and GABA permease (Uga4), as these genes were previously shown to be related to growth promotion in A. muscaria hyphae (42). Within 3 h, the fungal cells responded to both compounds (Fig. 6A). The level of Aacs expression was increased approximately twofold by auxofuran and threefold by WS-5995 B, the level of Cyp 40 expression was increased threefold by WS-5995 B, and the level of Uga4 expression was increased threefold by WS-5995 B. As the levels of Uga4 expression correlate with GABA concentrations in the budding yeast S. cerevisiae (1), we determined the GABA concentrations in an A. muscaria suspension culture treated like the culture used for the gene expression analysis. Indeed, in line with the increased level of Uga4 expression, the GABA concentration increased after the addition of WS-5995 B but not after the addition of auxofuran (Fig. 6B). Overall, these observations demonstrated the ability of A. muscaria to rapidly respond to the stimulatory and suppressive substances and to specifically alter its physiology in response to the compounds excreted by strain AcH 505.
FIG. 6.
(A) Levels of transcripts of growth- and stress-related A. muscaria genes under the influence of auxofuran and WS-5995 B. Cultures were harvested after 3 h of incubation with MeOH, 15 μM auxofuran (A), or 30 μM WS-5995 B (B). The levels of expression of A. muscaria acetoacyl-CoA synthetase (Aacs), cyclophilin 40 (Cyp40), and γ-aminobutyric acid permease (Uga4) genes were analyzed by Northern analysis. (B) WS-5995 B-based induction of the GABA concentration in A. muscaria mycelia. Changes in the GABA concentration during fungal growth in a submerged culture were investigated. Water (A), MeOH (B), 15 μM auxofuran (C), or 30 μM WS-5995 B (D) was added to actively growing mycelia. The levels of GABA were determined after 3 h of incubation. The means (±standard deviations) for three independent experiments are indicated.
DISCUSSION
This is the first report of the isolation and molecular characterization of a fungal-growth-promoting substance from an MHB belonging to the genus Streptomyces. However, the observation that streptomycetes are able to promote fungal growth is not novel, and streptomycete isolates having such activity with ECM fungi have been characterized previously (3, 39). Auxofuran, the growth-promoting compound produced by Streptomyces strain AcH 505, exhibits structural similarity to the biologically inactive compound ulufuranol (21) and to the benzopyran-type compound koninginin B (8) that exhibits herbicidal activity.
Strain AcH 505 produced the growth promoter auxofuran preferably in fermentations at an acidic pH, which simultaneously inhibited production of the antibiotics WS-5995 B and C. Acidification of the dual-culture medium by the fungus was observed to be an important external factor controlling the secondary metabolite pool of strain AcH 505, leading specifically to an increase in the auxofuran concentration in the dual-culture supernatant. Like A. muscaria, most ectomycorrhizal fungi that have been studied acidify culture media due to the release of oxalate and H+ (2). The acidic substances may be used to attack other organisms and to mobilize poorly soluble nutrients from the soil (7, 11). Here we report a novel function for these acidic fungal substances: modulation of secondary substance production in neighboring microbial species.
Induction of ECM fungal growth takes place not only in response to MHB but also in the vicinity of the host plant roots, where spore germination and mycelial growth can be promoted by root exudates (23, 34). To date, two ECM fungal growth-promoting secondary substances from plant root exudates have been characterized, abietic acid (17) and the flavonoid rutin (28). Interestingly, the structure of the fungal growth suppressor WS-5995 B is similar to the structure of plant flavonoids, indicating the strong sensitivity of ECM fungi to this group of secondary metabolites. The addition of a single hydroxy group to WS-5995 B rendered it significantly less active, suggesting that A. muscaria is very selective in its response.
