Abstract
The Wingless pathway plays an essential role during synapse development. Recent studies at Drosophila glutamatergic synapses suggest that Wingless is secreted by motor neuron terminals and binds to postsynaptic Drosophila Frizzled-2 (DFz2) receptors. DFz2 is, in turn, endocytosed and transported to the muscle perinuclear area, where it is cleaved, and the C-terminal fragment is imported into the nucleus, presumably to regulate transcription during synapse growth. Alterations in this pathway interfere with the formation of new synaptic boutons and lead to aberrant synaptic structures. Here, we show that the 7 PDZ protein dGRIP is necessary for the trafficking of DFz2 to the nucleus. dGRIP is localized to Golgi and trafficking vesicles, and dgrip mutants mimic the synaptic phenotypes observed in wg and dfz2 mutants. DFz2 and dGRIP colocalize in trafficking vesicles, and a severe decrease in dGRIP levels prevents the transport of endocytosed DFz2 receptors to the nucleus. Moreover, coimmunoprecipitation experiments in transfected cells and yeast two-hybrid assays suggest that the C terminus of DFz2 interacts directly with the PDZ domains 4 and 5. These results provide a mechanism by which DFz2 is transported from the postsynaptic membrane to the postsynaptic nucleus during synapse formation and implicate dGRIP as an essential molecule in the transport of this signal.
Recent studies have implicated the Wingless (Wg)/Wnt pathway as one of the signaling pathways central to synapse differentiation. In vertebrates, Wnt-7a functions as a retrograde messenger that promotes synapse maturation and, additionally, modulates dendritic development (1–3). At the fly neuromuscular junction (NMJ) the Wnt homologue Wg is secreted by presynaptic terminals, and its receptor, Drosophila Frizzled-2 (DFz2) is localized both pre- and postsynaptically (4). Wg secretion at the synapse is required for the normal proliferation of synaptic boutons during muscle growth and for the differentiation of active zones and postsynaptic specializations (4, 5).
Wg/Wnt signaling occurs through a variety of pathways. In some epithelial tissues, Wg operates through a canonical pathway in which Wg binding to DFz initiates a cascade that leads to the formation of a complex between Armadillo/β-catenin and transcription factors that are imported into the nucleus, where they regulate transcription (6). Wnt ligands can also signal through noncanonical pathways that involve G proteins or the release of calcium from intracellular stores (3). A recent study described an alternative pathway in which DFz2 is continuously endocytosed at the plasma membrane of postsynaptic muscles and transported to the perinuclear area. Upon Wg signaling, the C-terminal region of DFz2 is cleaved and imported into the nucleus. In the absence of nuclear import, synaptic proliferation and the formation of synaptic specializations are severely affected (7). The molecular mechanisms by which DFz2 receptors are trafficked to the nucleus are unknown.
In a search for synaptic proteins involved in synapse development, we identified the Drosophila homologue of GRIP, a 7-PDZ protein that, in vertebrates, is thought to be involved in trafficking and scaffolding of several synaptic molecules, including AMPA receptors (8–12), Ephrin ligands and receptors (13, 14), and Liprin (15). The elucidation of GRIP function in the mammalian nervous system has been complicated by the presence of multiple GRIP PDZ domains and the widespread localization of GRIP isoforms in many tissues. Furthermore, mouse grip1 knockouts generally die as embryos (16), and the few surviving adults have a wide range of developmental defects, but no neuronal phenotypes have been described (17). This early lethality, combined with pleiotropic defects, has precluded a study of the specific function of GRIP in the nervous system in an intact organism. A recent study examined the role of GRIP in cultured hippocampal neurons and found that GRIP is required for dendrite morphogenesis (14). However, the validity of these findings for the intact nervous system remains to be tested.
In Drosophila, there is a single dgrip gene, and a previous study has implicated dGRIP in muscle guidance during embryonic development (18); however, its potential function during synapse development was not addressed. By using a combination of genetic and tissue-specific RNA interference (RNAi) expression tools, we have been able to examine the synaptic phenotypes resulting from synaptic dGRIP loss of function in vivo.
We show that dGRIP is present in both presynaptic terminals and postsynaptic muscles in vesicles associated with the microtubule cytoskeleton. Perturbation of dGRIP levels interferes with normal synapse development, and the synaptic defects observed in dgrip loss-of-function conditions phenocopy those observed in wg and dfz2 mutants. We also show that dGRIP and the PDZ-binding domain of DFz2 interact and that this interaction is likely to be required for the retrograde transport of DFz2 from the synapse to the nucleus. These results suggest that, at synapses, dGRIP is involved in the transduction of the Wg signaling pathway required to specify the differentiation of pre- and postsynaptic structures.
Results
dGRIP Is Localized to Synapses and to Trafficking Vesicles.