Streptomycetes are capable of producing microbial antibiotics with a wide variety of chemical structures, including, for example, the majority of antibiotics developed for agricultural use (47). The two antifungal antibiotics found in the cultures of strain AcH 505 are identical to the previously identified anticoccidial antibiotics WS-5995 B and C (25). Our study shows that these substances have an effect not only on prokaryotic species but also on eukaryotic microbial species. Since Schrey et al. (42) observed antifungal activity of strain AcH 505 against the ectomycorrhizal fungus H. cylindrosporum, we examined the effect of WS-5995 B on this fungus and found that H. cylindrosporum is significantly more sensitive to WS-5995 B than A. muscaria is.
The effects of the antibiotics WS-5995 B and C in combination with the growth promoter auxofuran on target fungi represent a previously unreported mode of cooperative action between secondary metabolites from streptomycetes. The fungal strains tested thus far, A. muscaria, H. cylindrosporum (this study), and Heterobasidion annosum (N. Lehr and M. Tarkka, unpublished data), have responded similarly to auxofuran; the greatest promotion of mycelial growth occurred with 1 to 15 μM auxofuran, while significant promotion was still observed at concentrations in the nanomolar range. In contrast, the fungi exhibited different responses to WS-5995 B and C. Most importantly, the fungal strains whose growth was suppressed during coculture with strain AcH 505 were more sensitive to WS-5995 B than the strains whose growth was promoted by this streptomycete, indicating that the resistance to WS-5995 B and C might serve as a selector, leading to the growth promotion phenotype only in resistant organisms.
Three selected fungal genes from a strain AcH 505-induced fly agaric cDNA library (42) responded differently to the streptomycete substances. Acetoacyl-CoA synthetase (Aacs) catalyzes the activation of acetoacetate, which is needed for efficient sterol biosynthesis (4), and we have previously observed that levels of AmAacs transcription increased due to the activation of fungal growth in a dual culture with strain AcH 505 (42). The increased level of AmAacs transcription in response to auxofuran indicated that AmAacs is an early marker for fungal growth activation. Surprisingly, Aacs expression was also increased by WS-5995 B, perhaps in response to membrane damage and the resulting need for rapid ergosterol biosynthesis in the fungal cells.
Cyclophilin 40 (Cyp40) proteins are prolyl isomerases that are involved in the regulation of cell proliferation, in differentiation, and in the control of cell stress (10, 12, 49). The AmCyp40 transcript content increased both in a dual culture with AcH 505 and in the presence of AcH 505 culture supernatants (42). The data presented here show that the latter response can be attributed solely to the antibiotic WS-5995 B, indicating that cyclophilin 40 plays a role in enduring the cell stress caused by the antibiotic.
Furthermore, WS-5995 B caused induction of the putative GABA permease gene of A. muscaria, AmUga4. GABA transporter gene expression in fungi is subject to complex regulatory controls, and it can be increased, for example, by addition of GABA to the culture medium (24). The increased concentration of GABA in the fungus in the presence of WS-5995 B may thus have caused the increased level of AmUga4 expression. As GABA catabolism is involved in scavenging reactive oxygen species (5), WS-5995 B could cause oxidative stress in fungal hyphae. Analysis of auxofuran- and WS-5995 B-related gene expression in other fungal species would provide information about the specificity of the fungal response to AcH 505. This comparative analysis should preferably be performed with isolates of a single fungal species that are growth suppressed and not suppressed in dual culture. Recently, during an analysis of the spruce pathogen Heterobasidion annosum, we obtained such differently responding isolates.
There was a correlation between the promotion of A. muscaria strain 13 mycelium growth in dual culture and the increase in mycorrhization of spruce with A. muscaria in the presence of strain AcH 505, indicating that fungal growth promotion is an important factor behind the mycorrhiza helper bacterium effect. In our previous study, similar results were obtained when dual-culture and mycorrhization tests were performed with the fungal isolates A. muscaria strain 6 and Suillus bovinus (42). Nevertheless, a direct effect of strain AcH 505 on the receptivity of the plant roots leading to the enhanced formation of mycorrhizas has to be considered, since cocultivation of spruce, as well as Scots pine (Pinus sylvestris), with AcH 505 leads to increased fine-root formation (18, 42).
The taxonomic position of strain AcH 505, as determined by a comparative 16S rRNA gene sequence study, indicated that this streptomycete forms a distinct phyletic line within the Streptomyces tree. This observation confirmed the results of Maier et al. (31), which were obtained with a partial 16S rRNA gene sequence. Only recently, three close relatives of strain AcH 505, S. laceyi, Streptomyces aureus, and Streptomyces sanglieri, were classified based on rRNA gene sequences by Manfio et al. (32), and Höltzel et al. (22) reported isolation of lactonamycin Z, an antibiotic and antitumor compound from S. sanglieri culture supernatant. These observations and our results demonstrate the importance of further analysis of this novel taxonomic group of streptomycetes.
In conclusion, the present data show that the streptomycete AcH 505 increases the growth rate of A. muscaria due to the production of the novel secondary substance auxofuran. A low level of fungal sensitivity to the antibiotics WS-5995 B and WS-5995 C, on the other hand, is necessary for the responsiveness to auxofuran. This creates an advantage for A. muscaria over other microbial species that are more sensitive to these antibiotics.
Acknowledgments
We thank R. Süßmuth and his group at Technical University of Berlin for their stimulating work in structure elucidation, B. Stadelhofer and H. Stransky for the GABA analyses, M. Ecke for excellent technical assistance, A. Maier for advice and assistance in setting up the dual cultures, I. Kottke for advice concerning implementation of perlite-moss cultures, and G. Grewe for assistance with fermentations. We especially thank M. E. Maier, Institute for Organic Chemistry, University of Tübingen, for efforts in the synthesis of 7-dehydroxy-auxofuran. We are also indebted to U. Nehls for helpful suggestions. J.R. thanks E. Stackebrandt and R. M. Kroppenstedt from DSMZ for their introduction to taxonomic methods.
This work was supported by the German Research Foundation (graduate school “Infection Biology”) (J.R. and S.D.S.) and by the Strukturfonds of the University of Tübingen (M.T.T.).
Footnotes
Article no. 35 in “Biosynthetic Capacities of Actinomycetes.”
REFERENCES
- 1.Andre, B., C. Hein, M. Grenson, and J. C. Jauniaux. 1993. Cloning and expression of the UGA4 gene coding for the inducible GABA-specific transport protein of Saccharomyces cerevisiae. Mol. Gen. Genet. 237:17-25. [DOI] [PubMed] [Google Scholar]
- 2.Arvieu, J. C., F. Leprince, and C. Plassard. 2003. Release of oxalate and protons by ectomycorrhizal fungi in response to P-deficiency and calcium carbonate in nutrient solution. Ann. For. Sci. 60:815-821. [Google Scholar]
- 3.Becker, D. M., S. T. Bagley, and G. K. Podila. 1999. Effects of mycorrhizal-associated streptomycetes on growth of Laccaria bicolor, Cenococcum geophilum, and Armillaria species and on gene expression in Laccaria bicolor. Mycologia 91:718-723. [Google Scholar]
- 4.Bergstrom, J. D., G. A. Wong, P. A. Edwards, and J. Edmond. 1984. The regulation of acetoacetyl-CoA synthetase activity by modulators of cholesterol synthesis in vivo and the utilization of acetoacetate for cholesterogenesis. J. Biol. Chem. 259:14548-14553. [PubMed] [Google Scholar]
- 5.Coleman, S. T., T. K. Fang, S. A. Rovinsky, F. J. Turano, and W. S. Moye-Rowley. 2001. Expression of a glutamate decarboxylase homologue is required for normal oxidative stress tolerance in Saccharomyces cerevisiae. J. Biol. Chem. 276:244-250. [DOI] [PubMed] [Google Scholar]
- 6.Crawford, D. L., J. M. Lynch, J. M. Whipps, and M. A. Ousley. 1993. Isolation and characterization of actinomycete antagonists of a root fungal pathogen. Appl. Environ. Microbiol. 59:3899-3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cromack, K., P. Sollins, W. C. Granstein, K. Speidel, A. W. Todd, G. Spycher, Y.-L. Ching, and R. L. Todd. 1979. Calcium oxalate accumulation and soil weathering in mats of the hypogeus fungus Hysterangium crassum. Soil Biol. Biochem. 11:463-468. [Google Scholar]
- 8.Cutler, H. G., D. S. Himmelsbach, B. Yagan, R. F. Arrendale, J. M. Jacyno, P. D. Cole, and R. H. Cox. 1991. Koninginin B: a biologically active congener of koninginin A from Trichoderma koningii. J. Agric. Food Chem. 39:977-980. [Google Scholar]
- 9.Debaud, J. C., and G. Gay. 1987. In vitro fruiting under controlled conditions of the ectomycorrhizal fungus Hebeloma cylindrosporum associated with Pinus pinaster. New Phytol. 105:429-435. [DOI] [PubMed] [Google Scholar]
- 10.Dolinski, K., and J. Heitman. 1997. Peptidyl-prolyl isomerases—an overview of the cyclophilin, FKBP and parvulin families, p. 359-369. In M. J. Gething (ed.), Guidebook to molecular chaperones and protein folding catalysis. Oxford University Press, Oxford, United Kingdom.