To examine the pattern of dGRIP expression at the NMJ, we generated two antibodies directed against the N and C termini of dGRIP (Fig. 1A). The staining with these antibodies was specific because both antibodies gave rise to the same pattern of immunoreactivity at the body-wall muscles, and signal intensity was reduced in dgrip mutants or enhanced by dGRIP overexpression (see below). dGRIP was localized both at the pre- and postsynaptic compartments of the NMJ (Fig. 1 B and C). At the presynaptic compartment, dGRIP was observed in a punctate pattern that was enriched along the microtubule bundle that transverses NMJ branches and is labeled by antibodies against the MAP1B-related protein Futsch (19) (Fig. 1 E–H). dGRIP puncta were also found inside the bouton cytoplasm, especially at distal boutons within an NMJ branch (arrowheads in Fig. 1 D and I).
Fig. 1.
Localization of dGRIP at the NMJ. (A) Genomic structure of dgrip showing exons 1–10. Blue, localization of dgripf05600 and the 2 FRT insertions used to generate the null allele; red, region targeted by dGRIP-RNAi (Upper). Protein structure of dGRIP and organization of PDZ domains (Lower). Green, regions used to generate dGRIP antibodies. (B–D and I) dGRIP immunoreactivity at the NMJ and muscles in a preparation stained with antibodies against dGRIP (green) and HRP (red) shown at low (B and C) and high (D and I) magnification in muscles 6 and 7. Arrowheads, punctate dGRIP immunoreactivity at presynaptic boutons; arrows, punctate dGRIP at the postsynaptic muscle area. (E–H) Localization of dGRIP at the presynaptic microtubule bundle, shown in a preparation labeled with antibodies against dGRIP (green), Futsch (red), and HRP (blue). Arrows, large dGRIP muscle spots; arrowhead, punctate dGRIP immunoreactivity at the peribouton area. (J–M) Localization of large dGRIP spots to Golgi bodies shown at low (J) and high (K–M) magnification in a preparation stained with antibodies against dGRIP (green), Tubulin (blue), and Lva (red). Arrow, Lva-positive dGRIP spot; arrowheads, Lva-negative dGRIP puncta. (M Inset) High-magnification view of a GRIP-Lva spot. (N) Association of Lva-positive spots with microtubules shown in a preparation labeled with Lva (blue), Tubulin (red), and Spectrin (green). All are single confocal slices, except for B, C, and J, which are a Z-series projection. [Scale bar, 70 μm (B and C), 17 μm (E–H), 13 μm (J), 8 μm (D, I, and K–N), and 2.5 μm (M Inset).]
In muscles, dGRIP was localized in puncta and in large well defined spots (≈0.5 μm) throughout the muscle cytoplasm (Fig. 1 B and C and arrows in E and K). Muscle puncta were found in the muscle cortical region, and their frequency increased at the peribouton area (Fig. 1 D and I, long arrows and G and H, arrowheads). In contrast, the large spots had a uniform distribution (Fig. 1 B and C). We used several markers to identify the intracellular compartments labeled by dGRIP antibodies. A one-to-one correspondence was found between the large dGRIP spots and Lava lamp (Lva), a Golgi resident protein (20) (Fig. 1 J–M, arrows), but the smaller puncta were not associated with Lva (Fig. 1M, arrowhead). Although there was a near perfect one-to-one relationship between large dGRIP spots and Lva, Lva and dGRIP immunoreactivity were slightly offset, suggesting that the two proteins may occupy slightly different Golgi subcompartments (Fig. 1M Inset). dGRIP spots and Lva were also found to be associated with microtubules in muscles (Fig. 1N; see below). Neither large dGRIP-Lva spots nor Lva were found within presynaptic boutons (Fig. 1N). Thus, dGRIP is found both pre- and postsynaptically in a punctate pattern, with a fraction of the postsynaptic staining associated with Golgi bodies.
We next examined the relationship between microtubules and dGRIP puncta and spots in vivo by expressing dGRIP-red fluorescent protein (RFP) and Tubulin-GFP fusion proteins in muscles by using the UAS/Gal4 system (21). The specificity of the RFP label to dGRIP was confirmed with anti-dGRIP immunoreactivity (data not shown). Dissected larvae were imaged by time-lapse confocal microscopy. Large Lva-dGRIP-RFP spots were juxtaposed to microtubules and appeared to be stationary. In contrast, many of the smaller Lva-negative dGRIP-RFP puncta, which were also juxtaposed to microtubules, were motile (see Movie 1 and Fig. 7, which are published as supporting information on the PNAS web site).
Abnormal Synapse Development in Larvae with Decreased dGRIP Levels.