- 11.Duchesne, L. C., R. L. Peterson, and B. E. Ellis. 1988. Pine root exudate stimulates the synthesis of antifungal compounds by the ectomycorrhizal fungus Paxillus involutus. New Phytol. 108:471-476. [Google Scholar]
- 12.Duina, A. A., J. A. Marsh, and R. F. Gaber. 1996. Identification of two cyp-40 like cyclophilins in Saccharomyces cerevisiae, one of which is required for normal growth. Yeast 12:943-952. [DOI] [PubMed] [Google Scholar]
- 13.Felsenstein, J. 1989. PHYLIP—phylogeny inference package. Cladistics 5:164-166. [Google Scholar]
- 14.Fiedler, H.-P. 1993. Biosynthetic capacities of actinomycetes. 1. Screening for secondary metabolites by HPLC and UV-visible absorbance spectral libraries. Nat. Prod. Lett. 2:119-128. [Google Scholar]
- 15.Founoune, H., R. Duponnois, A. M. Ba, S. Sall, I. Branget, J. Lorquin, M. Neyra, and J. L. Chotte. 2002. Mycorrhiza helper bacteria stimulated ectomycorrhizal symbiosis of Acacia holosericea with Pisolithus alba. New Phytol. 153:81-89. [Google Scholar]
- 16.Frey-Klett, P., M. Chavatte, M.-L. Clausse, S. Courrier, C. Le Roux, J. Raaijmakers, M. G. Martinotti, J.-C. Pierrat, and J. Garbaye. 2005. Ectomycorrhizal symbiosis affects functional diversity of rhizosphere fluorescent pseudomonads. New Phytol. 165:317-328. [DOI] [PubMed] [Google Scholar]
- 17.Fries, N., K. Serck-Hanssen, L. Häll-Dimberg, and O. Theander. 1987. Abietic acid, an activator of basidiospore germination in ectomycorrhizal species of the genus Suillus. Exp. Mycol. 11:360 363. [Google Scholar]
- 18.Garbaye, J. 1994. Mycorrhiza helper bacteria: a new dimension to the mycorrhizal symbiosis. New Phytol. 128:197-210. [DOI] [PubMed] [Google Scholar]
- 19.Garbaye, J., and G. D. Bowen. 1989. Stimulation of mycorrhizal infection of Pinus radiata by some microorganisms associated with the mantle of ectomycorrhizas. New Phytol. 112:383-388. [Google Scholar]
- 20.Gupte, M., P. Kulkarni, and B. N. Ganguli. 2002. Antifungal antibiotics. Appl. Microbiol. Biotechnol. 58:46-57. [DOI] [PubMed] [Google Scholar]
- 21.Henne, P., S. Grabley, R. Thiericke, and A. Zeeck. 1997. Ulupyrinone and ulufuranol: new heteroaromatic metabolites from Streptomyces spina. Liebigs Ann. 1997:937-939. [Google Scholar]
- 22.Höltzel, A., A. Dieter, D. G. Schmid, R. Brown, M. Goodfellow, W. Beil, G. Jung, and H.-P. Fiedler. 2003. Lactonamycin Z, an antibiotic and antitumor compound produced by Streptomyces sanglieri strain AK 623. J. Antibiot. 56:1058-1061. [DOI] [PubMed] [Google Scholar]
- 23.Horan, D. P., and G. A. Chilvers. 1990. Chemotropism: the key to ectomycorrhizal function? New Phytol. 116:451-458. [Google Scholar]
- 24.Hutchings, H., K. P. Stahmann, S. Roels, E. A. Espeso, W. E. Timberlake, H. N. Arst, Jr., and J. Tilburn. 1999. The multiply-regulated gabA gene encoding the GABA permease of Aspergillus nidulans: a score of exons. Mol. Microbiol. 32:557-568. [DOI] [PubMed] [Google Scholar]
- 25.Ikushima, H., M. Okamoto, H. Tanaka, O. Ohe, M. Kohsaka, H. Aoki, and H. Imanaka. 1980. New anticoccidial antibiotics, WS-5995 A and B. I. Isolation and characterization. J. Antibiot. 33:1107-1113. [DOI] [PubMed] [Google Scholar]
- 26.Jeanmougin, F., J. D. Thompson, M. Gouy, D. G. Higgins, and T. J. Gibson. 1998. Multiple sequence alignment with ClustalX. Trends Biochem. Sci. 23:403-405. [DOI] [PubMed] [Google Scholar]
- 27.Keller, S., K. Schneider, and R. D. Süssmuth. Structure elucidation of auxofuran and other metabolites involved in stimulating growth of fly agaric, produced by the mycorrhiza helper bacterium Streptomyces AcH 505. J. Nat. Prod., in press. [DOI] [PubMed]
- 28.Lagrange, H., C. Jay-Allgmand, and F. Lapeyrie. 2001. Rutin, the phenolglycoside from eukalyptus root exudates, stimulates Pisolithus hyphal growth at picomolar concentrations. New Phytol. 149:349-355. [DOI] [PubMed] [Google Scholar]
- 29.Leake, J. R., D. P. Donnelly, and L. Boddy. 2002. Interactions between ectomycorrhizal and saprophytic fungi, p. 346-372. In M. A. G. van Heijden and I. Sanders I (ed.), Mycorrhizal ecology. Springer, Berlin, Germany.
- 30.Maier, A. 2003. Einfluss bakterieller Stoffwechselprodukte auf Wachstum und Proteom des Ektomykorrhizapilzes Amanita muscaria. Ph.D. thesis, University of Tubingen, Tubingen, Germany.
- 31.Maier, A., J. Riedlinger, H.-P. Fiedler, and R. Hampp. 2004. Actinomycetales bacteria from a spruce stand: characterization and effects on growth of root symbiotic and plant parasitic soil fungi in dual culture. Mycol. Progr. 3:129-136. [Google Scholar]
- 32.Manfio, G. P., E. Atalan, J. Zakrzewska-Czerwinska, M. Mordarski, C. Rodriguez, M. D. Collins, and M. Goodfellow. 2003. Classification of novel soil streptomycetes as Streptomyces aureus sp. nov., Streptomyces laceyi sp. nov. and Streptomyces sanglieri sp. nov. Antonie Leeuwenhoek 83:245-255. [DOI] [PubMed] [Google Scholar]
- 33.Marx, D. H. 1969. The influence of ectotrophic mycorrhizal fungi on the resistance of pine roots to pathogenic infections. I. Antagonism of mycorrhizal fungi to root pathogenic fungi and soil bacteria. Phytopathology 59:153-163. [PubMed] [Google Scholar]
- 34.Melin, E., and V. S. Rama Das. 1954. Influence of root-metabolites on the growth of tree mycorrhizal fungi. Physiol. Plant 7:851 858. [Google Scholar]
- 35.Nehls, U., J. Wiese, M. Guttenberger, and R. Hampp. 1998. Carbon allocation in ectomycorrhizas: identification and expression analysis of an Amanita muscaria monosaccharide transporter. Mol. Plant-Microbe Interact. 11:167-176. [DOI] [PubMed] [Google Scholar]