The role of dGRIP at the NMJ was investigated by generating mutants and dGRIP-RNAi (22). Two null dgrip alleles were available, dgripex36 (18), and dgripB-LO1 (see Fig. 8G, which is published as supporting information on the PNAS web site) generated here (23), which deleted the entire dgrip genomic region. However, in both alleles, muscle patterning was abnormal (ref. 18 and this report), making it difficult to discern which defects were solely of synaptic origin. Therefore, we used a dGRIP-RNAi construct fused to Gal4-binding sites (Fig. 1A), allowing us to temporally control dGRIP disruption and circumvent the early roles of dGRIP in muscle patterning when expressed in the postembryonic period. This construct decreased presynaptic dGRIP immunoreactivity by 30–50% when expressed with the presynaptic drivers C380 (24) or elav-Gal4 (Fig. 2A–F) and by ≈80% when expressed in muscles with the C57 driver (24) (Fig. 2 G–I). Real-time PCR analysis further confirmed a specific decrease in dgrip mRNA levels in these RNAi lines (Fig. 8G). We also examined a dgrip hypomorph, dgripf05600, containing a P-element insertion in the first intron of the gene (Fig. 1A). In this mutant, dGRIP was localized in aggregations at extrasynaptic sites at the muscle surface and showed a reduction of dGRIP immunoreactivity at the NMJ, but muscle patterning was mostly normal (Fig. 8).
Fig. 2.
dGRIP immunoreactivity and quantification of NMJ morphology in larvae expressing dGRIP-RNAi. (A–I) dGRIP immunoreactivity at the NMJ in preparations labeled with antibodies against dGRIP (green) and Spectrin (red) (A–C and G–I) or dGRIP (green) and HRP (red) (D–F), in wild-type larvae (A–C) and larvae expressing dGRIP-RNAi in motor neurons (D–F) or muscles (G–I). (J and K) Number of synaptic (J) and ghost (K) boutons at muscles 6 and 7 (A3) in third-instar larvae of various genotypes. (Scale bar, 13 μm.)
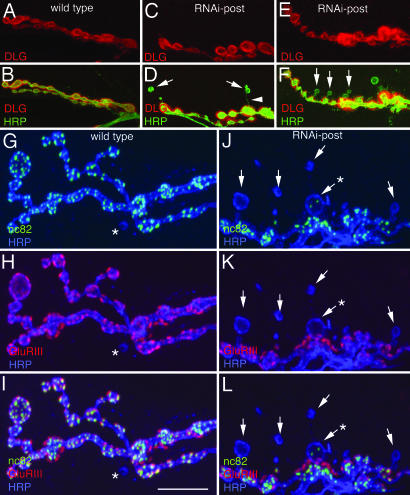
NMJs expressing the RNAi construct pre- or postsynaptically or from dgripf05600 were abnormal in similar ways, having a marked decrease in the number of synaptic boutons (Fig. 2J). We also observed boutons with an atypical shape (Fig. 3B, D, and F, arrows), which were clearly marked by the presynaptic marker anti-horseradish peroxidase (HRP), but were completely devoid of postsynaptic DLG immunoreactivity, suggesting that postsynaptic structure was abnormal (Figs. 3 A–F and 8K). These boutons, which we named “ghost” boutons, had a rounded appearance, as opposed to the more elliptical appearance of Type I boutons. In addition, a thin neuronal process connecting the ghost bouton with a main NMJ branch could occasionally be discerned (Fig. 3D, arrowhead).
Fig. 3.
Ghost boutons lack postsynaptic proteins and most active zones. (A–L) Third instar NMJs in wild-type larvae (A, B, and G–I) and in larvae expressing dGRIP-RNAi postsynaptically (C–F and J–L) in preparations labeled with anti-HRP (green) and anti-DLG (red) (A–F), and anti-HRP (blue), anti-GluRIII (red), and nc82 (green) (G–L). Arrows, ghost boutons; asterisks in G–I, uncommon ghost bouton observed in wild type; arrowhead, HRP-labeled process connecting a ghost bouton with the main arbor; asterisks in J–L, ghost bouton containing nc82 immunoreactivity. [Scale bar, 15 μm (A–F) and 12 μm (G–L).]
We examined the localization of several pre- and postsynaptic proteins in ghost boutons. None of the postsynaptic proteins examined, including glutamate receptors (GluRIIA and GluRIII) (Fig. 3 G–L, arrows), Scribble, Bazooka, DFz2, and Spectrin were present in ghost boutons, suggesting that the postsynaptic apparatus was missing or severely defective in ghost boutons. Most ghost boutons were also devoid of active zones, as visualized with monoclonal antibody nc82 (25, 26) (Fig. 3 J–L, arrows), although, rarely, a ghost bouton contained a few active zones (e.g., Fig. 3J, asterisk). In contrast, synaptic vesicle proteins, such as Synapsin (see Fig. 9, which is published as supporting information on the PNAS web site) and CSP (data not shown) were present in ghost boutons.