- 36.Pilot, G., H. Stransky, D. F. Bushey, R. Pratelli, U. Ludewig, V. P. Wingate, and W. B. Frommer. 2004. Overexpression of GLUTAMINE DUMPER1 leads to hypersecretion of glutamine from hydathodes of Arabidopsis leaves. Plant Cell 16:1827-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poole, E. J., G. D. Bending, J. M. Whipps, and D. J. Read. 2001. Bacteria associated with Pinus sylvestris-Lactarius rufus ectomycorrhizas and their effects on mycorrhiza formation in vitro. New Phytol. 151:743-751. [DOI] [PubMed] [Google Scholar]
- 38.Rainey, F. A., N. Ward-Rainey, R. M. Kroppenstedt, and E. Stackebrandt. 1996. The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage: proposal of Nocardiopsaceae fam. nov. Int. J. Syst. Bacteriol. 46:1088-1092. [DOI] [PubMed] [Google Scholar]
- 39.Richter, D. L., T. R. Zuellig, S. T. Bagley, and J. N. Bruhn. 1989. Effects of red pine (Pinus resinosa Ait.) mycorrhizoplane-associated actinomycetes on in vitro growth of ectomycorrhizal fungi. Plant Soil 115:109-116. [Google Scholar]
- 40.Rose, S. L., C.-Y. Li, and A. T. Hutchins. 1980. A streptomycete antagonist to Phellinus weirii, Fomes annosus and Phytophthora cinnamonii. Can. J. Microbiol. 26:583-587. [DOI] [PubMed] [Google Scholar]
- 41.Schaeffer, C., P. Johann, U. Nehls, and R. Hampp. 1996. Evidence for an up-regulation of the host and down-regulation of the fungal phosphofructokinase activity in ectomycorrhizas of Norway spruce and fly agaric. New Phytol. 134:697-702. [DOI] [PubMed] [Google Scholar]
- 42.Schrey, S. D., M. Schellhammer, M. Ecke, R. Hampp, and M. T. Tarkka. 2005. Mycorrhiza helper bacterium Streptomyces AcH 505 induces differential gene expression in the ectomycorrhizal fungus Amanita muscaria. New Phytol. 168:205-216. [DOI] [PubMed] [Google Scholar]
- 43.Shirling, E. B., and D. Gottlieb. 1966. Methods for characterization of Streptomyces species. Int. J. Syst. Bacteriol. 16:313-340. [Google Scholar]
- 44.Smith, S. E., and D. J. Read. 1997. Mycorrhizal symbiosis. Academic Press, London, United Kingdom.
- 45.Stackebrandt, E., F. A. Rainey, and N. L. Ward-Rainey. 1997. Proposal for a new hierarchic classification system, Actinobacteria classis nov. Int. J. Syst. Bacteriol. 47:479-491. [Google Scholar]
- 46.Staneck, J. L., and G. D. Roberts. 1974. Simplified approach to the identification of aerobic actinomycetes by thin-layer chromatography. Appl. Microbiol. 28:226-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanaka, Y., and S. Ōmura. 1993. Agroactive compounds of microbial origin. Annu. Rev. Microbiol. 47:57-87. [DOI] [PubMed] [Google Scholar]
- 48.Tindall, B. I. 1990. Lipid composition of Halobacterium lacusprofundi. FEMS Microbiol. Lett. 66:199-202. [Google Scholar]
- 49.Wrath, R., P. A. Briand, and D. Picard. 1997. Functional analysis of the yeast 40 kDa cyclophilin Cyp40 and its role for viability and steroid receptor regulation. Biol. Chem. 378:381-391. [DOI] [PubMed] [Google Scholar]