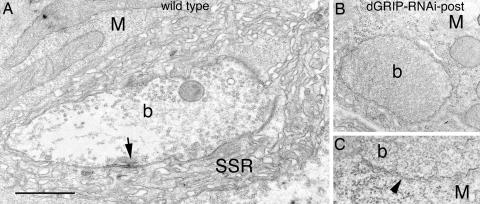
We also carried out an ultrastructural analysis of NMJs. We found that, in animals expressing dGRIP-RNAi in postsynaptic muscles, there was a population of synaptic boutons that were filled with synaptic vesicles but were devoid of active zones or postsynaptic specializations, including postsynaptic densities and subsynaptic reticulum (SSR) (Fig. 4).
Fig. 4.
Disrupted synaptic structure in larvae expressing dGRIP-RNAi-post. Cross-section through a type I bouton in wild type (A), and a ghost bouton lacking active zones and SSR (B and C) dGRIP-RNAi-post; arrow, active zone. (C) High-magnification view of a ghost bouton membrane (arrowhead), showing its abnormal ruffled appearance. M, muscle; b, bouton. [Scale bar, 1.2 μm (A and B) and 0.3 μm (C).]
The ultrastructural phenotype observed in dGRIP RNAi-post synapses was distinctly similar to the phenotype observed in wg mutations and in genetic manipulations that interfere with DFz2 in postsynaptic muscles (4, 7). In these mutants, which also have diminished synaptic boutons, a subset of boutons lacking active zones, postsynaptic densities, and SSR, yet containing synaptic vesicles, are observed. Therefore, we examined whether ghost boutons are formed when Wg signaling is disrupted specifically in the postsynaptic muscles by expressing a dominant-negative DFz2 transgene DFz2-C, consisting of just the cytoplasmic region of DFz2, in muscles. We found that, indeed, the number of ghost boutons was increased in DFz2-C-post NMJs, similar to dgripf05600 mutants and dGRIP-RNAi (Fig. 2K).
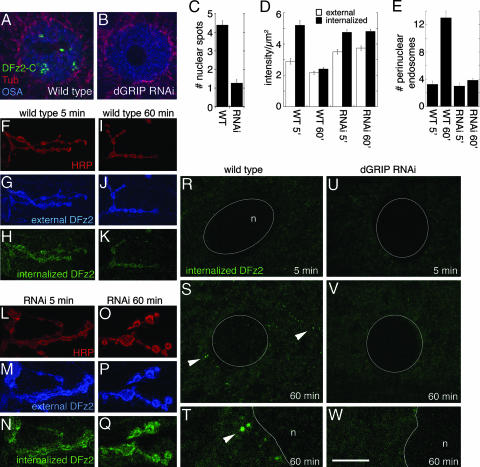
The similarity between these phenotypes pointed to a potential relationship between dGRIP and the Wg pathway at the NMJ. In this alternative transduction mechanism, synaptic DFz2 is endocytosed and transported to the nuclear area, where the C terminus of DFz2 is cleaved and imported into the nucleus (7). To determine whether dGRIP was involved in this process, we examined larvae expressing dGRIP-RNAi in muscle cells, and we used antibodies to the C-terminal region of DFz2 (DFz2-C) to determine the presence of intranuclear DFz2-C spots. We found that the number of DFz2-C-positive nuclear spots was drastically reduced in muscles expressing dGRIP-RNAi (Fig. 5A–C).
Fig. 5.
Abnormal trafficking of DFz2-C from the synapse to the nucleus in larvae expressing dGRIP-RNAi-post. (A and B) Muscle nucleus in wild type (A) and in a dGRIP-RNAi-post larva (B) in preparations stained with antibodies against DFz2-C (green), Tubulin (red), and OSA (blue). (C) Number of DFz2-C immunoreactive nuclear spots. (D) External and internalized DFz2 signal intensity at the NMJ. (E) Number of DFz2-N-positive perinuclear vesicles in the internalization assay. (F–Q) External (blue) and internalized (green) DFz2 at the NMJ of wild type (F–K) and dGRIP-RNAi post (L–Q), at 5 (F–H and L–N) and 60 (I–K and O–Q) min after the antibody-binding step in the internalization assay. NMJs are visualized by the anti-HRP staining (red). (R–W) Internalized DFz2 (green) at the perinuclear area in wild-type (R–T), and dGRIP-RNAi-post (U–W) larvae, at 5 (R and U) and 60 (S, T, V, and W) min after the antibody-binding step. (T and W) High-magnification views of a region in S and V. Arrowheads, internalized DFz2 vesicles at the perinuclear area. n, nucleus. [Scale bar, 10 μm (A and B), 20 μm (F–Q), 12 μm (R–V), and 3 μm (T and W).]
We next tested whether DFz2 trafficking was altered in dGRIP-RNAi-post flies by using a DFz2 internalization assay that has been used to characterize DFz2 trafficking from synapses to the nucleus (7). In this assay, body-wall muscles dissected in physiological saline are incubated in the cold with an antibody (DFz2-N) that labels the surface pool of DFz2. After the antibody-binding step, excess antibody is washed away, and preparations are shifted to room temperature and fixed at various times. DFz2–antibody complexes remaining at the surface are recognized by using an Alexa Fluor 647-conjugated secondary antibody applied under nonpermeabilizing conditions, and internalized DFz2–antibody complexes are subsequently labeled by a FITC-conjugated secondary antibody after permeabilization. As demonstrated in ref. 7, in wild type at 5 min after the antibody-binding step, internalized DFz2 accumulated around synaptic boutons (Fig. 5 D and F–H) but was not observed at the perinuclear area (Fig. 5 E and R). At 60 min, however, internalized DFz2 decreased at the NMJ, and this decrease was accompanied by the presence of DFz2 vesicles at the perinuclear area (Fig. 5 D–E, I–K, and S–T, arrowheads). In larvae expressing dGRIP-RNAi postsynaptically, the amount of surface and internalized DFz2 was significantly greater than wild type (Fig. 5 D and L–N). In striking contrast to wild type, 60 min after the antibody-binding step, the level of DFz2 at the NMJ was still unchanged, remaining as high as at 5 min (Fig. 5 D and O–Q). Also, no DFz2 vesicles were observed at the perinuclear area at either 5 or 60 min (Fig. 5 E and U–W). These results provide evidence that severe reductions in dGRIP levels prevent the transport of DFz2 to the perinuclear area and suggest that dGRIP is required for a step in DFz2 trafficking to the nucleus.
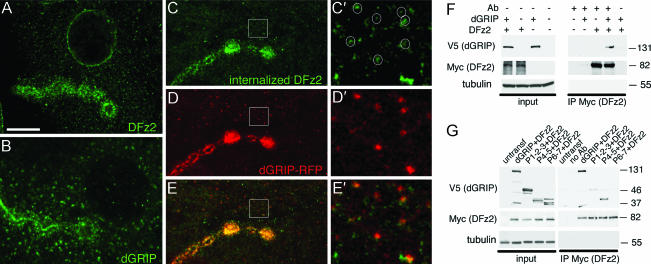
DFz2 contains a PDZ-binding motif (ASHV) at the C-terminal tail and is localized around synapses in a pattern similar to that of dGRIP (Fig. 6A and B). To determine whether dGRIP was likely to be associated with internalized DFz2 receptors and/or with DFz2-containing endosomes, the above DFz2 internalization assay was performed by using larvae expressing dGRIP-RFP. We found that dGRIP and endocytosed DFz2 strongly colocalized at the bouton and peribouton areas (Fig. 6 C–E) and in many DFz2-containing internalized vesicles in the muscle (Fig. 6 C′–E′). Thus, internalized DFz2 is present in a subset of dGRIP vesicles, and dGRIP is required to traffic DFz2 to the nucleus.
Fig. 6.
Colocalization of internalized DFz2 with dGRIP and interactions between dGRIP and DFz2. (A and B) Localization of DFz2 (A) and dGRIP (B) at the NMJ. (C–E) Colocalization between dGRIP-RFP (red) and internalized DFz2 (green) at the NMJ. (C′–E′) High-magnification views of the regions enclosed by squares in C–E. (F) Coimmunoprecipitation of V5 (dGRIP) by anti-Myc (DFz2) in S2 cells. (G) Coimmunoprecipitation of dGRIP deletion constructs. Input lanes in F and G correspond to 10% of the initial extract. Molecular masses are shown in kDa. [Scale bar, 10 μm (A–E) and 2 μm (C′–E′).]
We also tested the possibility that DFz2 and dGRIP may interact using Drosophila Schneider (S2) cells transfected with Myc-DFz2 and V5-dGRIP. We found that antibodies to Myc (DFz2) immunoprecipitated V5 (dGRIP), suggesting that, indeed, DFz2 and dGRIP can interact (Fig. 6F). To determine the likely regions for such an interaction, S2 cells were cotransfected with Myc-DFz2, and either the deletion constructs V5-PDZ1,2,3, V5-PDZ4,5, and V5-PDZ6,7 or full-length V5-dGRIP were cotransfected. As above, anti-Myc antibodies immunoprecipitated full-length V5-dGRIP, but also V5-PDZ4,5 alone (Fig. 6G). In contrast, very weak immunoprecipitation of V5-PDZ1,2,3 or V5-PDZ6,7 was observed, suggesting that the interaction is very likely through PDZ4 and -5. The specific interaction between PDZ4 and -5 of dGRIP and the C-terminal region of DFz2 was confirmed by using the yeast two-hybrid assay. This interaction was quite specific for the PDZ4 and -5 domains, because the DFz2 C-terminal did not show any interaction with any of the 3 PDZ domains of DLG or with the 4 PDZ domains of Scribble with the yeast two-hybrid assay (data not shown). Together, the colocalization of internalized DFz2 and dGRIP at the NMJ, the similarity of the phenotypes at synaptic boutons when either protein is decreased or eliminated, and the interactions between these proteins observed by immunoprecipitations and the yeast two-hybrid assay suggest that both proteins may function in the same synaptic development pathway.
Discussion
At the NMJ, the Wg pathway is initiated by the secretion of Wg from the presynaptic cells and its binding to DFz2 receptors present at the postsynaptic muscle cells (4). Upon Wg binding to DFz2, the receptor is internalized and transported to the perinuclear area, where it is cleaved, and the C-terminal DFz2 fragment (DFz2-C) is imported into the nucleus (7). Although the evidence suggested that endocytosis at the postsynaptic membrane and transport via microtubules are required for DFz2 trafficking from synapses to the muscle nucleus, the exact molecular mechanisms of trafficking were unknown. In this study, we provide evidence that the transport of DFz2 to the nucleus depends on interactions between a PDZ-binding motif at the C terminus of DFz2 and PDZ domains of dGRIP. We show that, in postsynaptic muscles, dGRIP is present in Golgi bodies and in a subset of vesicles that is highly concentrated at the postsynaptic area. These vesicles move along microtubules and colocalize with internalized DFz2. We also show that DFz2 and dGRIP can directly interact when expressed in heterologous systems. Manipulations that lead to severe reduction of dGRIP mimic all of the synaptic phenotypes resulting from mutations in wg or dfz2. Furthermore, in these dgrip mutants, internalized DFz2 accumulates at the postsynaptic region and is not transported to the nucleus. We suggest that dGRIP is required at synapses to mediate the trafficking of DFz2 to the nucleus to properly regulate the expansion of the NMJ during muscle growth.
Synaptic Functions of GRIP in Mammals and Flies.
Studies suggest that GRIP is involved in the clustering and trafficking of AMPA receptors at mammalian synapses (8, 9, 15, 27). GRIP has also been found to interact with Ephrin ligands and Eph receptors (13, 14), neuronal RAS guanine nucleotide exchange factor (GRASP1) (28), members of the Liprin-α/syd2 family of proteins (15), the KIF5 microtubule motor kinesin (27), and extracellular matrix protein FRAS1 (17). This large number of partners identified is perhaps not surprising, given that GRIP contains at least seven modular protein-interaction domains.
Studies have shown that, similar to dGRIP, rat GRIP is also localized to both presynaptic axons and postsynaptic dendritic structures and is enriched in vesicular profiles that closely associate with microtubules (29, 30). Furthermore, a recent study shows that knockdown of GRIP-1 using siRNA in primary hippocampal neurons interfered with the formation and growth of dendrites in developing neurons and the maintenance of dendrites in mature neurons (14). In this study, we similarly found that interfering with GRIP function hampered synaptic bouton formation and growth in larval glutamatergic synapses. Additionally, elimination of postsynaptic dGRIP led to loss of the postsynaptic apparatus and presynaptic active zones and, presumably, to either the retraction or deficient stabilization of new synaptic boutons (see below). These results imply that GRIP family proteins have a conserved role in both the formation and the stabilization of synapses in the nervous system.
In Drosophila, dGRIP is involved in the guidance of embryonic muscle precursors to establish the proper body-wall muscle pattern (18), whereas here, we show that dGRIP is required for synapse differentiation. These results are not surprising, given the recurrent theme that many molecules necessary for early pattern formation in the embryo, such as members of the TGF-β pathway, the Wg pathway, and the tumor suppressor proteins DLG and Scribble (Scrib) are used again during synapse development (5).
Involvement of dGRIP in DFz2 Trafficking During Synapse Development.
Evidence that dGRIP and DFz2 might interact arises from the observation that manipulations that lead to alterations in both proteins give rise to remarkably similar phenotypes, including decreased NMJ expansion and the presence of ghost boutons, which lack all postsynaptic proteins studied and the subsynaptic reticulum, and are devoid of active zones but filled with synaptic vesicles. These ghost boutons may represent boutons that initiated their differentiation presynaptically but never fully matured by forming corresponding pre- and postsynaptic specializations. Alternatively, the ghost boutons may represent boutons that are initially formed (including differentiation of both pre- and postsynaptic specializations) but subsequently retracted. However, studies have suggested that retraction of mature boutons at the Drosophila NMJ is accompanied by the presence of “synaptic footprints,” in which postsynaptic proteins are still present, despite the absence of a presynaptic bouton (31). In our mutants, we observe the opposite phenotype, where synapses have some presynaptic proteins but are devoid of a postsynaptic apparatus.
Interestingly, synaptic footprints are observed when the retrograde signaling mediated by TGFβ is disrupted (32). In contrast, ghost boutons are observed when the Wg pathway is abnormal. The Wg pathway at the NMJ has been shown to function in an anterograde manner, but the possibility that it also functions in a retrograde manner has not been studied. These findings suggest that, at the Drosophila NMJ, synapse retraction can be induced both pre- and postsynaptically, as in the vertebrate NMJ (33–35). It would be interesting to determine whether anterograde Wg and retrograde TGF-β signaling pathways crosstalk and coordinate synapse stability during development. Taken together, our studies suggest that one function of dGRIP at the NMJ is in trafficking DFz2 to the nucleus, which, in turn, regulates synaptic growth.
Materials and Methods
The following fly stocks were used: the wild-type strain Canton-S (CS), dgripB-L01, dgripf05600 (Exelixis, Harvard Medical School, Boston), UAS-Tubulin-GFP (36), UAS-6xMyc-DFz2-C (7), and the Gal4 strains BG487, C57, C380, and C164 (24, 37). We also generated the following UAS strains: UAS-dGRIP (ORF of RE14068), UAS-dGRIP-RFP, and UAS-dGRIP-RNAi.
For immunocytochemistry, Drosophila third-instar larvae were dissected and processed as in ref. 24. Antibody conditions, morphometric analysis, internalization assay, yeast two-hybrid assay, real-time PCR, and constructs used are described in Supporting Materials and Methods, which is published as supporting information on the PNAS web site. Electron microscopy was performed as in ref. 4. S2 cell transfection and immunoprecipitations from S2 cells were carried out as in ref. 38.
Supplementary Material
Acknowledgments
We thank Drs. S. Cumberledge and S. Speese for comments on the manuscript, Dr. C. Ruiz-Canada for preliminary characterization of dGRIP, and M. Walsh for the yeast two-hybrid assay. This work was supported by National Institutes of Health Grants NS042629 and MH070000 (to V.B.).
Abbreviations
- DFz2
Drosophila Frizzled-2
- HRP
horseradish peroxidase
- Lva
Lava lamp
- NMJ
neuromuscular junction
- RFP
red fluorescent protein
- RNAi
RNA interference
- Wg
Wingless.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Hall A. C., Lucas F. R., Salinas P. C. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- 2.Ciani L., Salinas P. C. Nat. Rev. Neurosci. 2005;6:351–362. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- 3.Rosso S. B., Sussman D., Wynshaw-Boris A., Salinas P. C. Nat. Neurosci. 2005;8:34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- 4.Packard M., Koo E. S., Gorczyca M., Sharpe J., Cumberledge S., Budnik V. Cell. 2002;111:319–330. doi: 10.1016/s0092-8674(02)01047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Packard M., Mathew D., Budnik V. Nat. Rev. Neurosci. 2003;4:113–120. doi: 10.1038/nrn1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moon R. T., Bowerman B., Boutros M., Perrimon N. Science. 2002;296:1644–1646. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- 7.Mathew D., Ataman B., Chen J., Zhang Y., Cumberledge S., Budnik V. Science. 2005;310:1344–1347. doi: 10.1126/science.1117051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong H., O'Brien R. J., Fung E. T., Lanahan A. A., Worley P. F., Huganir R. L. Nature. 1997;386:279–284. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- 9.Liu S. J., Cull-Candy S. G. Nat. Neurosci. 2005;8:768–775. doi: 10.1038/nn1468. [DOI] [PubMed] [Google Scholar]
- 10.Hirbec H., Francis J. C., Lauri S. E., Braithwaite S. P., Coussen F., Mulle C., Dev K. K., Coutinho V., Meyer G., Isaac J. T., et al. Neuron. 2003;37:625–638. doi: 10.1016/s0896-6273(02)01191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braithwaite S. P., Xia H., Malenka R. C. Proc. Natl. Acad. Sci. USA. 2002;99:7096–7101. doi: 10.1073/pnas.102156099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osten P., Khatri L., Perez J. L., Kohr G., Giese G., Daly C., Schulz T. W., Wensky A., Lee L. M., Ziff E. B. Neuron. 2000;27:313–325. doi: 10.1016/s0896-6273(00)00039-8. [DOI] [PubMed] [Google Scholar]
- 13.Contractor A., Rogers C., Maron C., Henkemeyer M., Swanson G. T., Heinemann S. F. Science. 2002;296:1864–1869. doi: 10.1126/science.1069081. [DOI] [PubMed] [Google Scholar]
- 14.Hoogenraad C. C., Milstein A. D., Ethell I. M., Henkemeyer M., Sheng M. Nat. Neurosci. 2005;8:906–915. doi: 10.1038/nn1487. [DOI] [PubMed] [Google Scholar]
- 15.Wyszynski M., Kim E., Dunah A. W., Passafaro M., Valtschanoff J. G., Serra-Pages C., Streuli M., Weinberg R. J., Sheng M. Neuron. 2002;34:39–52. doi: 10.1016/s0896-6273(02)00640-2. [DOI] [PubMed] [Google Scholar]
- 16.Bladt F., Tafuri A., Gelkop S., Langille L., Pawson T. Proc. Natl. Acad. Sci. USA. 2002;99:6816–6821. doi: 10.1073/pnas.092130099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takamiya K., Kostourou V., Adams S., Jadeja S., Chalepakis G., Scambler P. J., Huganir R. L., Adams R. H. Nat. Genet. 2004;36:172–177. doi: 10.1038/ng1292. [DOI] [PubMed] [Google Scholar]
- 18.Swan L. E., Wichmann C., Prange U., Schmid A., Schmidt M., Schwarz T., Ponimaskin E., Madeo F., Vorbruggen G., Sigrist S. J. Genes Dev. 2004;18:223–237. doi: 10.1101/gad.287604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruiz-Canada C., Ashley J., Moeckel-Cole S., Drier E., Yin J., Budnik V. Neuron. 2004;42:567–580. doi: 10.1016/s0896-6273(04)00255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sisson J. C., Field C., Ventura R., Royou A., Sullivan W. J. Cell Biol. 2000;151:905–918. doi: 10.1083/jcb.151.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brand A. H., Perrimon N. Development (Cambridge, U.K.) 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 22.Kalidas S., Smith D. P. Neuron. 2002;33:177–184. doi: 10.1016/s0896-6273(02)00560-3. [DOI] [PubMed] [Google Scholar]
- 23.Ryder E., Blows F., Ashburner M., Bautista-Llacer R., Coulson D., Drummond J., Webster J., Gubb D., Gunton N., Johnson G., et al. Genetics. 2004;167:797–813. doi: 10.1534/genetics.104.026658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Budnik V., Koh Y. H., Guan B., Hartmann B., Hough C., Woods D., Gorczyca M. Neuron. 1996;17:627–640. doi: 10.1016/s0896-6273(00)80196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wucherpfennig T., Wilsch-Brauninger M., Gonzalez-Gaitan M. J. Cell Biol. 2003;161:609–624. doi: 10.1083/jcb.200211087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marrus S. B., Portman S. L., Allen M. J., Moffat K. G., DiAntonio A. J. Neurosci. 2004;24:1406–1415. doi: 10.1523/JNEUROSCI.1575-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Setou M., Seog D. H., Tanaka Y., Kanai Y., Takei Y., Kawagishi M., Hirokawa N. Nature. 2002;417:83–87. doi: 10.1038/nature743. [DOI] [PubMed] [Google Scholar]
- 28.Ye B., Liao D., Zhang X., Zhang P., Dong H., Huganir R. L. Neuron. 2000;26:603–617. doi: 10.1016/s0896-6273(00)81198-8. [DOI] [PubMed] [Google Scholar]
- 29.Wyszynski M., Valtschanoff J. G., Naisbitt S., Dunah A. W., Kim E., Standaert D. G., Weinberg R., Sheng M. J. Neurosci. 1999;19:6528–6537. doi: 10.1523/JNEUROSCI.19-15-06528.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong H., Zhang P., Liao D., Huganir R. L. Ann. N.Y. Acad. Sci. 1999;868:535–540. doi: 10.1111/j.1749-6632.1999.tb11323.x. [DOI] [PubMed] [Google Scholar]
- 31.Eaton B. A., Fetter R. D., Davis G. W. Neuron. 2002;34:729–741. doi: 10.1016/s0896-6273(02)00721-3. [DOI] [PubMed] [Google Scholar]
- 32.Eaton B. A., Davis G. W. Neuron. 2005;47:695–708. doi: 10.1016/j.neuron.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Walsh M. K., Lichtman J. W. Neuron. 2003;37:67–73. doi: 10.1016/s0896-6273(02)01142-x. [DOI] [PubMed] [Google Scholar]
- 34.Balice-Gordon R. J., Chua C. K., Nelson C. C., Lichtman J. W. Neuron. 1993;11:801–815. doi: 10.1016/0896-6273(93)90110-d. [DOI] [PubMed] [Google Scholar]
- 35.Colman H., Nabekura J., Lichtman J. W. Science. 1997;275:356–361. doi: 10.1126/science.275.5298.356. [DOI] [PubMed] [Google Scholar]
- 36.Grieder N. C., de Cuevas M., Spradling A. C. Development (Cambridge, U.K.) 2000;127:4253–4264. doi: 10.1242/dev.127.19.4253. [DOI] [PubMed] [Google Scholar]
- 37.Torroja L., Packard M., Gorczyca M., White K., Budnik V. J. Neurosci. 1999;19:7793–7803. doi: 10.1523/JNEUROSCI.19-18-07793.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashley J., Packard M., Ataman B., Budnik V. J. Neurosci. 2005;25:5943–5955. doi: 10.1523/JNEUROSCI.1144-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.